Staged Surgery for Intra-Extracranial Communicating Jugular Foramen Paraganglioma: A Case Report and Systematic Review
Abstract
1. Introduction
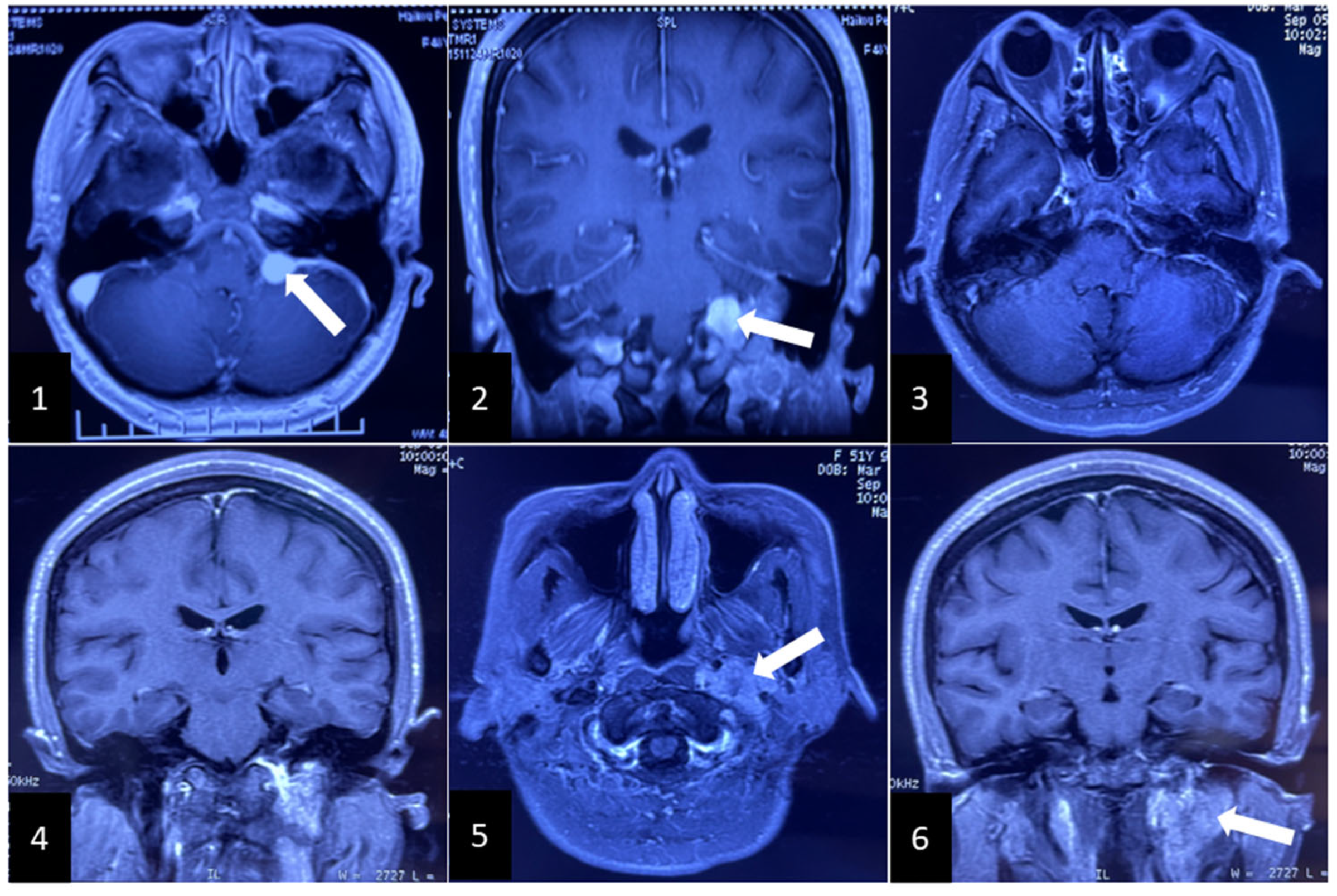
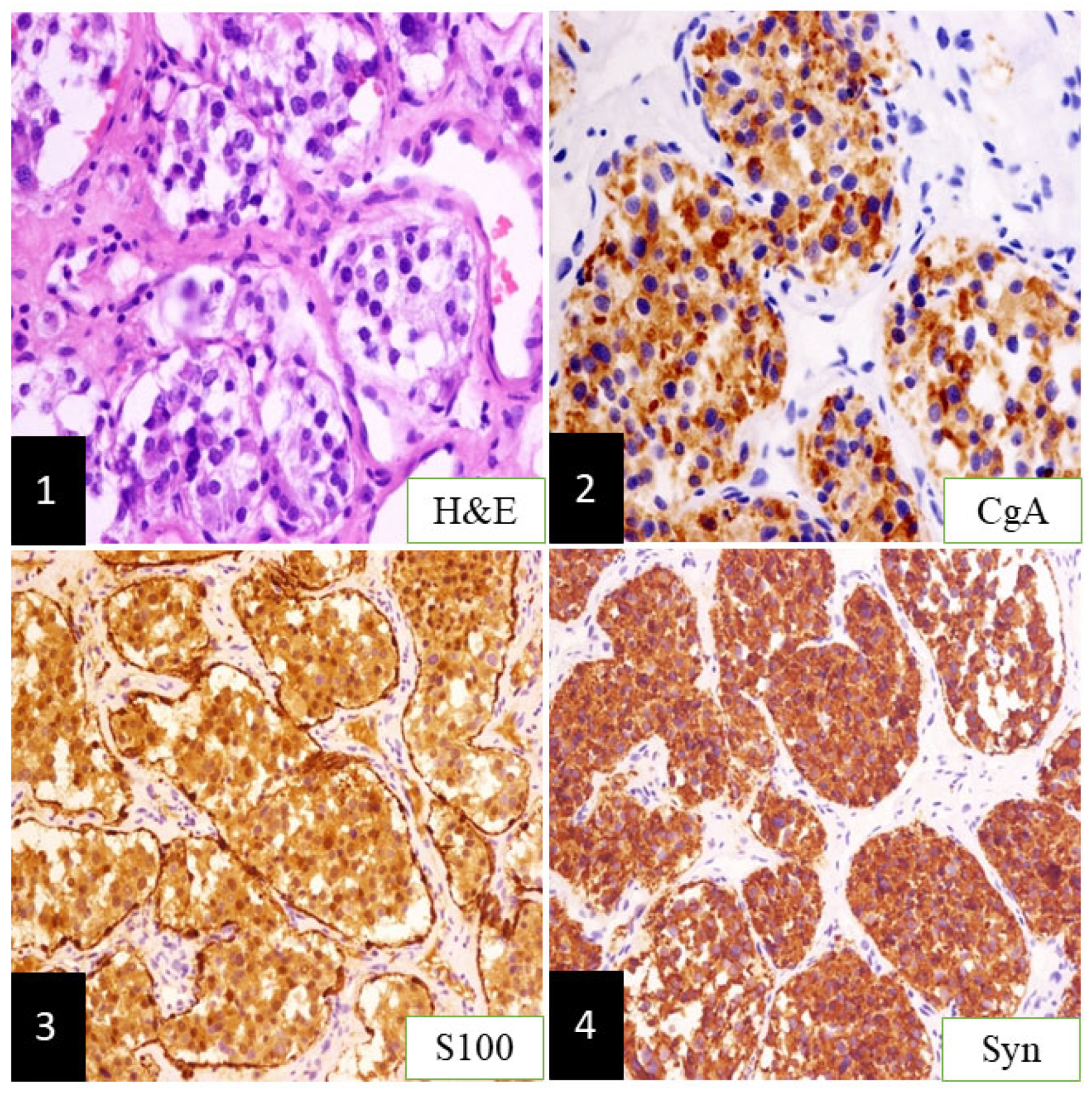
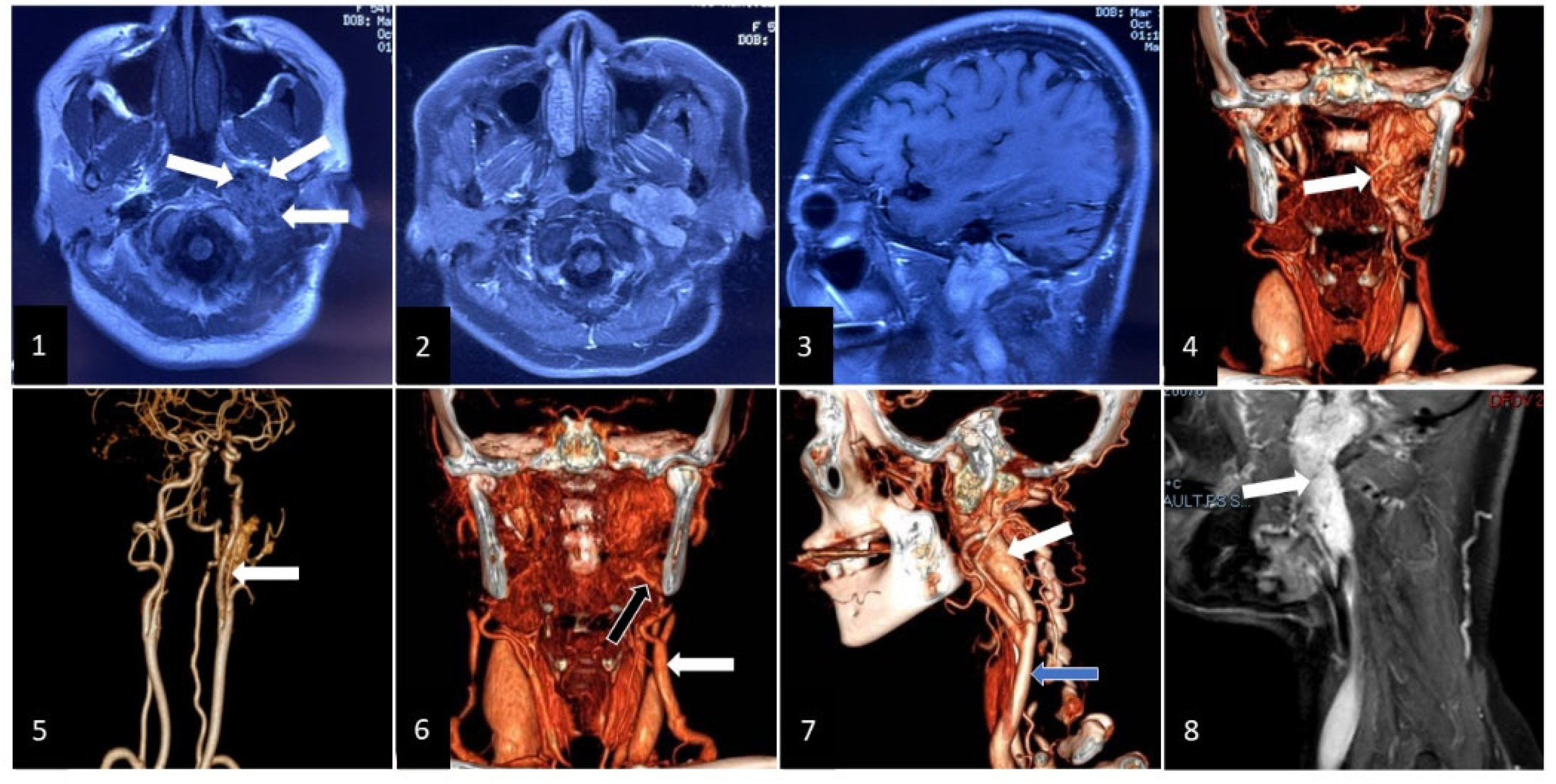
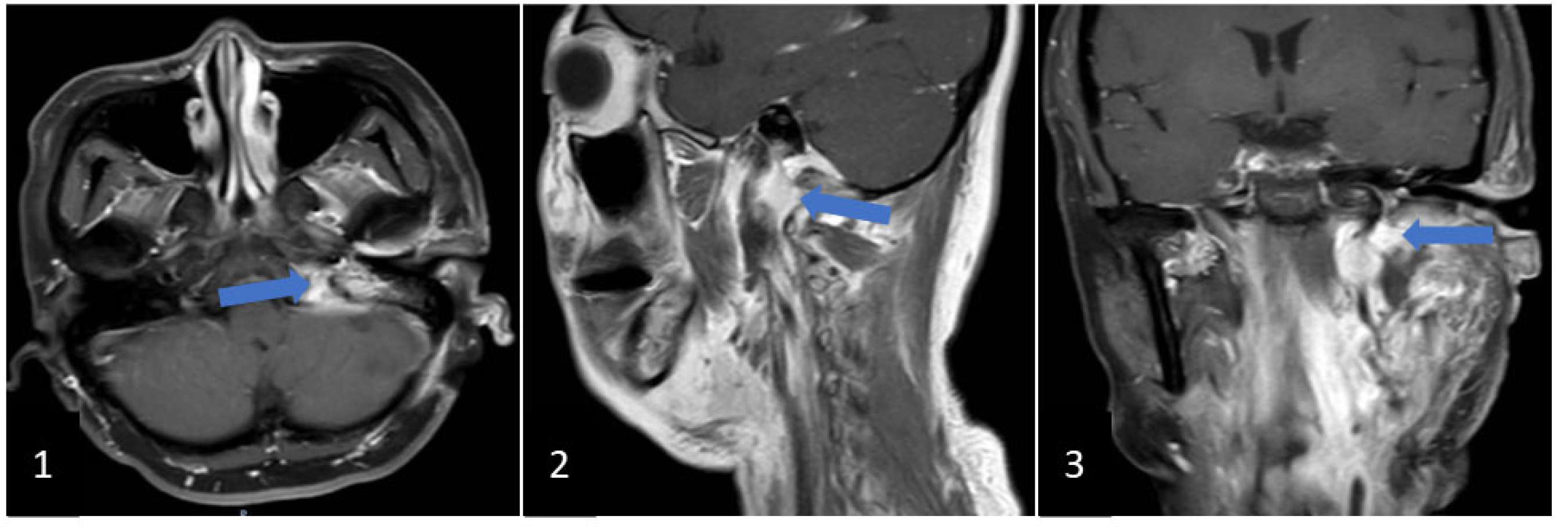
2. Clinical Case
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Myssiorek, D. Head and neck paragangliomas: An overview. Otolaryngol. Clin. N. Am. 2001, 34, 829–836. [Google Scholar] [CrossRef]
- Wasserman, P.G.; Savargaonkar, P. Paragangliomas: Classification, pathology, and differential diagnosis. Otolaryngol. Clin. N. Am. 2001, 34, 845–862. [Google Scholar] [CrossRef]
- Thedinger, B.A.; Glasscock, M.E., 3rd; Cueva, R.A.; Jackson, G.G. Postoperative Radiographic Evaluation After Acoustic Neuroma and Glomus Jugulare Tumor Removal. Laryngoscope 1992, 102, 261–266. [Google Scholar] [CrossRef]
- Al-Mefty, O.; Teixeira, A. Complex tumors of the glomus jugulare: Criteria, treatment, and outcome. J. Neurosurg. 2002, 97, 1356–1366. [Google Scholar] [CrossRef]
- Jackson, C.G.; McGrew, B.M.; Forest, J.A.; Netterville, J.; Hampf, C.F.; Glasscock, M.E., 3rd. Lateral Skull Base Surgery for Glomus Tumors: Long-Term Control. Otol. Neurotol. 2001, 22, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.C.; Mimoune, H.A.; Khardaly, M.; Piazza, P.; Russo, A.; Sanna, M. Strategies and long-term outcomes in the surgical management of tympanojugular paragangliomas. Head Neck 2015, 38, 871–885. [Google Scholar] [CrossRef]
- Jackson, C.G.; Kaylie, D.M.; Coppit, G.; Gardner, E.K. Glomus jugulare tumors with intracranial extension. Neurosurg. Focus 2004, 17, 45–50. [Google Scholar] [CrossRef]
