Regulation of Microglia-Activation-Mediated Neuroinflammation to Ameliorate Ischemia-Reperfusion Injury via the STAT5-NF-κB Pathway in Ischemic Stroke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Administration
2.3. Oxygen-Glucose Deprivation-Reperfusion
2.4. Cell Viability Assays with CCK-8 and LDH
2.5. Calcein Acetoxymethylester/Propidium Iodide AM/PI Staining Assay
2.6. Middle Cerebral Artery Occlusion in Mice
2.7. Infarct Volume Calculation
2.8. Neurological Deficit Scoring
2.9. Cerebral Blood Flow Measure
2.10. RNA Isolation and Quantitative Real-Time PCR
2.11. RNA Sequencing
2.12. Cytokine Analyses in Cellular Supernatant
2.13. Western Blot Assay
2.14. Immunofluorescence Staining in Brain Sections and Microglia Cells
2.15. Ligand–Receptor Docking and Analysis
2.16. Plasmid Construction and Transfection
2.17. Statistical Analysis
3. Results
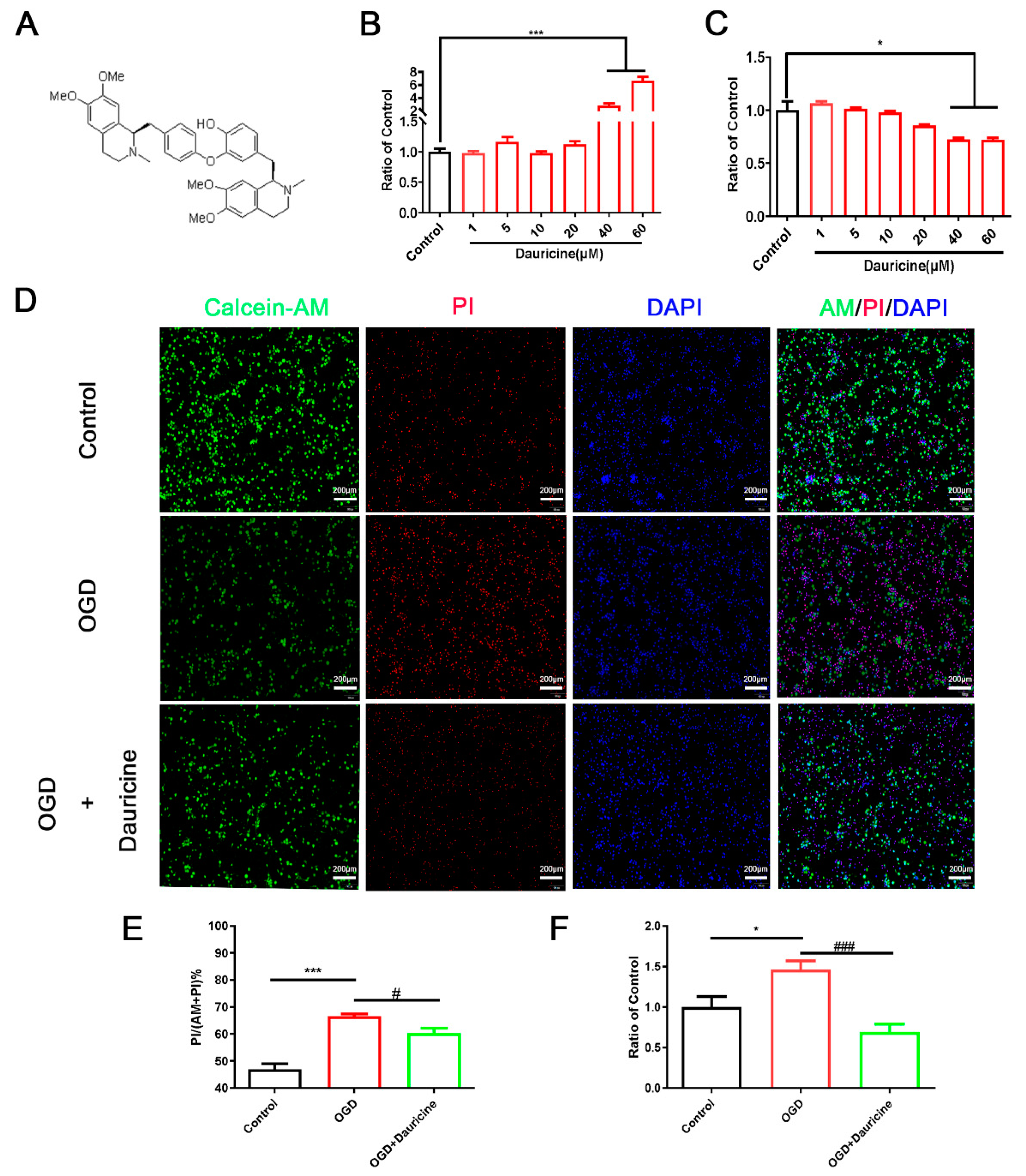
3.1. Dauricine Decreased Neuron Death Induced by OGD-R
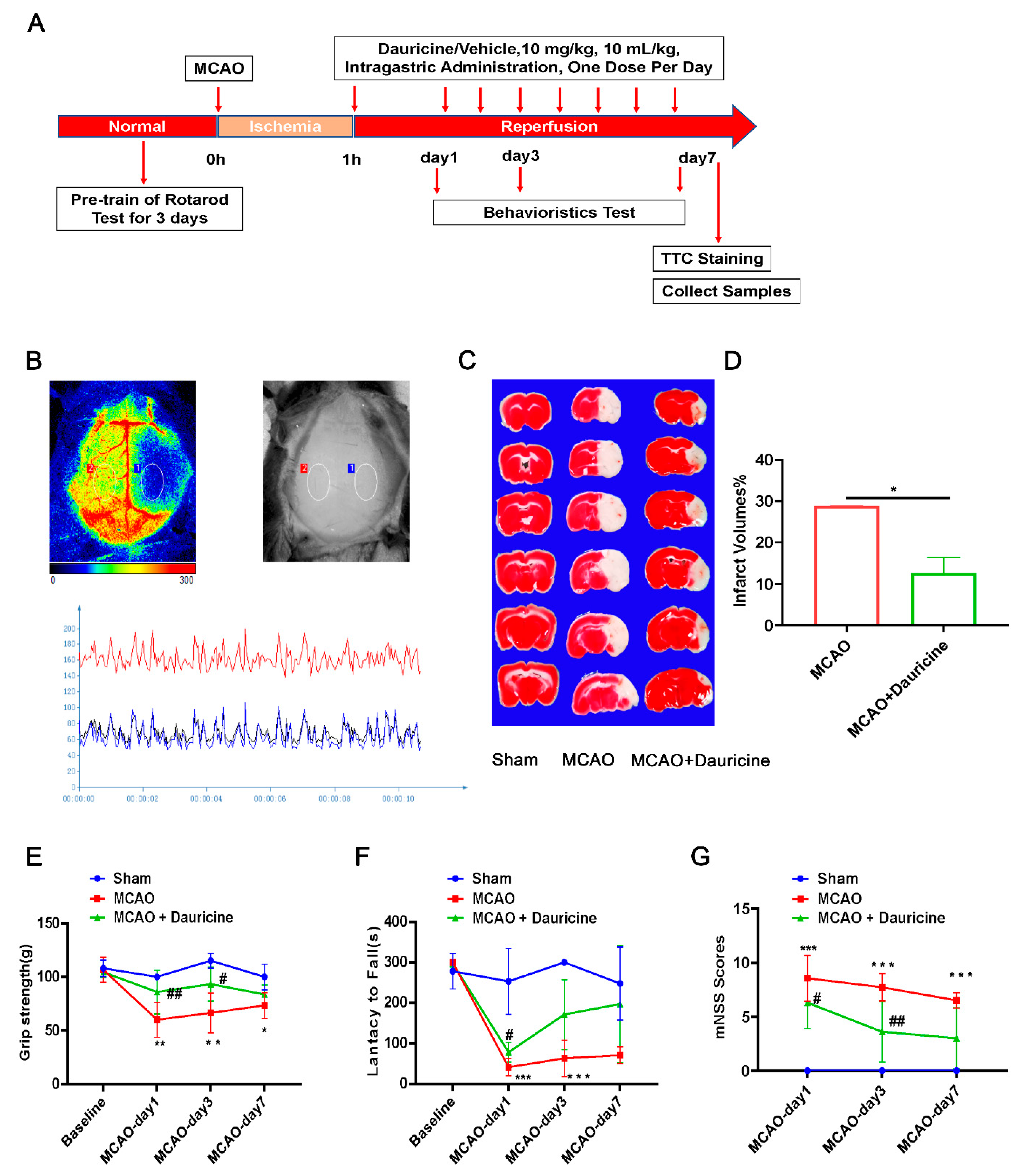
3.2. Dauricine Decreased Brain Infarct Size and Neurological Deficits after tMCAO Injury
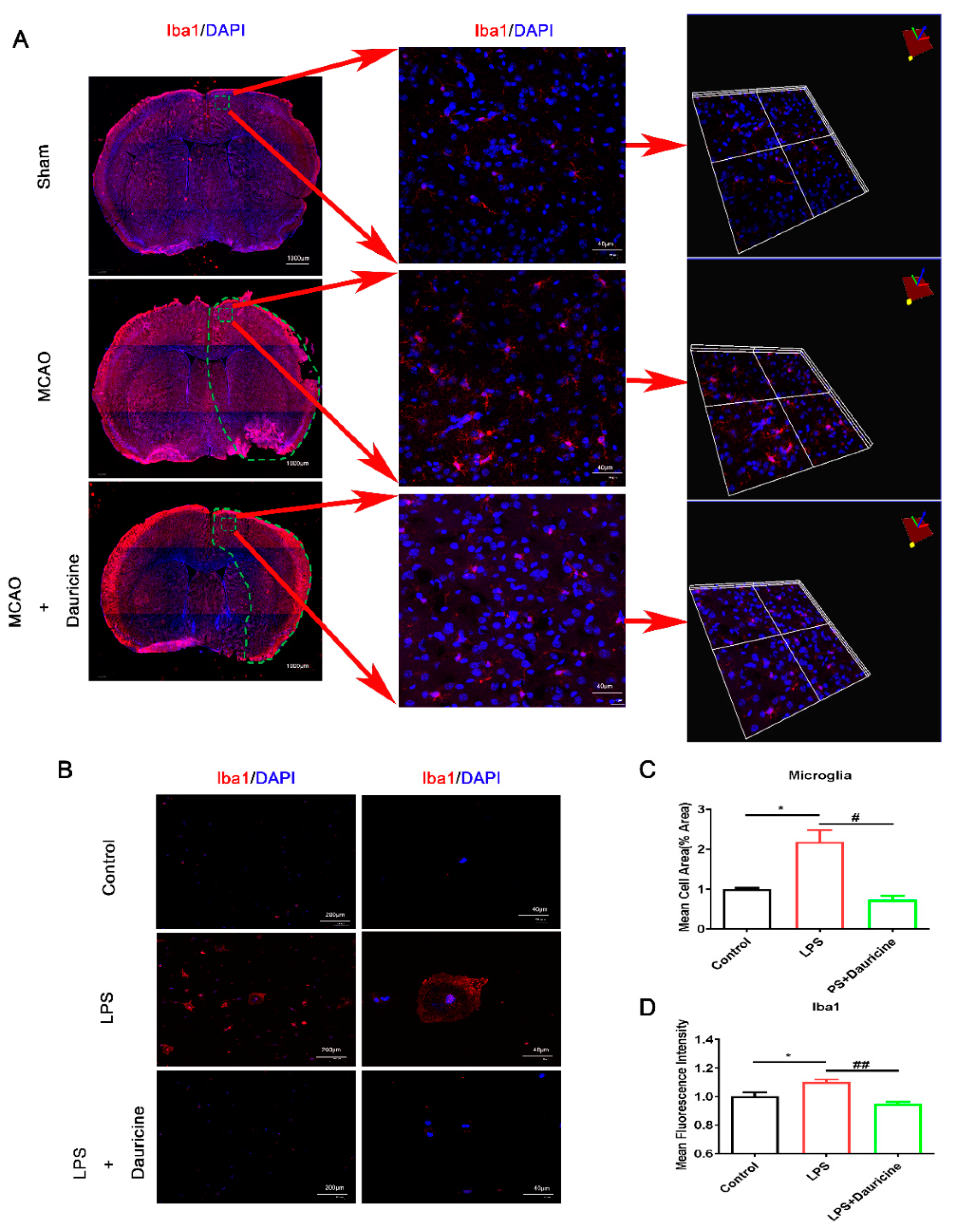
3.3. Dauricine Inhibited the Activation of Microglia In Vitro and In Vivo
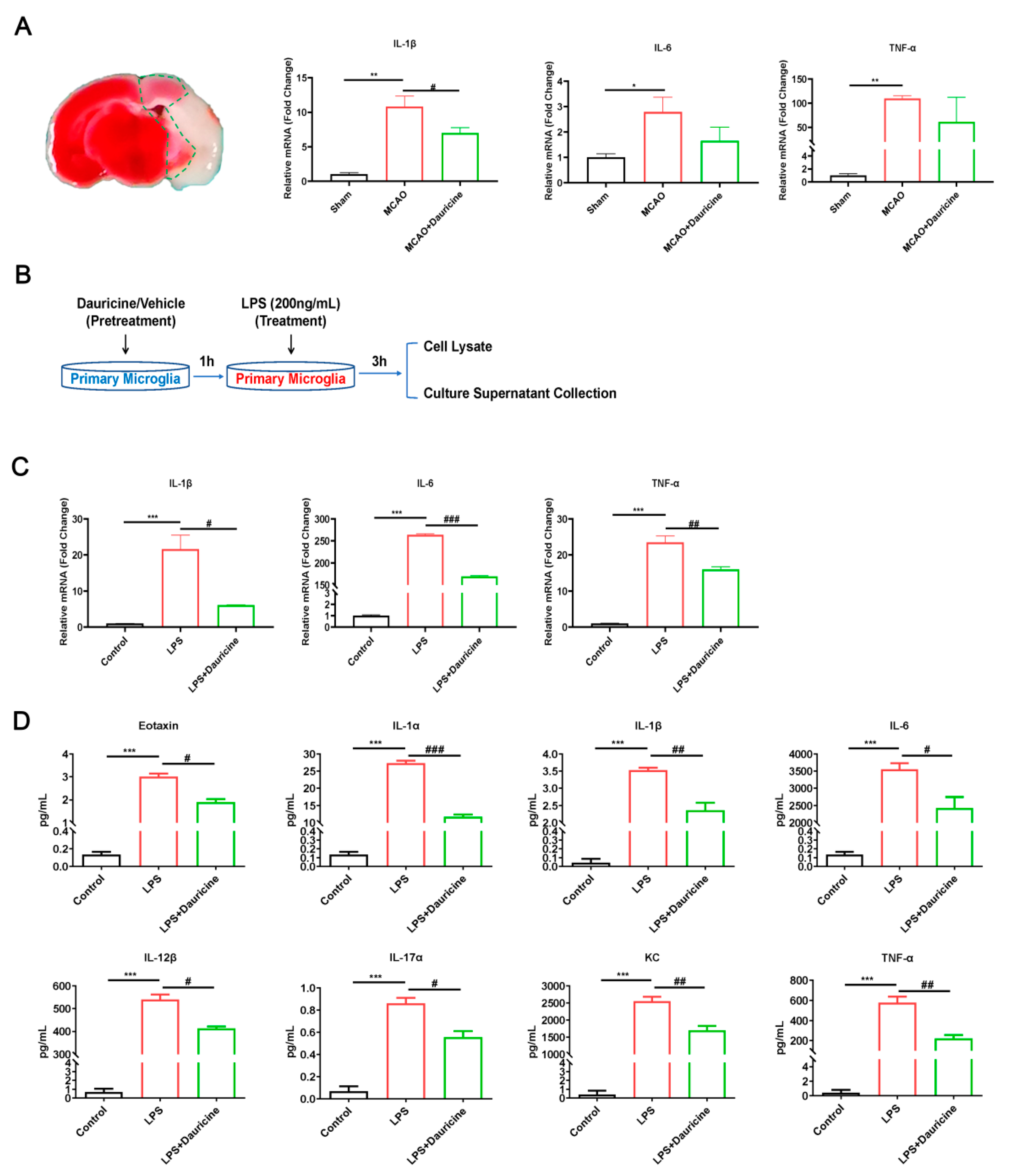
3.4. Dauricine Reduced Inflammation Induced by LPS or tMCAO Injury
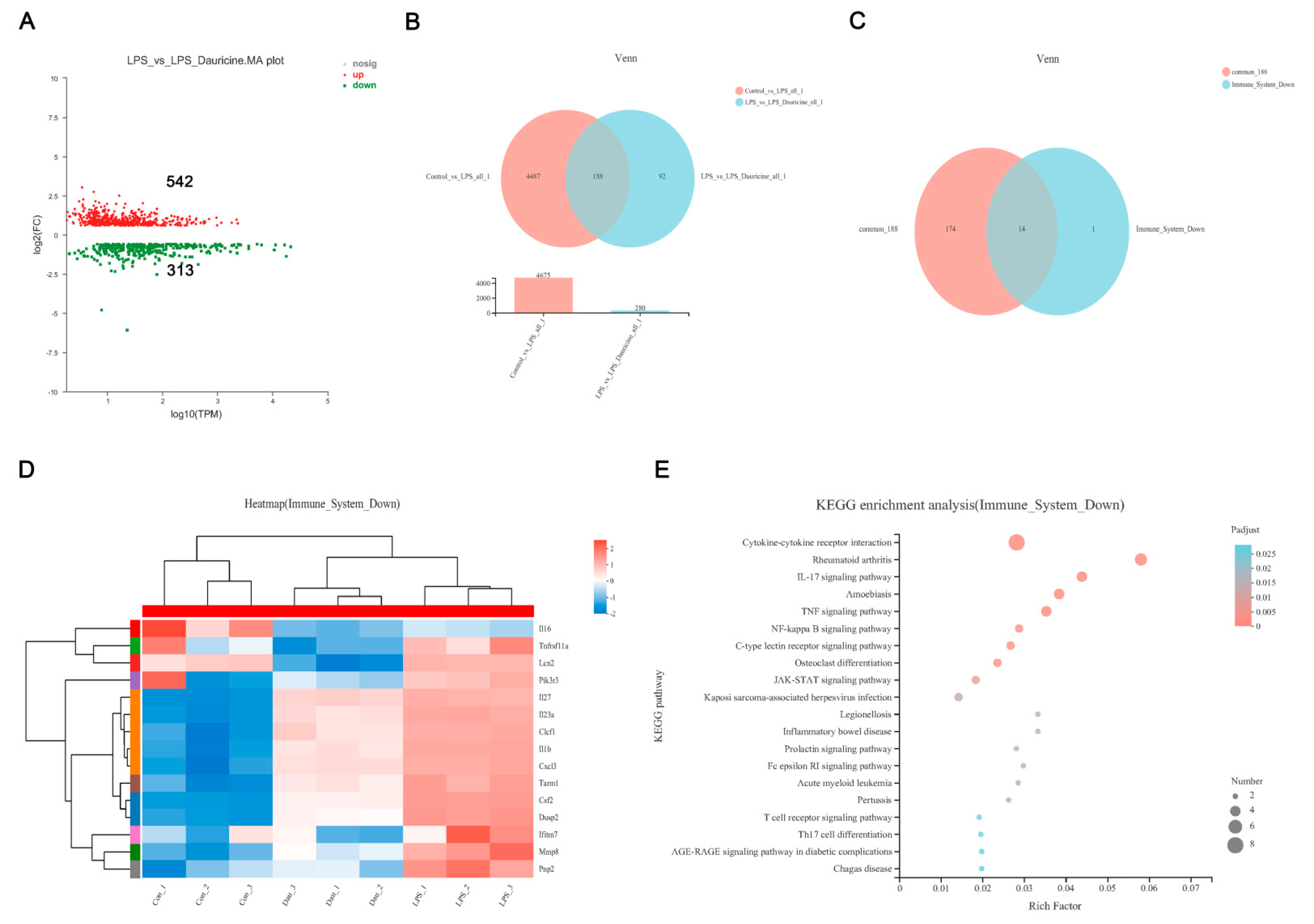
3.5. Dauricine Down-Regulated Expression of Immune Factors
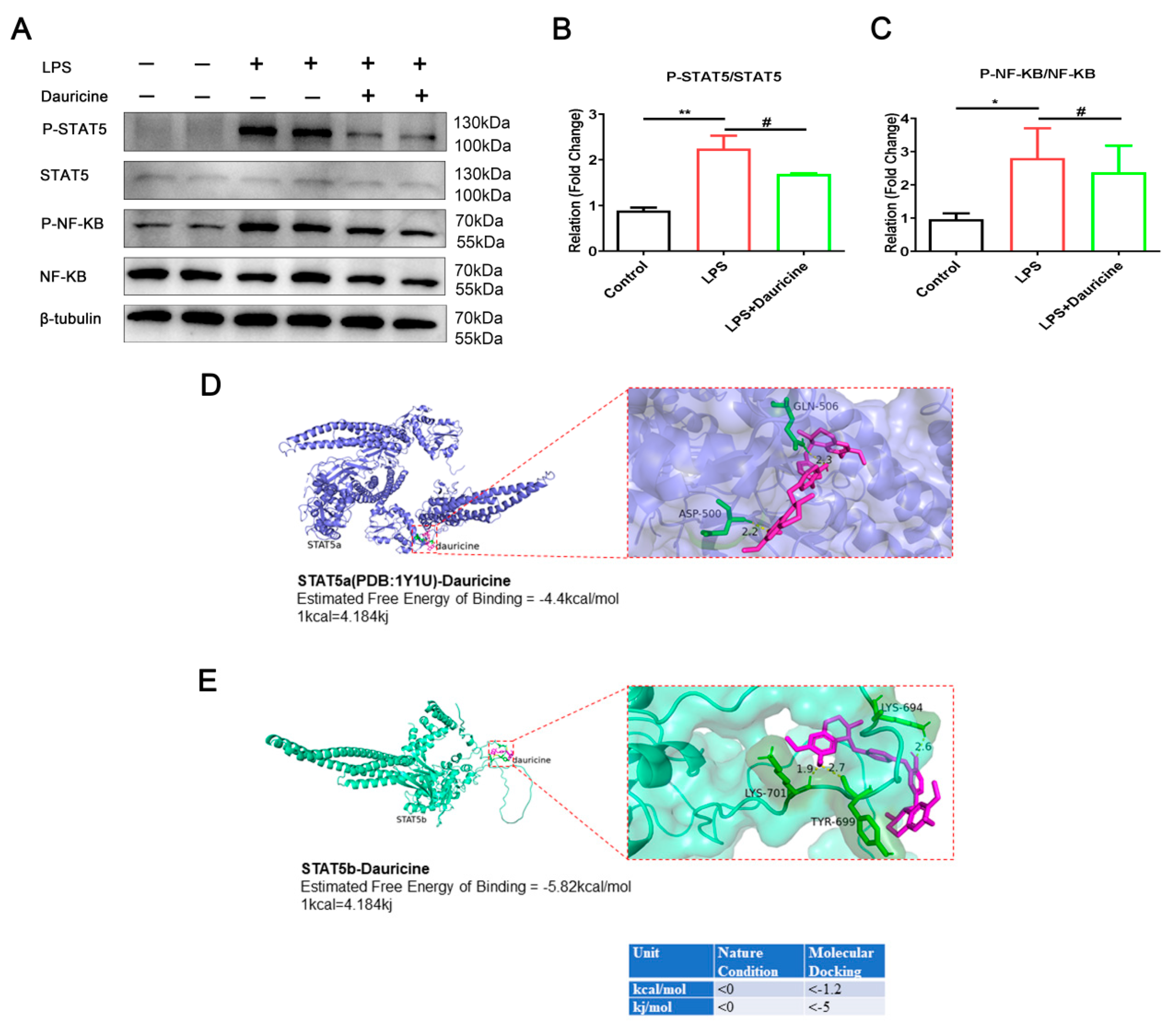
3.6. Dauricine Inhibited the Activation of STAT5 and NF-κB Pathways
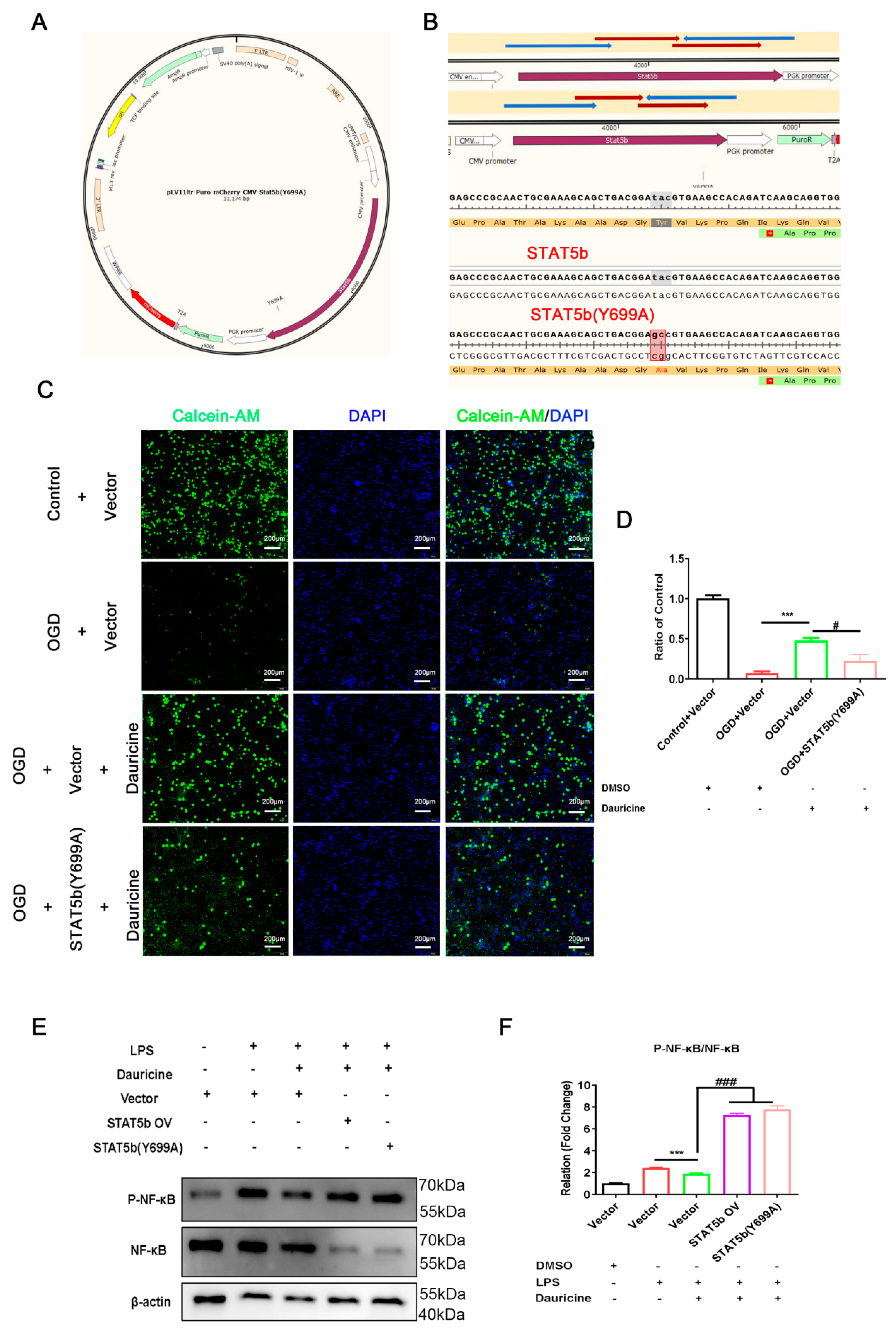
3.7. STAT5b (Tyr699 mutant) Reversed the Neuroprotection of Neuron and Activated the NF-κB Pathway of Microglia
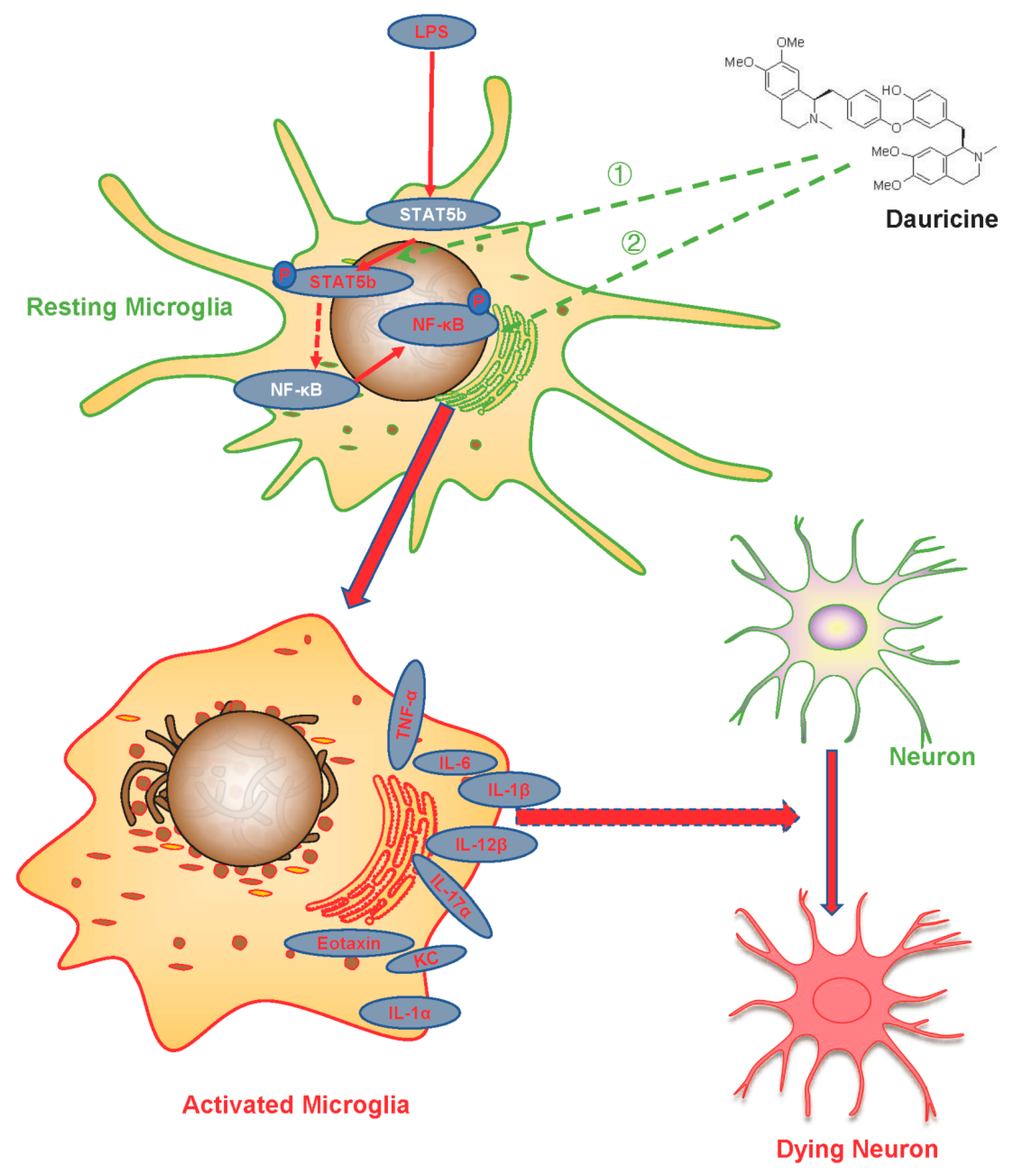
