A Mini-Review Regarding the Modalities to Study Neurodevelopmental Disorders-Like Impairments in Zebrafish—Focussing on Neurobehavioural and Psychological Responses
Abstract
:1. Introduction
2. Methods
3. Pathophysiology and Mechanisms
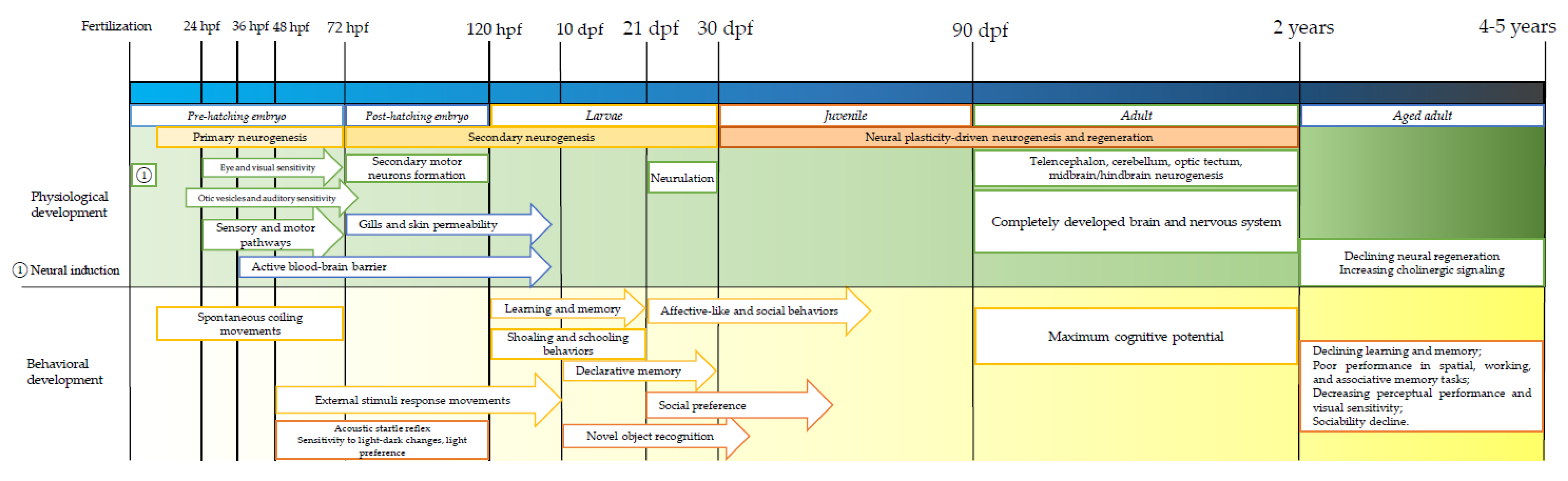
4. Zebrafish Physiology, Neurodevelopment, and Behavior
5. Autism and Schizophrenia Zebrafish Animal Models
| Study | Modulatory Agent | Stage of Development | Observed Effects in Zebrafish | Interspecific Translation |
|---|---|---|---|---|
| [102,117] | Valproic acid | Embryos |
Dose-depend response | In valproic acid in utero exposed rats [118,119]:
Repetitive behaviour. |
| [120,121] | Valproic acid | Embryos/larvae | Telencephalon: neural progenitor cell proliferation in the mutagenesis: adsl, mdbs, tsclb, shank3 | |
| [122] | Valproic acid | Juveniles |
| |
| [6,123,124] | Valproic acid | Embryos, larvae |
| In rodents: similar developmental and behavioural outcomes |
| [6,93,125,126,127,128,129] | MK-801 (NMDAR antagonist) | Larvae |
|
|
| [113,129,130,131] | Shank3a/shank3b mutated model | Embryos | ASD-typical behavior and digestive impairments | In juvenile Shank3 deficient rats: social communication deficits |
| [132,133,134,135] | CHD8 mutated models | Larvae |
| In transgenic mice:
|
| [136,137,138] | Chd7 null mutant models | Larvae |
| In mice: cerebellar hypoplasia |
| [139,140] |
| Embryos/larvae |
| In mice: impaired synaptic transmission |
| [102,117] | Caffeine | Embryos |
| Adults rats:
|
| [22,34] | Nicotine | Embryos (46 hpf) | Hyperactivity at high doses | Rodents: hyperactivity |
| [102,141] | Eclipta prostrata, Spilanthes acmella (Linn.) Murr | Embryos |
| |
| [142] | Polygonum multiflorum | Embryos | Morphological defects were observed from 105 mg/L | |
| [102,142] | Millettia pachycarpa | Embryos (blastula stage) |
| |
| [102,136] | Celastrol | Embryos |
| |
| [102,143] | Arecoline | Embryos |
| In mice:
|
| [36,102] | Cannabinoid | Embryos |
| Similar effects on locomotor activity, but also may be harmful to the development of the foetus and behaviour of the mother. |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Thapar, A.; Cooper, M.; Rutter, M. Neurodevelopmental disorders. Lancet Psychiatry 2017, 4, 339–346. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. America’s Children and the Environment, 3rd ed.; U.S. Environmental Protection Agency: Washington, DC, USA, 2013. [Google Scholar]
- D’Souza, H.; Karmiloff-Smith, A. Neurodevelopmental disorders. Wiley Interdiscip. Rev. Cogn. Sci. 2017, 8, e1398. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.W.; Dawes, S.E.; Heaton, R.K. What do we know about neuropsychological aspects of schizophrenia? Neuropsychol. Rev. 2009, 19, 365–384. [Google Scholar] [CrossRef] [PubMed]
