Sex Differences in the Level of Homocysteine in Alzheimer’s Disease and Parkinson’s Disease Patients: A Meta-Analysis
Abstract
1. Introduction
1.1. Alzheimer’s Disease
1.2. Parkinson’s Disease
1.3. Homocysteine
1.4. Sex Differences
2. Materials and Methods
2.1. Search Methodology
2.2. Criteria for Inclusion/Exclusion
2.3. Risk of Bias Assessment
2.4. Data Extraction and Statistical Analysis
3. Results
3.1. Number of Studies Included
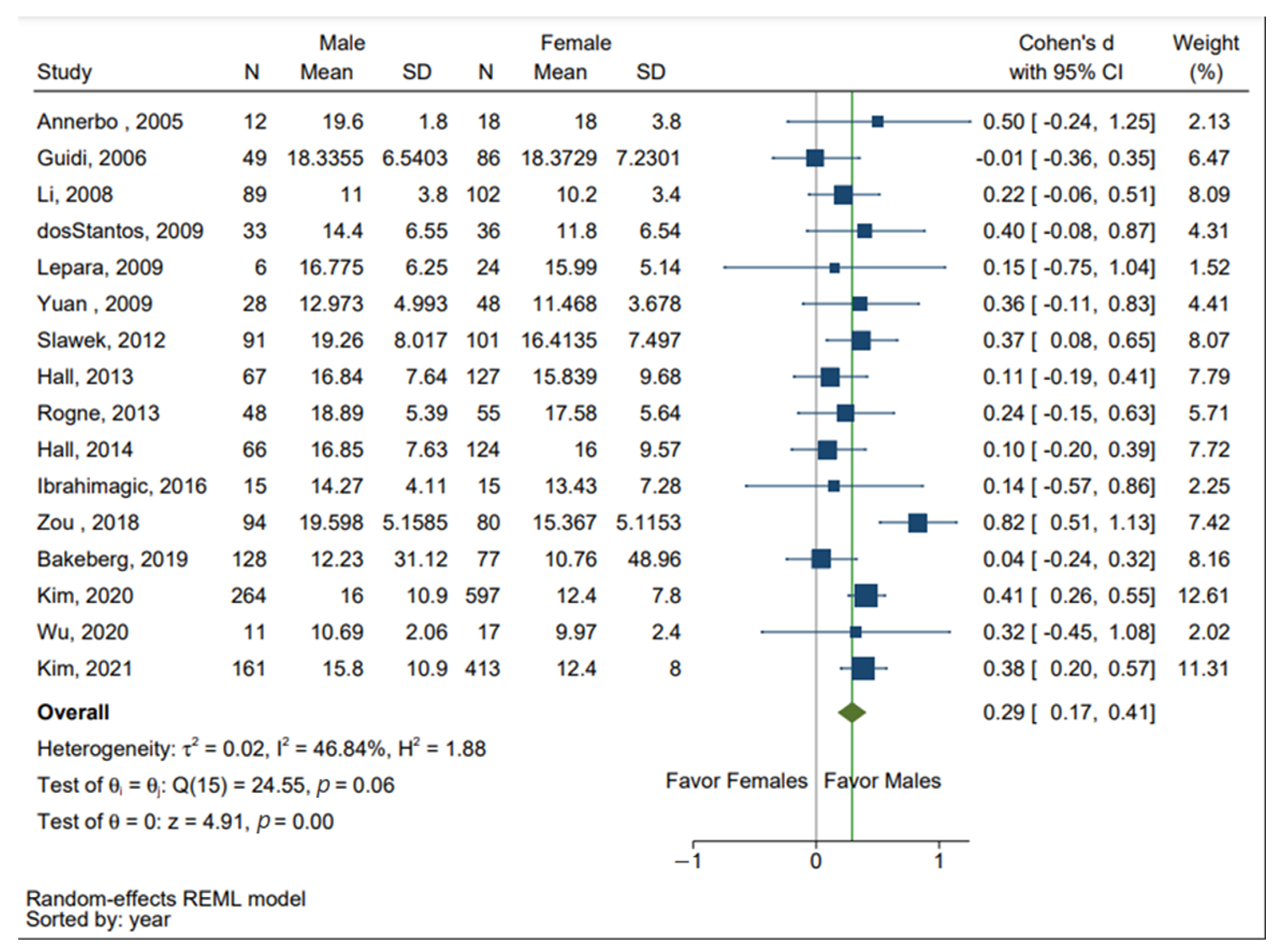
3.2. Sex Differences in the Levels of Hcy in Both AD and PD Patients
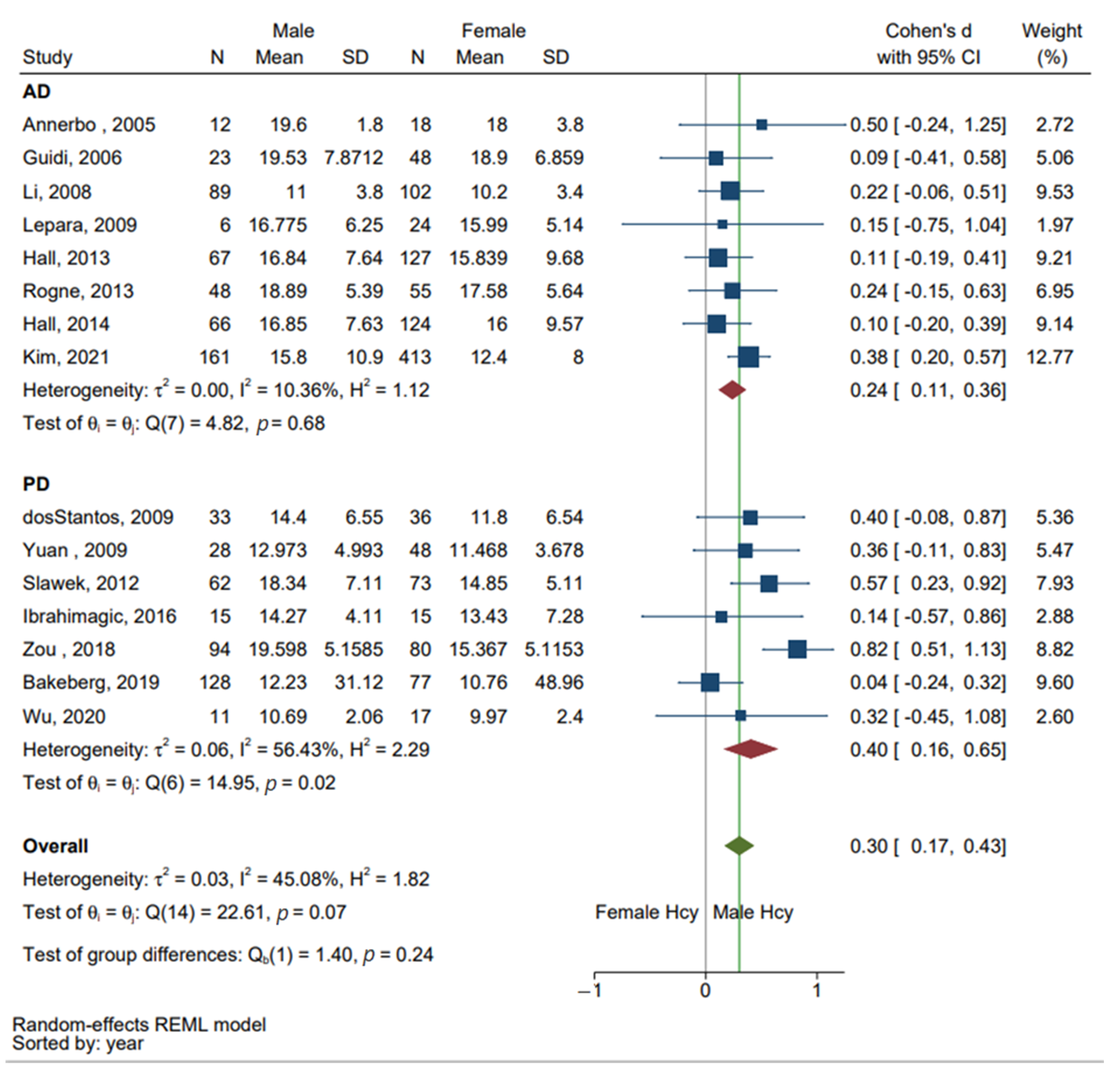
3.3. Subgroup Analysis of AD and PD
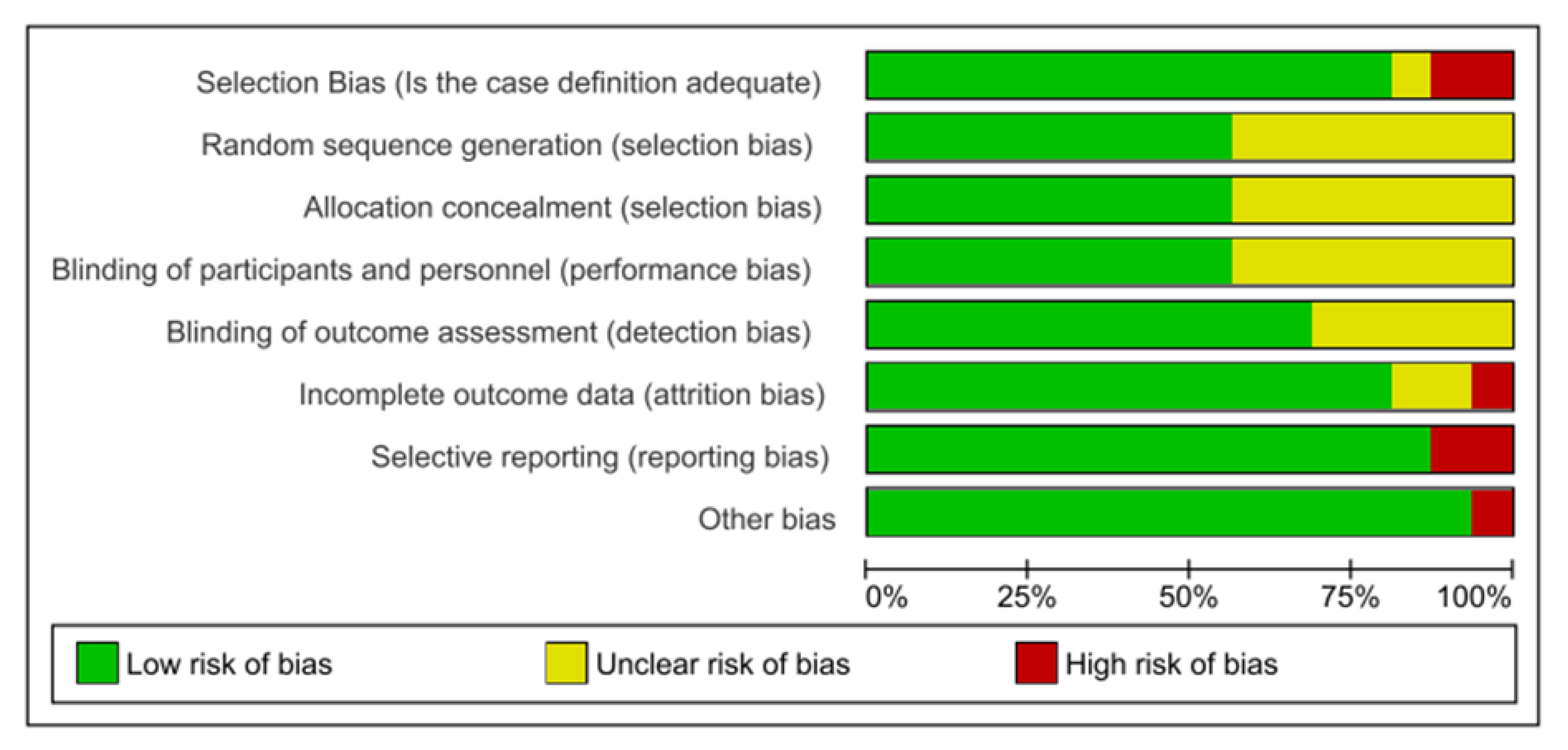
3.4. Risk of Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, R.L.; Ellis, C.E. Alzheimer’s Disease and Parkinson’s Disease. N. Engl. J. Med. 2003, 348, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Jagust, W.J.; Zheng, L.; Harvey, D.J.; Mack, W.J.; Vinters, H.V.; Weiner, M.W.; Ellis, W.G.; Zarow, C.; Mungas, D.; Reed, B.R.; et al. Neuropathological basis of magnetic resonance images in aging and dementia. Ann. Neurol. 2008, 63, 72–80. [Google Scholar] [CrossRef]
- Annerbo, S.; Wahlund, L.-O.; Lökk, J. The Relation between Homocysteine Levels and Development of Alzheimer’s Disease in Mild Cognitive Impairment Patients. Dement. Geriatr. Cogn. Disord. 2005, 20, 209–214. [Google Scholar] [CrossRef]
- Du, H.; Guo, L.; Yan, S.; Sosunov, A.A.; McKhann, G.M.; Yan, S.S.D. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proc. Natl. Acad. Sci. USA 2010, 107, 18670–18675. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- DeMaagd, G.; Philip, A. Parkinson’s Disease and Its Management: Part 1: Disease Entity, Risk Factors, Pathophysiology, Clinical Presentation, and Diagnosis. P & T 2015, 40, 504–532. [Google Scholar]
