Characteristics of Microstructural Changes Associated with Glioma Related Epilepsy: A Diffusion Tensor Imaging (DTI) Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. MRI Acquisition
2.3. Tumor ROI Extraction
2.4. Diffusion Data Processing
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Atlas-Based General Analysis of Diffusion Parametric
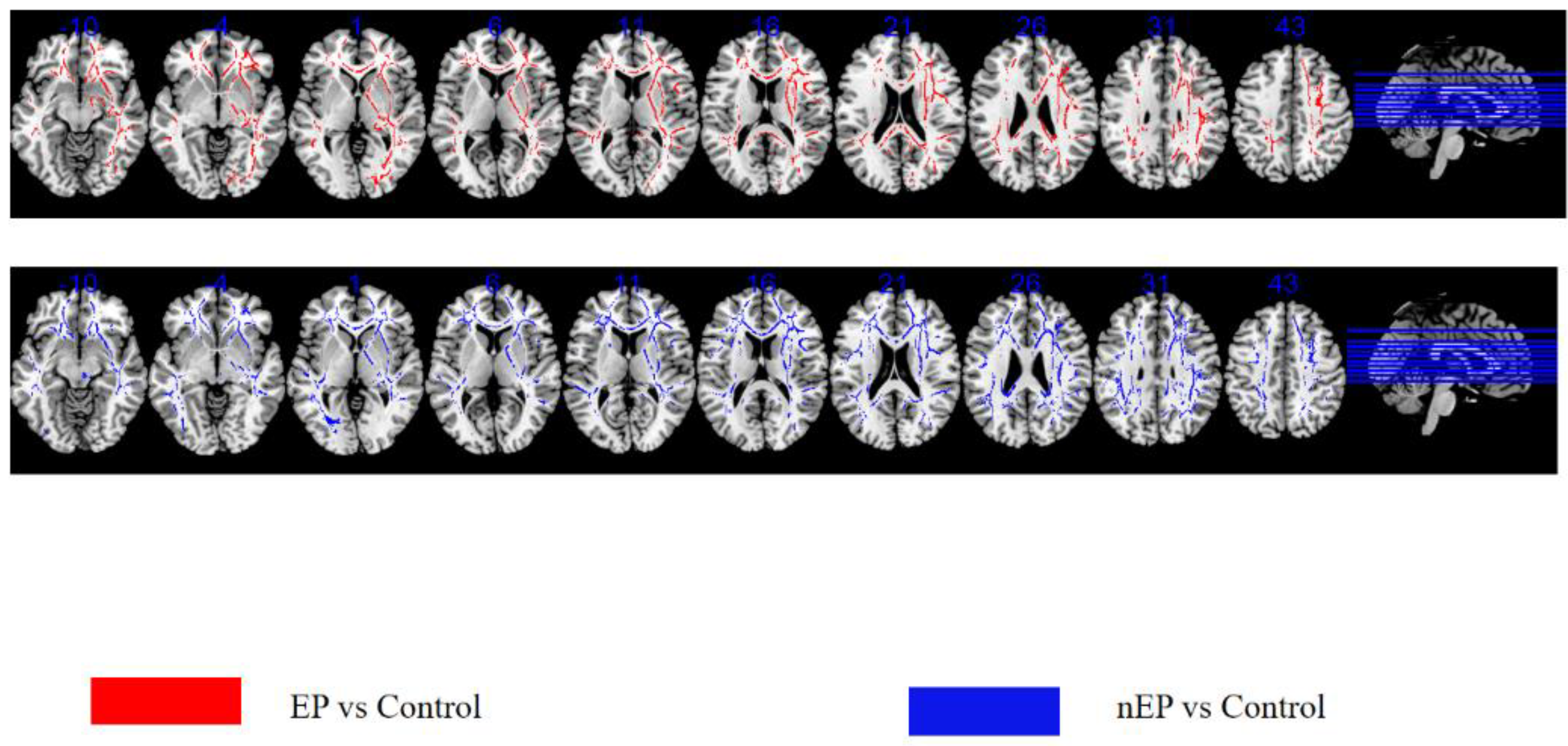
3.3. TBSS-EP Group versus Control
3.4. TBSS-nEP Group versus Control
4. Discussion
5. Limitation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rudà, R.; Bello, L.; Duffau, H.; Soffietti, R. Seizures in low-grade gliomas: Natural history, pathogenesis, and outcome after treatments. Neuro-Oncology 2012, 14 (Suppl. S4), iv55–iv64. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.F.; Potts, M.B.; Keles, G.E.; Lamborn, K.R.; Chang, S.M.; Barbaro, N.M.; Berger, M.S. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J. Neurosurg. 2008, 108, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qian, T.; You, G.; Peng, X.; Chen, C.; You, Y.; Yao, K.; Wu, C.; Ma, J.; Sha, Z.; et al. Localizing seizure-susceptible brain regions associated with low-grade gliomas using voxel-based lesion-symptom mapping. Neuro-Oncology 2015, 17, 282–288. [Google Scholar] [CrossRef]
- Li, Y.; Shan, X.; Wu, Z.; Wang, Y.; Ling, M.; Fan, X. IDH1 mutation is associated with a higher preoperative seizure incidence in low-grade glioma: A systematic review and meta-analysis. Seizure 2018, 55, 76–82. [Google Scholar] [CrossRef]
- Neal, A.; Kwan, P.; O’Brien, T.J.; Buckland, M.E.; Gonzales, M.; Morokoff, A. IDH1 and IDH2 mutations in postoperative diffuse glioma-associated epilepsy. Epilepsy Behav. 2018, 78, 30–36. [Google Scholar] [CrossRef]
- Zhou, C.; Fang, S.; Yang, J.; Wang, Y.; Wang, L. Epilepsy-related white matter network changes in patients with frontal lobe glioma. J. Neuroradiol. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Bouwen, B.L.J.; Pieterman, K.J.; Smits, M.; Dirven, C.M.F.; Gao, Z.; Vincent, A.J.P.E. The Impacts of Tumor and Tumor Associated Epilepsy on Subcortical Brain Structures and Long Distance Connectivity in Patients With Low Grade Glioma. Front. Neurol. 2018, 9, 1004. [Google Scholar] [CrossRef] [PubMed]
- Gonen, T.; Grossman, R.; Sitt, R.; Nossek, E.; Yanaki, R.; Cagnano, E.; Korn, A.; Hayat, D.; Ram, Z. Tumor location and IDH1 mutation may predict intraoperative seizures during awake craniotomy. J. Neurosurg. 2014, 121, 1133–1138. [Google Scholar] [CrossRef]