- Bitsch, F.; Berger, P.; Nagels, A.; Falkenberg, I.; Straube, B. Characterizing the theory of mind network in schizophrenia reveals a sparser network structure. Schizophr. Res. 2020, 228, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Lysaker, P.H.; Cheli, S.; Dimaggio, G.; Buck, B.; Bonfils, K.A.; Huling, K.; Wiesepape, C.; Lysaker, J.T. Metacognition, social cognition, and mentalizing in psychosis: Are these distinct constructs when it comes to subjective experience or are we just splitting hairs? BMC Psychiatry 2021, 21, 329. [Google Scholar] [CrossRef] [PubMed]
- Meshalkina, D.A.; Kizlyk, M.N.; Kysil, E.V.; Collier, A.D.; Echevarria, D.J.; Abreu, M.S.; Barcellos, L.J.G.; Song, C.; Warnick, J.E.; Kyzar, E.J.; et al. Zebrafish models of autism spectrum disorder. Exp. Neurol. 2018, 299, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef]
- Spence, R.; Gerlach, G.; Lawrence, C.; Smith, C. The behaviour and ecology of the zebrafish, Danio rerio. Biol. Rev. 2007, 83, 13–34. [Google Scholar] [CrossRef]
- Vaz, R.; Hofmeister, W.; Lindstrand, A. Zebrafish Models of Neurodevelopmental Disorders: Limitations and Benefits of Current Tools and Techniques. Int. J. Mol. Sci. 2019, 20, 1296. [Google Scholar] [CrossRef]
- Snyder, M.A.; Gao, W.J. NMDA hypofunction as a convergence point for progression and symptoms of schizophrenia. Front. Cell. Neurosci. 2013, 7, 31. [Google Scholar] [CrossRef] [Green Version]
- Silverman, J.L.; Thurm, A.; Ethridge, S.B.; Soller, M.M.; Petkova, S.P.; Abel, T.; Bauman, M.D.; Brodkin, E.S.; Harony-Nicolas, H.; Wöhr, M.; et al. Reconsidering animal models used to study autism spectrum disorder: Current state and optimizing future. Genes Brain Behav. 2022, 21, e12803. [Google Scholar] [CrossRef] [PubMed]
- Ozonoff, S.; Heung, K.; Byrd, R.; Hansen, R.; Hertz-Picciotto, I. The onset of autism: Patterns of symptom emergence in the first years of life. Autism Res. 2008, 1, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.S.; Andrade, C. The MMR vaccine and autism: Sensation, refutation, retraction, and fraud. Indian. J. Psychiatry 2011, 53, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Meguid, N.A.; El-Bana, M.A.; Tinkov, A.A.; Saad, K.; Dadar, M.; Hemimi, M.; Skalny, A.V.; Hosnedlová, B.; Kizek, R.; et al. Oxidative Stress in Autism Spectrum Disorder. Mol. Neurobiol. 2020, 57, 2314–2332. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.J.; O’Donovan, M.C. Schizophrenia and the neurodevelopmental continuum:evidence from genomics. World Psychiatry 2017, 16, 227–235. [Google Scholar] [CrossRef]
- Barlati, S.; Deste, G.; Ariu, C.; Vita, A. Autism Spectrum Disorder and Schizophrenia: Do They Overlap? Int. J. Emerg. Ment. Health 2016, 18, 760–763. [Google Scholar] [CrossRef]
- Willborn, R.J.; Hall, C.P.; Fuller, M.A. Recycling N-acetylcysteine: A review of evidence for adjunctive therapy in schizophrenia. Ment. Health Clin. 2019, 9, 116–123. [Google Scholar] [CrossRef]
- Jastrzębska, K.; Walczak, M.; Cieślak, P.E.; Szumiec, Ł.; Turbasa, M.; Engblom, D.; Błasiak, T.; Parkitna, J.R. Loss of NMDA receptors in dopamine neurons leads to the development of affective disorder-like symptoms in mice. Sci. Rep. 2016, 6, 37171. [Google Scholar] [CrossRef]