- Brochard, V.; Combadière, B.; Prigent, A.; Laouar, Y.; Perrin, A.; Beray-Berthat, V.; Bonduelle, O.; Alvarez-Fischer, D.; Callebert, J.; Launay, J.-M.; et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Investig. 2008, 119, 182–192. [Google Scholar] [CrossRef]
- Rizek, P.; Kumar, N.; Jog, M.S. An update on the diagnosis and treatment of Parkinson disease. Can. Med. Assoc. J. 2016, 188, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cao, D.; Desmond, R.; Rahman, A.; Lah, J.J.; Levey, A.I.; Zamrini, E. Cognitive Performance and Plasma Levels of Homocysteine, Vitamin B12, Folate and Lipids in Patients with Alzheimer Disease. Dement. Geriatr. Cogn. Disord. 2008, 26, 384–390. [Google Scholar] [CrossRef]
- Lipton, S.A.; Kim, W.-K.; Choi, Y.-B.; Kumar, S.; D’Emilia, D.M.; Rayudu, P.V.; Arnelle, D.R.; Stamler, J.S. Neurotoxicity associated with dual actions of homocysteine at the N -methyl- d -aspartate receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 5923–5928. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, E.F.; Busanello, E.N.B.; Miglioranza, A.; Zanatta, A.; Barchak, A.G.; Vargas, C.R.; Saute, J.; Rosa, C.; Carrion, M.J.; Camargo, D.; et al. Evidence that folic acid deficiency is a major determinant of hyperhomocysteinemia in Parkinson´s disease. Metab. Brain Dis. 2009, 24, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, X.; Li, X.; Wang, H.; Wang, T. Elevated cerebrospinal fluid homocysteine is associated with blood-brain barrier disruption in amyotrophic lateral sclerosis patients. Neurol. Sci. 2020, 41, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Muddapu, V.R.; Dharshini, S.A.P.; Chakravarthy, V.S.; Gromiha, M.M. Neurodegenerative Diseases—Is Metabolic Deficiency the Root Cause? Front. Neurosci. 2020, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Wald, D.S.; Kasturiratne, A.; Simmonds, M. Serum homocysteine and dementia: Meta-analysis of eight cohort studies including 8669 participants. Alzheimer’s Dement. 