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelly, P.J.; Lemmens, R.; Tsivgoulis, G. Inflammation and Stroke Risk: A New Target for Prevention. Stroke 2021, 52, 2697–2706. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Wang, C.; Ye, X.; Zhong, P.; Wu, D. Persistent inflammation worsens short-term outcomes in massive stroke patients. BMC Neurol. 2021, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Goshi, N.; Morgan, R.K.; Lein, P.J.; Seker, E. A primary neural cell culture model to study neuron, astrocyte, and microglia interactions in neuroinflammation. J. Neuroinflamm. 2020, 17, 155. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, J.; Wang, Y.; Yang, G.Y. The biphasic function of microglia in ischemic stroke. Prog. Neurobiol. 2017, 157, 247–272. [Google Scholar] [CrossRef]
- Kelly, P.J.; Camps-Renom, P.; Giannotti, N.; Marti-Fabregas, J.; McNulty, J.P.; Baron, J.C.; Barry, M.; Coutts, S.B.; Cronin, S.; Delgado-Mederos, R.; et al. A Risk Score Including Carotid Plaque Inflammation and Stenosis Severity Improves Identification of Recurrent Stroke. Stroke 2020, 51, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J.; Camps-Renom, P.; Giannotti, N.; Marti-Fabregas, J.; Murphy, S.; McNulty, J.; Barry, M.; Barry, P.; Calvet, D.; Coutts, S.B.; et al. Carotid Plaque Inflammation Imaged by (18)F-Fluorodeoxyglucose Positron Emission Tomography and Risk of Early Recurrent Stroke. Stroke 2019, 50, 1766–1773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yan, C.; Wei, C.; Yao, Y.; Ma, X.; Gong, Z.; Liu, S.; Zang, D.; Chen, J.; Shi, F.D.; et al. Vinpocetine Inhibits NF-κB-Dependent Inflammation in Acute Ischemic Stroke Patients. Transl. Stroke Res. 2018, 9, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, R.; An, J.; Wang, Y.; Liang, M.; Huang, K. Dauricine Attenuates Vascular Endothelial Inflammation Through Inhibiting NF-κB Pathway. Front. Pharm. 