- Balu, D.T. The NMDA Receptor and Schizophrenia: From Pathophysiology to Treatment. Adv. Pharmacol. 2016, 76, 351–382. [Google Scholar]
- Mielnik, C.A.; Binko, M.A.; Chen, Y.; Funk, A.J.; Johansson, E.M.; Intson, K.; Sivananthan, N.; Islam, R.; Milenkovic, M.; Horsfall, W.; et al. Consequences of NMDA receptor deficiency can be rescued in the adult brain. Mol Psychiatry 2021, 26, 2929–2942. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, J.; Wu, R. Drugs Based on NMDAR Hypofunction Hypothesis in Schizophrenia. Front. Neurosci. 2021, 15, 641047. [Google Scholar] [CrossRef] [PubMed]
- Filice, F.; Janickova, L.; Henzi, T.; Bilella, A.; Schwaller, B. The Parvalbumin Hypothesis of Autism Spectrum Disorder. Front. Cell. Neurosci. 2020, 14, 577525. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, G.E.; Do, K.Q. Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia patho-genesis. Nat. Publ. Gr. 2016, 17, 1–9. [Google Scholar] [CrossRef]
- Mavroudis, I.A.; Petrides, F.; Manani, M.; Chatzinikolaou, F.; Ciobică, A.S.; Pădurariu, M.; Kazis, D.; Njau, S.N.; Costa, V.G.; Baloyannis, S.J. Purkinje cells pathology in schizophrenia. A morphometric approach. Rom. J. Morphol. Embryol. 2017, 58, 419–424. [Google Scholar]
- Ashwin, C.; Chapman, E.; Howells, J.; Rhydderch, D.; Walker, I.; Baron-Cohen, S. Enhanced olfactory sensitivity in autism spectrum conditions. Mol. Autism 2014, 5, 53. [Google Scholar] [CrossRef]
- Porter, B.A.; Mueller, T. The Zebrafish Amygdaloid Complex–Functional Ground Plan, Molecular Delineation, and Everted Topology. Front. Neurosci. 2020, 14, 608. [Google Scholar] [CrossRef]
- Urban-Kowalczyk, M.; Śmigielski, J.; Strzelecki, D. Olfactory identification in patients with schizophrenia–the influence of β-endorphin and calcitonin gene-related peptide concentrations. Eur. Psychiatry 2017, 41, 16–20. [Google Scholar] [CrossRef]
- Gawel, K.; Banono, S.; Michalak, A.; Esguerra, C.V. A critical review of zebrafish schizophrenia models: Time for validation? Neuroscience & Biobehavioral Reviews. Neurosci. Biobehav. Rev. 2019, 107, 6–22. [Google Scholar]
- Bigdai, E.V.; Samoilov, V.O.; Sinegubov, A.A. Complex Impairments to the Olfactory Sensory System in Schizophrenia. Neurosci. Behav. Physiol. 2022, 52, 598–606. [Google Scholar] [CrossRef]
- López-Schier, H. Neuroplasticity in the acoustic startle reflex in larval zebrafish. Curr. Opin. Neurobiol. 2019, 54, 134–139. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S.; et al. Towards a Comprehensive Catalog of Zebrafish Behavior 1.0 and Beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Mora-Zamorano, F.X.; Svoboda, K.R.; Carvan, M.J. The Nicotine-Evoked Locomotor Response: A Behavioral Paradigm for Toxicity Screening in Zebrafish (Danio rerio) Embryos and Eleutheroembryos Exposed to Methylmercury. PLoS ONE 2016, 11, e0154570. [Google Scholar] [CrossRef]
- Wagner, S. Developing Sensory Behavioral Assays for Zebrafish Autism Model. Honors College Theses, 2019. Available online: https://digitalcommons.georgiasouthern.edu/honors-theses/441 (accessed on 3 March 2021).