2011, 7, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Spilt, A.; Weverling-Rijnsburger, A.W.E.; Middelkoop, H.A.M.; Van Der Flier, W.M.; Gussekloo, J.; De Craen, A.J.M.; Bollen, E.L.E.M.; Blauw, G.J.; Van Buchem, M.A.; Westendorp, R.G.J. Late-Onset Dementia: Structural Brain Damage and Total Cerebral Blood Flow. Radiology 2005, 236, 990–995. [Google Scholar] [CrossRef] [PubMed]
- O’Suilleabhain, P.E.; Sung, V.; Hernandez, C.; Lacritz, L.; Jr, R.B.D.; Bottiglieri, T.; Diaz-Arrastia, R. Elevated Plasma Homocysteine Level in Patients With Parkinson Disease: Motor, Affective, and Cognitive Associations. Arch. Neurol. 2004, 61, 865–868. [Google Scholar] [CrossRef]
- Podcasy, J.L.; Epperson, C.N. Considering sex and gender in Alzheimer disease and other dementias. Dialog. Clin. Neurosci. 2016, 18, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Beam, C.R.; Kaneshiro, C.; Jang, J.Y.; Reynolds, C.A.; Pedersen, N.L.; Gatz, M. Differences Between Women and Men in Incidence Rates of Dementia and Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, 1077–1083. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Tufanaru, C.; Munn, Z.; Stephenson, M.; Aromataris, E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. JBI Evid. Implement. 2015, 13, 196–207. [Google Scholar] [CrossRef]
- Guidi, I.; Galimberti, D.; Lonati, S.; Novembrino, C.; Bamonti, F.; Tiriticco, M.; Fenoglio, C.; Venturelli, E.; Baron, P.; Bresolin, N.; et al. Oxidative imbalance in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2006, 27, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Lepara, O.; Alajbegovic, A.; Zaciragic, A.; Nakaš-Ićindić, E.; Valjevac, A.; Lepara, D.; Hadzovic-Dzuvo, A.; Fajkic, A.; Kulo, A.; Sofic, E. Elevated serum homocysteine level is not associated with serum C-reactive protein in patients with probable Alzheimer’s disease. J. Neural Transm. 2009, 116, 1651–1656. [Google Scholar] [CrossRef]