- Sanna, M.; Shin, S.-H.; De Donato, G.; Sivalingam, S.; Lauda, L.; Vitullo, F.; Piazza, P. Management of complex tympanojugular paragangliomas including endovascular intervention. Laryngoscope 2011, 121, 1372–1382. [Google Scholar] [CrossRef]
- Woolen, S.; Gemmete, J.J. Paragangliomas of the Head and Neck. Neuroimaging Clin. N. Am. 2016, 26, 259–278. [Google Scholar] [CrossRef]
- Fisch, U. Infratemporal Fossa Approach for Lesions in the Temporal Bone and Base of the Skull. Adv. Otorhinolaryngol. 1984, 34, 254–266. [Google Scholar] [CrossRef]
- Mazzoni, A.; Cazzador, D.; D’Avella, D.; Zanoletti, E. Large Intradural Tympanojugular Paragangliomas. A Contribution on Surgery and Management. World Neurosurg. 2018, 122, e1482–e1490. [Google Scholar] [CrossRef] [PubMed]
- Neskey, D.M.; Hatoum, G.; Modh, R.; Civantos, F.; Telischi, F.F.; I Angeli, S.; Weed, D.; Sargi, Z. Outcomes after Surgical Resection of Head and Neck Paragangliomas: A Review of 61 Patients. Skull Base 2011, 21, 171–176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moe, K.S.; Li, D.; Linder, T.E.; Schmid, S.; Fisch, U. An Update on the Surgical Treatment of Temporal Bone Paraganglioma. Skull Base 1999, 9, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.G.; Netterville, J.L.; Mendenhall, W.M.; Isaacson, B.; Nussenbaum, B. Head and Neck Paragangliomas: An Update on Evaluation and Management. Otolaryngol. Head Neck Surg. 2016, 154, 597–605. [Google Scholar] [CrossRef]