- Pallud, J.; Audureau, E.; Blonski, M.; Sanai, N.; Bauchet, L.; Fontaine, D.; Mandonnet, E.; Dezamis, E.; Psimaras, D.; Guyotat, J.; et al. Epileptic seizures in diffuse low-grade gliomas in adults. Brain 2014, 137 Pt 2, 449–462. [Google Scholar] [CrossRef]
- Yunhe, M.; Yuan, Y.; Xiang, W.; Yanhui, L.; Qing, M. Mapping seizure foci and tumor genetic factors in glioma associated seizure patients. J. Neurosurg. Sci. 2020, 64, 456–463. [Google Scholar] [CrossRef]
- Prakash, O.; Lukiw, W.J.; Peruzzi, F.; Reiss, K.; Musto, A.E. Gliomas and seizures. Med. Hypotheses 2012, 79, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Trimmel, K.; van Graan, A.L.; Caciagli, L.; Haag, A.; Koepp, M.J.; Thompson, P.J.; Duncan, J.S. Left temporal lobe language network connectivity in temporal lobe epilepsy. Brain 2018, 141, 2406–2418. [Google Scholar] [CrossRef] [PubMed]
- Burianová, H.; Faizo, N.L.; Gray, M.; Hocking, J.; Galloway, G.; Reutens, D. Altered functional connectivity in mesial temporal lobe epilepsy. Epilepsy Res. 2017, 137, 45–52. [Google Scholar] [CrossRef]
- Rosch, R.; Baldeweg, T.; Moeller, F.; Baier, G. Network dynamics in the healthy and epileptic developing brain. Netw. Neurosci. 2018, 2, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Schoene-Bake, J.C.; Faber, J.; Trautner, P.; Kaaden, S.; Tittgemeyer, M.; Elger, C.E.; Weber, B. Widespread affections of large fiber tracts in postoperative temporal lobe epilepsy. Neuroimage 2009, 46, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Deppe, M.; Kellinghaus, C.; Duning, T.; Möddel, G.; Mohammadi, S.; Deppe, K.; Schiffbauer, H.; Kugel, H.; Keller, S.S.; Ringelstein, E.B.; et al. Nerve fiber impairment of anterior thalamocortical circuitry in juvenile myoclonic epilepsy. Neurology 2008, 71, 1981–1985. [Google Scholar] [CrossRef]
- Ferda, J.; Kastner, J.; Mukensnabl, P.; Choc, M.; Horemuzová, J.; Ferdová, E.; Kreuzberg, B. Diffusion tensor magnetic resonance imaging of glial brain tumors. Eur. J. Radiol. 2010, 74, 428–436. [Google Scholar] [CrossRef]
- Gimenez, U.; Perles-Barbacaru, A.T.; Millet, A.; Appaix, F.; El-Atifi, M.; Pernet-Gallay, K.; van der Sanden, B.; Berger, F.; Lahrech, H. Microscopic DTI accurately identifies early glioma cell migration: Correlation with multimodal imaging in a new glioma stem cell model. NMR Biomed. 2016, 29, 1553–1562. [Google Scholar] [CrossRef]
- Basser, P.J.; Pierpaoli, C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reason. 2011, 213, 560–570. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Johansen-Berg, H.; Rueckert, D.; Nichols, T.E.; Mackay, C.E.; Watkins, K.E.; Ciccarelli, O.; Cader, M.Z.; Matthews, P.M.; et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage 2006, 31, 1487–1505. [Google Scholar] [CrossRef]