- Akhtar, M.T.; Ali, S.; Rashidi, H.; van der Kooy, F.; Verpoorte, R.; Richardson, M.K. Developmental Effects of Cannabinoids on Zebrafish Larvae. Zebrafish 2013, 10, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Kermen, F.; Darnet, L.; Wiest, C.; Palumbo, F.; Bechert, J.; Uslu, O.; Yaksi, E. Stimulus-specific behavioral responses of zebrafish to a large range of odors exhibit individual variability. BMC Biol. 2020, 18, 66. [Google Scholar] [CrossRef]
- Webb, K.J.; Norton, W.H.; Trümbach, D.; Meijer, A.H.; Ninkovic, J.; Topp, S.; Heck, D.; Marr, C.; Wurst, W.; Theis, F.J.; et al. Zebrafish reward mutants reveal novel transcripts mediating the behavioral effects of amphetamine. Genome Biol. 2009, 10, R81. [Google Scholar] [CrossRef]
- Adámek, P.; Langová, V.; Horáček, J. Early-stage visual perception impairment in schizophrenia, bottom-up and back again. Schizophrenia 2022, 8, 27. [Google Scholar] [CrossRef]
- Fornetto, C.; Tiso, N.; Pavone, F.S.; Vanzi, F. Colored visual stimuli evoke spectrally tuned neuronal responses across the central nervous system of zebrafish larvae. BMC Biol. 2020, 18, 172. [Google Scholar] [CrossRef]
- Chung, S.; Son, J.W. Visual Perception in Autism Spectrum Disorder: A Review of Neuroimaging Studies. Soa Chongsonyon Chongsin Uihak. 2020, 31, 105–120. [Google Scholar] [CrossRef]
- Santacà, M.; Dadda, M.; Miletto Petrazzini, M.E.; Bisazza, A. Stimulus characteristics, learning bias and visual discrimination in zebrafish (Danio rerio). Behav. Proc. 2021, 192, 104499. [Google Scholar] [CrossRef]
- Bollmann, J.H. The Zebrafish Visual System: From Circuits to Behavior. Ann. Rev. Vis. Sci. 2019, 5, 269–293. [Google Scholar] [CrossRef]
- Kyzar, E.; Stewart, A.M.; Landsman, S.; Collins, C.; Gebhardt, M.; Robinson, K.; Kalueff, A.V. Behavioral effects of bidirectional modulators of brain monoamines reserpine and d-amphetamine in zebrafish. Brain Res. 2013, 1527, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Zada, D.; Tovin, A.; Lerer-Goldshtein, T.; Vatine, G.D.; Appelbaum, L. Altered Behavioral Performance and Live Imaging of Circuit-Specific Neural Deficiencies in a Zebrafish Model for Psychomotor Retardation. PLoS Genet. 2014, 10, e1004615. [Google Scholar] [CrossRef] [PubMed]
- Teles, M.C.; Cardoso, S.D.; Oliveira, R.F. Social Plasticity Relies on Different Neuroplasticity Mechanisms across the Brain Social Decision-Making Network in Zebrafish. Front. Behav. Neurosci. 2016, 10, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Reynolds, K.; Ji, Y.; Gu, R.; Rai, S.; Zhou, C.J. Impaired neurodevelopmental pathways in autism spectrum disorder: A review of signaling mechanisms and crosstalk. J. Neurodev. Disord. 2019, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Białoń, M.; Wąsik, A. Advantages and Limitations of Animal Schizophrenia Models. Int. J. Mol. Sci. 2022, 23, 5968. [Google Scholar] [CrossRef]
- Barendse, E.M.; Hendriks, M.P.; Jansen, J.F.; Backes, W.H.; Hofman, P.A.; Thoonen, G.; Kessels, R.P.; Aldenkamp, A.P. Working memory deficits in high-functioning adolescents with autism spectrum disorders: Neuropsychological and neuroimaging correlates. J. Neurodev. Disord. 2013, 5, 14. [Google Scholar] [CrossRef]
- Rabiee, A.; Vasaghi-Gharamaleki, B.; Samadi, S.A.; Amiri-Shavaki, Y.; Alaghband-Rad, J. Working Memory Deficits and its Relationship to Autism Spectrum Disorders. Iran. J. Med. Sci. 2020, 45, 100–109. [Google Scholar] [CrossRef]
- Meshalkina, D.A.; Kysil, E.V.; Warnick, J.E.; Demin, K.A.; Kalueff, A.V. Adult zebrafish in CNS disease modeling: A tank that’s half-full, not half-empty, and still filling. Lab. Anim. 2017, 46, 378–387. [Google Scholar] [CrossRef]