- Yuan, R.-Y.; Sheu, J.-J.; Yu, J.-M.; Hu, C.-J.; Tseng, I.-J.; Ho, C.-S.; Yeh, C.-Y.; Hung, Y.-L.; Chiang, T.-R. Methylenetetrahydrofolate reductase polymorphisms and plasma homocysteine in levodopa-treated and non-treated Parkinson’s disease patients. J. Neurol. Sci. 2009, 287, 64–68. [Google Scholar] [CrossRef]
- Sławek, J.; Roszmann, A.; Robowski, P.; Dubaniewicz, M.; Sitek, E.J.; Honczarenko, K.; Gorzkowska, A.; Budrewicz, S.; Mak, M.; Gołąb-Janowska, M.; et al. The Impact of MRI White Matter Hyperintensities on Dementia in Parkinson’s Disease in Relation to the Homocysteine Level and Other Vascular Risk Factors. Neurodegener. Dis. 2012, 12, 1–12. [Google Scholar] [CrossRef]
- Hall, J.R.; Wiechmann, A.R.; Johnson, L.A.; Edwards, M.; Barber, R.C.; Winter, A.S.; Singh, M.; O’Bryant, S.E. Biomarkers of Vascular Risk, Systemic Inflammation, and Microvascular Pathology and Neuropsychiatric Symptoms in Alzheimer’s Disease. J. Alzheimer’s Dis. 2013, 35, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Rogne, S.; Vangberg, T.; Eldevik, P.; Wikran, G.; Mathiesen, E.B.; Schirmer, H. Mild Cognitive Impairment, Risk Factors and Magnetic Resonance Volumetry: Role of Probable Alzheimer’s Disease in the Family. Dement. Geriatr. Cogn. Disord. 2013, 36, 87–98. [Google Scholar] [CrossRef]
- Hall, J.R.; Wiechmann, A.R.; Johnson, L.A.; Edwards, M.; Barber, R.C.; Cunningham, R.; Singh, M.; O’Bryant, S.E. The Impact of APOE Status on Relationship of Biomarkers of Vascular Risk and Systemic Inflammation to Neuropsychiatric Symptoms in Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 40, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimagic, O.; Smajlovic, D.; Dostovic, Z.; Pasic, Z.; Kunic, S.; Iljazovic, A.; Hajdarevic, D. Hyperhomocysteinemia and Its Treatment in Patients with Parkinson’s Disease. Mater. Socio Medica 2016, 28, 303–306. [Google Scholar] [CrossRef]