2021, 12, 758962. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Wang, H.; Wang, C.; Liang, M.; Huang, K.; Li, Y. Dauricine negatively regulates lipopolysaccharide- or cecal ligation and puncture-induced inflammatory response via NF-κB inactivation. Arch. Biochem. Biophys. 2019, 666, 99–106. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Zhou, S.J. Dauricine combined with clindamycin inhibits severe pneumonia co-infected by influenza virus H5N1 and Streptococcus pneumoniae in vitro and in vivo through NF-κB signaling pathway. J. Pharmacol. Sci. 2018, 137, 12–19. [Google Scholar] [CrossRef]
- Peng, C.; Fu, X.; Wang, K.; Chen, L.; Luo, B.; Huang, N.; Luo, Y.; Chen, W. Dauricine alleviated secondary brain injury after intracerebral hemorrhage by upregulating GPX4 expression and inhibiting ferroptosis of nerve cells. Eur. J. Pharmacol. 2022, 914, 174461. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Jiang, S.Q.; Zhang, L.; Liu, Q.N.; Gong, P.L. Inhibitory effect of dauricine on inflammatory process following focal cerebral ischemia/reperfusion in rats. Am. J. Chin. Med. 2007, 35, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lian, Y.; Lu, C.; Jing, L.; Yuan, H.; Peng, S. Inhibitory effects of a bisbenzylisoquinline alkaloid dauricine on HERG potassium channels. J. Ethnopharmacol. 2012, 141, 685–691. [Google Scholar] [CrossRef]
- Meng, H.; Zhao, H.; Cao, X.; Hao, J.; Zhang, H.; Liu, Y.; Zhu, M.S.; Fan, L.; Weng, L.; Qian, L.; et al. Double-negative T cells remarkably promote neuroinflammation after ischemic stroke. Proc. Natl. Acad. Sci. USA 2019, 116, 5558–5563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cao, X.; Bao, X.; Zhang, Y.; Xu, Y.; Sha, D. Cocaine- and amphetamine-regulated transcript protects synaptic structures in neurons after ischemic cerebral injury. Neuropeptides 2020, 81, 102023. [Google Scholar] [CrossRef]