- Prasad, S.C.; Mimoune, H.A.; D’Orazio, F.; Medina, M.; Bacciu, A.; Mariani-Costantini, R.; Piazza, P.; Sanna, M. The Role of Wait-and-Scan and the Efficacy of Radiotherapy in the Treatment of Temporal Bone Paragangliomas. Otol. Neurotol. 2014, 35, 922–931. [Google Scholar] [CrossRef]
- Sivalingam, S.; Konishi, M.; Shin, S.-H.; Ahmed, R.A.R.L.; Piazza, P.; Sanna, M. Surgical Management of Tympanojugular Paragangliomas with Intradural Extension, with a Proposed Revision of the Fisch Classification. Audiol. Neurotol. 2012, 17, 243–255. [Google Scholar] [CrossRef]
- Guss, Z.D.; Batra, S.; Limb, C.J.; Li, G.; Sughrue, M.E.; Redmond, K.; Rigamonti, D.; Parsa, A.T.; Chang, S.; Kleinberg, L.; et al. Radiosurgery of Glomus Jugulare Tumors: A Meta-Analysis. Int. J. Radiat. Oncol. 2011, 81, e497–e502. [Google Scholar] [CrossRef]
- Sheehan, J.P.; Tanaka, S.; Link, M.J.; Pollock, B.E.; Kondziolka, D.; Mathieu, D.; Duma, C.; Young, A.B.; Kaufmann, A.M.; McBride, H.; et al. Gamma Knife surgery for the management of glomus tumors: A multicenter study. J. Neurosurg. 2012, 117, 246–254. [Google Scholar] [CrossRef]
- Carlson, M.L.; Sweeney, A.D.; Wanna, G.B.; Netterville, J.; Haynes, D.S. Natural History of Glomus Jugulare: A review of 16 tumors managed with primary observation. Otolaryngol. Neck Surg. 2014, 152, 98–105. [Google Scholar] [CrossRef]
- Li, D.; Zeng, X.-J.; Hao, S.-Y.; Wang, L.; Tang, J.; Xiao, X.-R.; Meng, G.-L.; Jia, G.-J.; Zhang, L.-W.; Wu, Z.; et al. Less-aggressive surgical management and long-term outcomes of jugular foramen paragangliomas: A neurosurgical perspective. J. Neurosurg. 2016, 125, 1143–1154. [Google Scholar] [CrossRef]