- Zou, J.; Chen, Z.; Liang, C.; Fu, Y.; Wei, X.; Lu, J.; Pan, M.; Guo, Y.; Liao, X.; Xie, H.; et al. Trefoil Factor 3, Cholinesterase and Homocysteine: Potential Predictors for Parkinson’s Disease Dementia and Vascular Parkinsonism Dementia in Advanced Stage. Aging Dis. 2018, 9, 51–65. [Google Scholar] [CrossRef]
- Bakeberg, M.C.; Jefferson, A.; Riley, M.; Byrnes, M.; Ghosh, S.; Mastaglia, F.L.; Horne, M.K.; McGregor, S.; Stell, R.; Kenna, J.; et al. Elevated Serum Homocysteine Levels Have Differential Gender-Specific Associations with Motor and Cognitive States in Parkinson’s Disease. Park. Dis. 2019, 2019, 3124295. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Sohn, I.W.; Kim, Y.S.; Jun, J.-B. The Different Relationship between Homocysteine and Uric Acid Levels with Respect to the MTHFR C677T Polymorphism According to Gender in Patients with Cognitive Impairment. Nutrients 2020, 12, 1147. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, M.; Yu, M.; Fu, J. Sex differences in underweight and body mass index in Chinese early de novo patients with Parkinson’s disease. Brain Behav. 2020, 10, e01893. [Google Scholar] [CrossRef]
- Kim, H.; Park, J.; Kim, Y.S.; Park, J. The sex-specific effect of the apolipoprotein E allele and methylenetetrahydrofolate reductase gene polymorphism on the biochemical, anatomical, and cognitive profiles of patients clinically diagnosed with probable Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2020, 36, 588–597. [Google Scholar] [CrossRef]
- Wang, A.-F.; Zhang, M.; Li, Q.-Y. Gender: A primary homocysteine level-effecting factor for patients suffering homocysteine-related diseases. Biomed. Res. 2017, 28, 353–356. [Google Scholar]
- Xu, R.; Huang, F.; Wang, Y.; Liu, Q.; Lv, Y.; Zhang, Q. Gender- and age-related differences in homocysteine concentration: A cross-sectional study of the general population of China. Sci. Rep. 2020, 10, 17401. [Google Scholar] [CrossRef] [PubMed]