- Stewart, A.M.; Braubach, O.; Spitsbergen, J.; Gerlai, R.; Kalueff, A.V. Zebrafish models for translational neuroscience research: From tank to bedside. Trends Neurosci. 2014, 37, 264–278. [Google Scholar] [CrossRef]
- de Abreu, M.S.; Demin, K.A.; Giacomini, A.C.; Amstislavskay, T.G.; Strekalov, T.; Maslov, G.O.; Kositsin, Y.; Petersen, E.; Kaluef, A.V. Understanding how stress responses and stress-related behaviors have evolved in zebrafish and mammals. Neurobiol. Stress 2021, 15, 100405. [Google Scholar] [CrossRef]
- Engeszer, R.E.; Barbiano, L.A.; Ryan, M.J.; Parichy, D.M. Timing and plasticity of shoaling behaviour in the zebrafish, Danio rerio. Anim. Behav. 2007, 74, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Dreosti, E.; Lopes, G.; Kampff, A.; Wilson, S. Development of social behavior in young zebrafish. Front. Neural Circuits 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Fontana, B.D.; Gibbon, A.J.; Cleal, M.; Sudwarts, A.; Pritchett, D.; Miletto Petrazzini, M.E.; Brennan, C.H.; Parker, M.O. Moderate early life stress improves adult zebrafish (Danio rerio) working memory but does not affect social and anxiety-like responses. Dev. Psychobiol. 2021, 63, 54–64. [Google Scholar] [CrossRef]
- Peterson, T. The zebrafish subcortical social brain as a model for studying social behavior disorders. Dis. Model. Mech. 2019, 12, dmm039446. [Google Scholar] [CrossRef]
- Maruska, K.; Soares, M.C.; Lima-Maximino, M.; Henrique de Siqueira-Silva, D.; Maximino, C. Social plasticity in the fish brain: Neuroscientific and ethological aspects. Brain Res. 2019, 1711, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.C.; Pearce, K.C.; Choe, R.C.; Alzagatiti, J.B.; Yeung, A.K.; Bill, B.R. Neurobiology of Learning and Memory Long-term habituation of the C-start escape response in zebrafish larvae. Neurobiol. Learn. Mem. 2016, 134, 360–368. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Goldsmith, P. Zebrafish as a pharmacological tool: The how, why and when. Curr. Opin. Pharmacol. 2004, 4, 504–512. [Google Scholar] [CrossRef]
- Wullimann, M.F. Secondary neurogenesis and telencephalic organization in zebrafish and mice: A brief review. Integr. Zool. 2009, 4, 123–133. [Google Scholar] [CrossRef]
- Bond, A.M.; Berg, D.A.; Lee, S.; Garcia-Epelboim, A.S.; Adusumilli, V.S.; Ming, G.L.; Song, H. Differential Timing and Coordination of Neurogenesis and Astrogenesis in Developing Mouse Hippocampal Subregions. Brain Sci. 2020, 10, 909. [Google Scholar] [CrossRef]
- Parker, M.O.; Brock, A.J.; Walton, R.T.; Brennan, C.H. The role of zebrafish (Danio rerio) in dissecting the genetics and neural circuits of executive function. Front. Neural Circ. 2013, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Potrich, D.; Schiona, I.; Sovrano, V.A.; Fraser, S.E.; Brennan, C.H.; Vallortigara, G. Neurons in the Dorso-Central Division of Zebrafish Pallium Respond to Change in Visual Numerosity. Cereb. Cortex 2022, 32, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Torigoe, M.; Islam, T.; Kakinuma, H.; Fung, C.C.A.; Isomura, T.; Shimazaki, H.; Aoki, T.; Fukai, T.; Okamoto, H. Zebrafish capable of generating future state prediction error show improved active avoidance behavior in virtual reality. Nat. Commun. 2021, 12, 5712. [Google Scholar] [CrossRef] [PubMed]
- Bahl, A.; Engert, F. Neural circuits for evidence accumulation and decision making in larval zebrafish. Nat. Neurosci. 2020, 23, 94–102. [Google Scholar] [CrossRef]
- Xu, Z.; Cheng, X. Zebrafish tracking using convolutional neural networks. Sci. Rep. 2017, 7, 42815. [Google Scholar] [CrossRef]