- Makiese, O.; Chibbaro, S.; Marsella, M.; Huy, P.T.B.; George, B. Jugular foramen paragangliomas: Management, outcome and avoidance of complications in a series of 75 cases. Neurosurg. Rev. 2012, 35, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, E.; Dahm, V.; Gstoettner, W.; Rössler, K.; Bauer, B.; Wressnegger, A.; Schwarz-Nemec, U.; Gatterbauer, B.; Matula, C.; Arnoldner, C. Long-Term Outcome and Comparison of Treatment Modalities of Temporal Bone Paragangliomas. Cancers 2021, 13, 5083. [Google Scholar] [CrossRef] [PubMed]
- Wanna, G.B.; Sweeney, A.D.; Carlson, M.L.; Latuska, R.F.; Rivas, A.; Bennett, M.L.; Netterville, J.; Haynes, D.S. Subtotal Resection for Management of Large Jugular Paragangliomas with Functional Lower Cranial Nerves. Otolaryngol. Neck Surg. 2014, 151, 991–995. [Google Scholar] [CrossRef]
- Ivan, M.E.; Sughrue, M.E.; Clark, A.J.; Kane, A.J.; Aranda, D.; Barani, I.J.; Parsa, A.T. A meta-analysis of tumor control rates and treatment-related morbidity for patients with glomus jugulare tumors. J. Neurosurg. 2011, 114, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- De Marini, P.; Greget, M.; Boatta, E.; Jahn, C.; Enescu, I.; Garnon, J.; Dalili, D.; Cazzato, R.L.; Gangi, A. Safety and technical efficacy of pre-operative embolization of head and neck paragangliomas: A 10-year mono-centric experience and systematic review. Clin. Imaging 2021, 80, 292–299. [Google Scholar] [CrossRef]
- Catapano, J.S.; Almefty, R.O.; Ding, D.; Whiting, A.C.; Pines, A.R.; Richter, K.R.; Ducruet, A.F.; Albuquerque, F.C. Onyx embolization of skull base paragangliomas: A single-center experience. Acta Neurochir. 2020, 162, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Sallabanda, K.; Barrientos, H.; Romero, D.A.I.; Vargas, C.; Diaz, J.A.G.; Peraza, C.; del Campo, E.R.; Praena-Fernandez, J.M.; López-Guerra, J.L. Long-term outcomes after radiosurgery for glomus jugulare tumors. Tumori J. 2018, 104, 300–306. [Google Scholar] [CrossRef]