- Lussier-Cacan, S.; Xhignesse, M.; A Piolot, A.; Selhub, J.; Davignon, J.; Genest, J. Plasma total homocysteine in healthy subjects: Sex-specific relation with biological traits. Am. J. Clin. Nutr. 1996, 64, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Gillies, G.E.; Pienaar, I.S.; Vohra, S.; Qamhawi, Z. Sex differences in Parkinson’s disease. Front. Neuroendocr. 2014, 35, 370–384. [Google Scholar] [CrossRef]
- Wooten, G.F.; Currie, L.J.; Bovbjerg, V.E.; Lee, J.K.; Patrie, J. Are men at greater risk for Parkinson’s disease than women? J. Neurol. Neurosurg. Psychiatry 2004, 75, 637–639. [Google Scholar] [CrossRef]
- Bertogliat, M.J.; Morris-Blanco, K.C.; Vemuganti, R. Epigenetic mechanisms of neurodegenerative diseases and acute brain injury. Neurochem. Int. 2019, 133, 104642. [Google Scholar] [CrossRef]
- Nugent, B.M.; McCarthy, M.M. Epigenetic Underpinnings of Developmental Sex Differences in the Brain. Neuroendocrinology 2011, 93, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Cerri, S.; Mus, L.; Blandini, F. Parkinson’s Disease in Women and Men: What’s the Difference? J. Park. Dis. 2019, 9, 501–515. [Google Scholar] [CrossRef] [PubMed]


| First Author | Year | Country | Diagnosis | Diagnostic Test | Sample Size F/M | Mean Age (Years) F/M * | Reference |
|---|---|---|---|---|---|---|---|
| Annerbo | 2005 | Sweden | AD | MMSE | 18/12 | 69.2/65.6 | 4 |
| Guidi | 2006 | Italy | AD | NINCDS-ADRDA | 48/23 | 78 | 22 |
| Li | 2008 | United States of America | AD | NINCDS-ADRDA | 102/89 | 72.1/73.1 | 10 |
| dosSantos | 2009 | Brazil | PD | UDPRS-motor score, HY staging and L-DOPA response | 36/33 | 61.6 | 12 |
| Lepara | 2009 | Bosnia and Herzegovina | AD | NINCDS-ADRDA | 24/6 | 79.96 | 23 |
| Yuan | 2009 | Taiwan | PD | HY staging | 68/28 | 71.37 | 24 |
| Slawek | 2012 | Poland | PD | UDPRS-motor score, HY staging | 101/91 | 63.7 | 25 |