- van Dellen, E.; Douw, L.; Hillebrand, A.; Ris-Hilgersom, I.H.; Schoonheim, M.M.; Baayen, J.C.; De Witt Hamer, P.C.; Velis, D.N.; Klein, M.; Heimans, J.J.; et al. MEG network differences between low- and high-grade glioma related to epilepsy and cognition. PLoS ONE 2012, 7, e50122. [Google Scholar] [CrossRef] [PubMed]
- Maesawa, S.; Bagarinao, E.; Fujii, M.; Futamura, M.; Wakabayashi, T. Use of Network Analysis to Establish Neurosurgical Parameters in Gliomas and Epilepsy. Neurol. Med. Chir. 2016, 56, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Goubran, M.; de Ribaupierre, S.; Hammond, R.R.; Burneo, J.G.; Parrent, A.G.; Peters, T.M. Quantitative relaxometry and diffusion MRI for lateralization in MTS and non-MTS temporal lobe epilepsy. Epilepsy Res. 2014, 108, 506–516. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Concha, L.; Kim, H.; Bernasconi, A.; Bernhardt, B.C.; Bernasconi, N. Spatial patterns of water diffusion along white matter tracts in temporal lobe epilepsy. Neurology 2012, 79, 455–462. [Google Scholar] [CrossRef]
- Otte, W.M.; van Eijsden, P.; Sander, J.W.; Duncan, J.S.; Dijkhuizen, R.M.; Braun, K.P. A meta-analysis of white matter changes in temporal lobe epilepsy as studied with diffusion tensor imaging. Epilepsia 2012, 53, 659–667. [Google Scholar] [CrossRef]
- Chiang, S.; Levin, H.S.; Wilde, E.; Haneef, Z. White matter structural connectivity changes correlate with epilepsy duration in temporal lobe epilepsy. Epilepsy Res. 2016, 120, 37–46. [Google Scholar] [CrossRef]
- Rugg-Gunn, F.J.; Eriksson, S.H.; Symms, M.R.; Barker, G.J.; Duncan, J.S. Diffusion tensor imaging of cryptogenic and acquired partial epilepsies. Brain 2001, 124 Pt 3, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.R.; Vardal, J.; Bjørnerud, A.; Larsson, C.; Arnesen, M.R.; Salo, R.A.; Groote, I.R. Serial diffusion tensor imaging for early detection of radiation-induced injuries to normal-appearing white matter in high-grade glioma patients. J. Magn. Reason. Imaging 2015, 41, 414–423. [Google Scholar] [CrossRef]
- Sternberg, E.J.; Lipton, M.L.; Burns, J. Utility of diffusion tensor imaging in evaluation of the peritumoral region in patients with primary and metastatic brain tumors. AJNR Am. J. Neuroradiol. 2014, 35, 439–444. [Google Scholar] [CrossRef]
- Hoefnagels, F.W.; De Witt Hamer, P.; Sanz-Arigita, E.; Idema, S.; Kuijer, J.P.; Pouwels, P.J.; Barkhof, F.; Vandertop, W.P. Differentiation of edema and glioma infiltration: Proposal of a DTI-based probability map. J. Neurooncol. 2014, 120, 187–198. [Google Scholar] [CrossRef]