- Li, Y.H.; Gong, P.L. Neuroprotective effects of dauricine against apoptosis induced by transient focal cerebral ischaemia in rats via a mitochondrial pathway. Clin. Exp. Pharmacol. Physiol. 2007, 34, 177–184. [Google Scholar] [CrossRef]
- Yang, X.Y.; Liu, Q.N.; Zhang, L.; Jiang, S.Q.; Gong, P.L. Neuroprotective effect of dauricine after transient middle cerebral artery occlusion in rats: Involvement of Bcl-2 family proteins. Am. J. Chin. Med. 2010, 38, 307–318. [Google Scholar] [CrossRef]
- Guo, S.; Tjarnlund-Wolf, A.; Deng, W.; Tejima-Mandeville, E.; Lo, L.J.; Xing, C.; Arai, K.; Ning, M.; Zhou, Y.; Lo, E.H. Comparative transcriptome of neurons after oxygen-glucose deprivation: Potential differences in neuroprotection versus reperfusion. J. Cereb. Blood Flow Metab. 2018, 38, 2236–2250. [Google Scholar] [CrossRef]
- Egashira, Y.; Suzuki, Y.; Azuma, Y.; Takagi, T.; Mishiro, K.; Sugitani, S.; Tsuruma, K.; Shimazawa, M.; Yoshimura, S.; Kashimata, M.; et al. The growth factor progranulin attenuates neuronal injury induced by cerebral ischemia-reperfusion through the suppression of neutrophil recruitment. J. Neuroinflamm. 2013, 10, 105. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, X.; Bao, Y.; Yu, H.; Jia, D.; Ma, C. Down-regulation of GAS5 ameliorates myocardial ischaemia/reperfusion injury via the miR-335/ROCK1/AKT/GSK-3beta axis. J. Cell. Mol. Med. 2019, 23, 8420–8431. [Google Scholar] [CrossRef]
- Cen, J.; Liu, L.; Li, M.S.; He, L.; Wang, L.J.; Liu, Y.Q.; Liu, M.; Ji, B.S. Alteration in P-glycoprotein at the blood-brain barrier in the early period of MCAO in rats. J. Pharm. Pharmacol. 2013, 65, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Fan, L.; Zhang, C.J.; Zhu, L.; Liu, P.; Chen, J.; Bao, X.; Pu, Z.; Zhu, M.S.; Xu, Y. Synthetic VSMCs induce BBB disruption mediated by MYPT1 in ischemic stroke. Iscience 2021, 24, 103047. [Google Scholar] [CrossRef] [PubMed]