| Hall | 2013 | United States of America | AD | NINCDS-ADRDA | 127/67 | 78.31/75.17 | 26 |
| Rogne | 2013 | Norway | AD | NINCDS-ADRDA | 55/48 | 73.97/74.49 | 27 |
| Hall | 2014 | United States of America | AD | NINCDS-ADRDA | 124/66 | 78.57/75.43 | 28 |
| Ibrahimagic | 2016 | Bosnia and Herzegovina | PD | tremor, bradykinesia, rigidity and postural abnormalities | 15/15 | 62.73/65.6 | 29 |
| Zou | 2018 | China | PD | UDPRS and HY staging | 80/94 | 67.88 | 30 |
| Bakeberg | 2019 | Australia | PD | UDPRS and HY staging | 77/128 | 64 | 31 |
| Kim | 2020 | South Korea | AD | NINCDS-ADRDA | 597/264 | 75.3/73.2 | 32 |
| Wu | 2020 | China | PD | MDS criteria | 107/146 | 62/63.8 | 33 |
| Kim | 2021 | South Korea | AD | NIA-AA | 413/161 | 73.2 | 34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.P.; Collins, A.E.; Hickey, J.P.; Pfeifer, J.A.; Kalisch, B.E. Sex Differences in the Level of Homocysteine in Alzheimer’s Disease and Parkinson’s Disease Patients: A Meta-Analysis. Brain Sci. 2023, 13, 153. https://doi.org/10.3390/brainsci13010153
Nguyen VP, Collins AE, Hickey JP, Pfeifer JA, Kalisch BE. Sex Differences in the Level of Homocysteine in Alzheimer’s Disease and Parkinson’s Disease Patients: A Meta-Analysis. Brain Sciences. 2023; 13(1):153. https://doi.org/10.3390/brainsci13010153
Chicago/Turabian StyleNguyen, V. Phu, Andrila E. Collins, Jordan P. Hickey, Julia A. Pfeifer, and Bettina E. Kalisch. 2023. "Sex Differences in the Level of Homocysteine in Alzheimer’s Disease and Parkinson’s Disease Patients: A Meta-Analysis" Brain Sciences 13, no. 1: 153. https://doi.org/10.3390/brainsci13010153
APA StyleNguyen, V. P., Collins, A. E., Hickey, J. P., Pfeifer, J. A., & Kalisch, B. E. (2023). Sex Differences in the Level of Homocysteine in Alzheimer’s Disease and Parkinson’s Disease Patients: A Meta-Analysis. Brain Sciences, 13(1), 153. https://doi.org/10.3390/brainsci13010153