- Price, S.J.; Peña, A.; Burnet, N.G.; Jena, R.; Green, H.A.; Carpenter, T.A.; Pickard, J.D.; Gillard, J.H. Tissue signature characterisation of diffusion tensor abnormalities in cerebral gliomas. Eur. Radiol. 2004, 14, 1909–1917. [Google Scholar] [CrossRef]
- Bieza, A.; Krumina, G. Magnetic resonance study on fractional anisotropy and neuronal metabolite ratios in peritumoral area of cerebral gliomas. Medicina 2012, 48, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Provenzale, J.M.; McGraw, P.; Mhatre, P.; Guo, A.C.; Delong, D. Peritumoral brain regions in gliomas and meningiomas: Investigation with isotropic diffusion-weighted MR imaging and diffusion-tensor MR imaging. Radiology 2004, 232, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Lhatoo, S.D.; Moghimi, N.; Schuele, S. Tumor-related epilepsy: Epidemiology, pathogenesis and management. J. Neurooncol. 2018, 139, 13–21. [Google Scholar]
- Kim, S.H.; Lim, S.C.; Kim, W.; Kwon, O.H.; Jeon, S.; Lee, J.M.; Shon, Y.M. Extrafrontal structural changes in juvenile myoclonic epilepsy: A topographic analysis of combined structural and microstructural brain imaging. Seizure 2015, 30, 124–131. [Google Scholar] [CrossRef]
- Domin, M.; Bartels, S.; Geithner, J.; Wang, Z.I.; Runge, U.; Grothe, M.; Langner, S.; von Podewils, F. Juvenile Myoclonic Epilepsy Shows Potential Structural White Matter Abnormalities: A TBSS Study. Front. Neurol. 2018, 9, 509. [Google Scholar] [CrossRef]
- Beaulieu, C. The basis of anisotropic water diffusion in the nervous system-a technical review. NMR Biomed. 2002, 15, 435–455. [Google Scholar] [CrossRef]
- Alexander, A.L.; Hurley, S.A.; Samsonov, A.A.; Adluru, N.; Hosseinbor, A.P.; Mossahebi, P.; Tromp, D.P.; Zakszewski, E.; Field, A.S. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connect. 2011, 1, 423–446. [Google Scholar] [CrossRef]
- von Podewils, F.; Runge, U.; Krüger, S.; Geithner, J.; Wang, Z.I.; Khaw, A.V.; Angermaier, A.; Gaida, B.; Domin, M.; Kessler, C.; et al. Diffusion tensor imaging abnormalities in photosensitive juvenile myoclonic epilepsy. Eur. J. Neurol. 2015, 22, 1192–1200. [Google Scholar] [CrossRef]
- Focke, N.K.; Diederich, C.; Helms, G.; Nitsche, M.A.; Lerche, H.; Paulus, W. Idiopathic-generalized epilepsy shows profound white matter diffusion-tensor imaging alterations. Hum. Brain Mapp. 2014, 35, 3332–3342. [Google Scholar] [CrossRef]