- Ota, Y.; Liao, E.; Capizzano, A.; Kurokawa, R.; Bapuraj, J.; Syed, F.; Baba, A.; Moritani, T.; Srinivasan, A. Diagnostic Role of Diffusion-Weighted and Dynamic Contrast-Enhanced Perfusion MR Imaging in Paragangliomas and Schwannomas in the Head and Neck. Am. J. Neuroradiol. 2021, 42, 1839–1846. [Google Scholar] [CrossRef]
- Ota, Y.; Naganawa, S.; Kurokawa, R.; Bapuraj, J.; Capizzano, A.; Kim, J.; Moritani, T.; Srinivasan, A. Assessment of MR Imaging and CT in Differentiating Hereditary and Nonhereditary Paragangliomas. Am. J. Neuroradiol. 2021, 42, 1320–1326. [Google Scholar] [CrossRef]
- Ota, Y.; Liao, E.; Capizzano, A.A.; Yokota, H.; Baba, A.; Kurokawa, R.; Kurokawa, M.; Moritani, T.; Yoshii, K.; Srinivasan, A. MR diffusion and dynamic-contrast enhanced imaging to distinguish meningioma, paraganglioma, and schwannoma in the cerebellopontine angle and jugular foramen. J. Neuroimaging 2021, 32, 502–510. [Google Scholar] [CrossRef]
- Ota, Y.; Liao, E.; Kurokawa, R.; Syed, F.; Baba, A.; Kurokawa, M.; Moritani, T.; Srinivasan, A. Diffusion-Weighted and dynamic contrast-enhanced MRI to assess radiation therapy response for head and neck paragangliomas. J. Neuroimaging 2021, 31, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Sahyouni, R.; Mahboubi, H.; Moshtaghi, O.; Goshtasbi, K.; Sahyouni, S.; Lin, H.W.; Djalilian, H.R. Radiosurgery of Glomus Tumors of Temporal Bone: A Meta-analysis. Otol. Neurotol. 