- Alamri, F.F.; Shoyaib, A.A.; Biggers, A.; Jayaraman, S.; Guindon, J.; Karamyan, V.T. Applicability of the grip strength and automated von Frey tactile sensitivity tests in the mouse photothrombotic model of stroke. Behav. Brain Res. 2018, 336, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.J.; Sun, J.J.; Qian, L.; Liu, Z.; Zhang, Z.; Cao, W.; Li, W.; Xu, Y. Human umbilical mesenchymal stem cells enhance the expression of neurotrophic factors and protect ataxic mice. Brain Res. 2011, 1402, 122–131. [Google Scholar] [CrossRef]
- Chen, J.; Sanberg, P.R.; Li, Y.; Wang, L.; Lu, M.; Willing, A.E.; Sanchez-Ramos, J.; Chopp, M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 2001, 32, 2682–2688. [Google Scholar] [CrossRef]
- Wei, G.; Jiang, D.; Hu, S.; Yang, Z.; Zhang, Z.; Li, W.; Cai, W.; Liu, D. Polydopamine-Decorated Microcomposites Promote Functional Recovery of an Injured Spinal Cord by Inhibiting Neuroinflammation. ACS Appl. Mater. Interfaces 2021, 13, 47341–47353. [Google Scholar] [CrossRef]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput.-Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef]
- Anrather, J.; Iadecola, C. Inflammation and Stroke: An Overview. Neurotherapeutics 2016, 13, 661–670. [Google Scholar] [CrossRef]
- Lambertsen, K.L.; Finsen, B.; Clausen, B.H. Post-stroke inflammation-target or tool for therapy? Acta Neuropathol. 2019, 137, 693–714. [Google Scholar] [CrossRef]
- Jin, R.; Liu, L.; Zhang, S.; Nanda, A.; Li, G. Role of inflammation and its mediators in acute ischemic stroke. J. Cardiovasc. Transl. Res. 2013, 6, 834–851. [Google Scholar] [CrossRef] [Green Version]