- Vollmar, C.; O’Muircheartaigh, J.; Symms, M.R.; Barker, G.J.; Thompson, P.; Kumari, V.; Stretton, J.; Duncan, J.S.; Richardson, M.P.; Koepp, M.J. Altered microstructural connectivity in juvenile myoclonic epilepsy: The missing link. Neurology 2012, 78, 1555–1559. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Suh, S.I.; Seo, W.K.; Oh, K.; Koh, S.B.; Kim, J.H. Altered thalamocortical functional connectivity in idiopathic generalized epilepsy. Epilepsia 2014, 55, 592–600. [Google Scholar] [CrossRef] [PubMed]

| Ep | nEp | Con | p-Value | |
|---|---|---|---|---|
| Age range (mean ± SE) | 43.92 ± 10.82 | 45.00 ± 9.03 | 44.85 ± 3.31 | 0.94 * |
| Sex (female/male) | 8/4 | 6/7 | 6/7 | 0.77 ^ |
| Histology (HGG/LGG) | 3/9 | 5/8 | / | 0.91 ^ |
| Tumor volume (mL) | 50.88 ± 40.48 | 27.76 ± 17.36 | / | 0.25 u |
| Cluster Location | Voxel Size of Mean Diffusivity | |
|---|---|---|
| Ep | nEp | |
| Superior.longitudinal.fasciculus.R | 1755 | 1577 |
| Superior.corona.radiata.R | 1342 | 1150 |
| Inferior.fronto-occipital.fasciculus.R | 488 | 481 |
| Cluster Location | Atlas-Based Results | p-Value | ||
|---|---|---|---|---|
| Ep | nEP | Ep vs. Control | nEp vs. Control | |
| Superior.longitudinal.fasciculus.R | 0.4626 ± 0.0286 | 0.4565 ± 0.0304 | 0.03 u | 0.026 u |
| Superior.corona.radiata.R | 0.4813 ± 0.0270 | 0.2484 ± 0.0310 | 0.028 u | 0.012 u |
| Inferior.fronto-occipital.fasciculus.R | 0.4479 ± 0.0590 | 0.4861 ± 0.0193 | 0.034 u | 0.025 u |
| Cluster Location | Voxel Size of Mean Diffusivity | |
|---|---|---|
| Ep | nEp | |
| Cingulum.(cingulate.gyrus).R | 279 | 243 |
| Uncinate.fasciculus.R | 46 | 32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhou, C.; Zhu, Q.; Li, T.; Wang, Y.; Wang, L. Characteristics of Microstructural Changes Associated with Glioma Related Epilepsy: A Diffusion Tensor Imaging (DTI) Study. Brain Sci. 2022, 12, 1169. https://doi.org/10.3390/brainsci12091169
Zhang H, Zhou C, Zhu Q, Li T, Wang Y, Wang L. Characteristics of Microstructural Changes Associated with Glioma Related Epilepsy: A Diffusion Tensor Imaging (DTI) Study. Brain Sciences. 2022; 12(9):1169. https://doi.org/10.3390/brainsci12091169
Chicago/Turabian StyleZhang, Hong, Chunyao Zhou, Qiang Zhu, Tianshi Li, Yinyan Wang, and Lei Wang. 2022. "Characteristics of Microstructural Changes Associated with Glioma Related Epilepsy: A Diffusion Tensor Imaging (DTI) Study" Brain Sciences 12, no. 9: 1169. https://doi.org/10.3390/brainsci12091169
APA StyleZhang, H., Zhou, C., Zhu, Q., Li, T., Wang, Y., & Wang, L. (2022). Characteristics of Microstructural Changes Associated with Glioma Related Epilepsy: A Diffusion Tensor Imaging (DTI) Study. Brain Sciences, 12(9), 1169. https://doi.org/10.3390/brainsci12091169