2018, 39, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Suárez, C.; Rodrigo, J.P.; Bödeker, C.C.; Llorente, J.L.; Silver, C.E.; Jansen, J.; Takes, R.P.; Strojan, P.; Pellitteri, P.K.; Rinaldo, A.; et al. Jugular and vagal paragangliomas: Systematic study of management with surgery and radiotherapy. Head Neck 2013, 35, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.T.; Timmers, H.J.; Marres, H.A.; Kaanders, J.; Kunst, H.P. Results of a systematic literature review of treatment modalities for jugulotympanic paraganglioma, stratified per Fisch class. Clin. Otolaryngol. 2018, 43, 652–661. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Yu, Y.; Zhang, L.; Liu, J.; Ren, H.; Zhen, X. Staged Surgery for Intra-Extracranial Communicating Jugular Foramen Paraganglioma: A Case Report and Systematic Review. Brain Sci. 2022, 12, 1257. https://doi.org/10.3390/brainsci12091257
Li Q, Yu Y, Zhang L, Liu J, Ren H, Zhen X. Staged Surgery for Intra-Extracranial Communicating Jugular Foramen Paraganglioma: A Case Report and Systematic Review. Brain Sciences. 2022; 12(9):1257. https://doi.org/10.3390/brainsci12091257
Chicago/Turabian StyleLi, Qiang, Yanbing Yu, Li Zhang, Jiang Liu, Hongxiang Ren, and Xueke Zhen. 2022. "Staged Surgery for Intra-Extracranial Communicating Jugular Foramen Paraganglioma: A Case Report and Systematic Review" Brain Sciences 12, no. 9: 1257. https://doi.org/10.3390/brainsci12091257
APA StyleLi, Q., Yu, Y., Zhang, L., Liu, J., Ren, H., & Zhen, X. (2022). Staged Surgery for Intra-Extracranial Communicating Jugular Foramen Paraganglioma: A Case Report and Systematic Review. Brain Sciences, 12(9), 1257. https://doi.org/10.3390/brainsci12091257