- Kadekar, D.; Agerholm, R.; Rizk, J.; Neubauer, H.A.; Suske, T.; Maurer, B.; Vinals, M.T.; Comelli, E.M.; Taibi, A.; Moriggl, R.; et al. The neonatal microenvironment programs innate gammadelta T cells through the transcription factor STAT5. J. Clin. Investig. 2020, 130, 2496–2508. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, K.L.; Aesoph, D.; Ammer, A.G.; Zheng, W.; Rahimpour, S.; Farris, B.Y.; Spinner, C.A.; Li, P.; Lin, J.X.; Yu, Z.X.; et al. Tetramerization of STAT5 promotes autoimmune-mediated neuroinflammation. Proc. Natl. Acad. Sci. USA 2021, 118, e2116256118. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, J.; Panangipalli, G.; Ulrich, B.J.; Koh, B.; Xu, C.; Kharwadkar, R.; Chu, X.; Wang, Y.; Gao, H.; et al. STAT5 promotes accessibility and is required for BATF-mediated plasticity at the Il9 locus. Nat. Commun. 2020, 11, 4882. [Google Scholar] [CrossRef] [PubMed]
- Hedl, M.; Sun, R.; Huang, C.; Abraham, C. STAT3 and STAT5 Signaling Thresholds Determine Distinct Regulation for Innate Receptor-Induced Inflammatory Cytokines, and STAT3/STAT5 Disease Variants Modulate These Outcomes. J. Immunol. 2019, 203, 3325–3338. [Google Scholar] [CrossRef]
- Jin, G.; Wang, L.; Ma, J. Inhibiting STAT5 significantly attenuated Ang II-induced cardiac dysfunction and inflammation. Eur. J. Pharm. 2022, 915, 174689. [Google Scholar] [CrossRef]
- Wang, X.; Ding, X.; Yan, J.; Lu, Z.; Cao, H.; Ni, X.; Ying, Y. STAT5 inhibitor attenuates atherosclerosis via inhibition of inflammation: The role of STAT5 in atherosclerosis. Am. J. Transl. Res. 2021, 13, 1422–1431. [Google Scholar]
- Lawrence, T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Lai, J.L.; Liu, Y.H.; Liu, C.; Qi, M.P.; Liu, R.N.; Zhu, X.F.; Zhou, Q.G.; Chen, Y.Y.; Guo, A.Z.; Hu, C.M. Indirubin Inhibits LPS-Induced Inflammation via TLR4 Abrogation Mediated by the NF-kB and MAPK Signaling Pathways. Inflammation 2017, 40, 1–12. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; Eid, B.G. Icariin modulates carrageenan-induced acute inflammation through HO-1/Nrf2 and NF-kB signaling pathways. Biomed Pharm. 2019, 120, 109567. [Google Scholar] [CrossRef]
- Davari, M.; Hashemi, R.; Mirmiran, P.; Hedayati, M.; Sahranavard, S.; Bahreini, S.; Tavakoly, R.; Talaei, B. Effects of cinnamon supplementation on expression of systemic inflammation factors, NF-kB and Sirtuin-1 (SIRT1) in type 2 diabetes: A randomized, double blind, and controlled clinical trial. Nutr. J. 2020, 19, 1. [Google Scholar] [CrossRef]
- Li, M.; Li, R. IL-2 regulates oral mucosa inflammation through inducing endoplasmic reticulum stress and activating the NF-kB pathway. J. Recept. Signal Transduct. 2020, 40, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Brockmueller, A.; Mueller, A.L.; Shayan, P.; Shakibaei, M. Curcumin Attenuates Environment-Derived Osteoarthritis by Sox9/NF-kB Signaling Axis. Int. J. Mol. Sci. 2021, 22, 7645. [Google Scholar] [CrossRef] [PubMed]
- Swierkot, J.; Sokolik, R.; Czarny, A.; Zaczynska, E.; Nowak, B.; Chlebicki, A.; Korman, L.; Madej, M.; Wojtala, P.; Lubinski, L.; et al. Activity of JAK/STAT and NF-kB in patients with axial spondyloarthritis. Postepy Hig. I Med. Dosw. 2015, 69, 1291–1298. [Google Scholar] [CrossRef]
- Lan, W.; Wang, Z.; Liu, J.; Liu, H. Methionyl-Methionine Exerts Anti-Inflammatory Effects through the JAK2-STAT5-NF-κB and MAPK Signaling Pathways in Bovine Mammary Epithelial Cells. J. Agric. Food Chem. 2020, 68, 13742–13750. [Google Scholar] [CrossRef]
- Sun, S.L.; Li, T.J.; Yang, P.Y.; Qiu, Y.; Rui, Y.C. Modulation of signal transducers and activators of transcription (STAT) factor pathways during focal cerebral ischaemia: A gene expression array study in rat hippocampus after middle cerebral artery occlusion. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
| Gene | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| TNF-α | CCTGTAGCCCACGTCGTAG | GGGAGTAGACAAGGTACAACCC |
| IL-1β | GAAATGCCACCTTTTGACAGTG | TGGATGCTCTCATCAGGACAG |
| IL-6 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| GAPDH | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
| β-Actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, Z.; Xia, S.; Shao, P.; Bao, X.; Wu, D.; Xu, Y. Regulation of Microglia-Activation-Mediated Neuroinflammation to Ameliorate Ischemia-Reperfusion Injury via the STAT5-NF-κB Pathway in Ischemic Stroke. Brain Sci. 2022, 12, 1153. https://doi.org/10.3390/brainsci12091153
Pu Z, Xia S, Shao P, Bao X, Wu D, Xu Y. Regulation of Microglia-Activation-Mediated Neuroinflammation to Ameliorate Ischemia-Reperfusion Injury via the STAT5-NF-κB Pathway in Ischemic Stroke. Brain Sciences. 2022; 12(9):1153. https://doi.org/10.3390/brainsci12091153
Chicago/Turabian StylePu, Zhijun, Shengnan Xia, Pengfei Shao, Xinyu Bao, Dan Wu, and Yun Xu. 2022. "Regulation of Microglia-Activation-Mediated Neuroinflammation to Ameliorate Ischemia-Reperfusion Injury via the STAT5-NF-κB Pathway in Ischemic Stroke" Brain Sciences 12, no. 9: 1153. https://doi.org/10.3390/brainsci12091153
APA StylePu, Z., Xia, S., Shao, P., Bao, X., Wu, D., & Xu, Y. (2022). Regulation of Microglia-Activation-Mediated Neuroinflammation to Ameliorate Ischemia-Reperfusion Injury via the STAT5-NF-κB Pathway in Ischemic Stroke. Brain Sciences, 12(9), 1153. https://doi.org/10.3390/brainsci12091153