- Pereida-Jaramillo, E.; Gómez-González, G.B.; Espino-Saldaña, A.E.; Martínez-Torres, A. Calcium Signaling in the Cerebellar Radial Glia and Its Association with Morphological Changes during Zebrafish Development. Int. J. Mol. Sci. 2021, 22, 13509. [Google Scholar] [CrossRef]
- Zylbertal, A.; Bianco, I.H. A recurrent network architecture explains tectal activity dynamics and experience-dependent behavior. bioRxiv 2022, 1, 1. [Google Scholar] [CrossRef]
- Tropepe, V.; Sive, H.L. Can zebrafish be used as a model to study the neurodevelopmental causes of autism ? Genes Brain Behav. 2003, 2, 268–281. [Google Scholar] [CrossRef]
- Tohid, H.; Faizan, M.; Faizan, U. Alterations of the occipital lobe in schizophrenia. Neurosciences 2015, 20, 213–224. [Google Scholar] [CrossRef]
- Girault, J.B.; Piven, J. The Neurodevelopment of Autism from Infancy Through Toddlerhood. Neuroimaging Clin. N. Am. 2020, 30, 97–114. [Google Scholar] [CrossRef]
- McGrath, J.J.; Féron, F.P.; Burne, T.H.; Mackay-Sim, A.; Eyles, D.W. The neurodevelopmental hypothesis of schizophrenia: A review of recent developments. Ann. Med. 2003, 35, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Guilbeault, N.C.; Guerguiev, J.; Martin, M.; Tate, I.; Thiele, T.R. BonZeb: Open-source, modular software tools for high-resolution zebrafish tracking and analysis. Sci. Rep. 2021, 11, 8148. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yan, H.; Yu, G.; Su, R. Flumazenil-insensitive benzodiazepine binding sites in GABAA receptors contribute to benzodiazepine-induced immobility in zebrafish larvae. Life Sci. 2019, 239, 117033. [Google Scholar] [CrossRef] [PubMed]
- Poh, J. Zebrafish (Danio rerio) embryo-larvae locomotor activity data analysis: Evaluating anxiolytic effects of the antidepressant compound citalopram. Data Briefs 2019, 27, 104812. [Google Scholar] [CrossRef]
- Shen, Q.; Truong, L.; Simonich, M.T.; Huang, C.; Tanguay, R.L.; Dong, Q. Rapid well-plate assays for motor and social behaviors in larval zebrafish. Behav. Brain Res. 2020, 391, 112625. [Google Scholar] [CrossRef] [PubMed]
- Cadena, P.G.; Cadena, M.R.S.; Sarmah, S.; Marrs, J.A. Folic acid reduces the ethanol-induced morphological and behavioral defects in embryonic and larval zebrafish (Danio rerio) as a model for fetal alcohol spectrum disorder (FASD). Reprod. Toxicol. 2020, 96, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, T.; Lin, J.; Zhang, Y.; Li, F.; Chen, X.; Wang, X.; Li, Q. Deficiency of nde1 in zebrafish induces brain inflammatory responses and autism-like behavior. IScience 2022, 25, 103876. [Google Scholar] [CrossRef]
- Beppi, C.; Penner, M.; Straumann, D.; Bögli, S.Y. A non-invasive biomechanical model of mild TBI in larval zebrafish. PLoS ONE 2022, 17, e0268901. [Google Scholar] [CrossRef]
- Roberts, A.C.; Reichl, J.; Song, M.Y.; Dearinger, A.D.; Moridzadeh, N.; Lu, E.D.; Pearce, K.; Esdin, J.; Glanzman, D.L. Habituation of the C-Start Response in Larval Zebrafish Exhibits Several Distinct Phases and Sensitivity to NMDA Receptor Blockade. PLoS ONE 2011, 6, e29132. [Google Scholar] [CrossRef]
- Beppi, C.; Straumann, D.; Bögli, S.Y. A model-based quantification of startle reflex habituation in larval zebrafish. Sci. Rep. 2021, 11, 846. [Google Scholar] [CrossRef]
- Burgess, H.A.; Granato, M. Sensorimotor Gating in Larval Zebrafish. J. Neurosci. 2007, 27, 4984–4994. [Google Scholar] [CrossRef] [PubMed]
- Szalontay, A.; Radu, D.; Bolos, A.; Untu, I. The importance of early initiation of antipsychotic treatment and maintenance of good therapeutic adherence in schizophrenia. Eur. Neuropsychopharmacol. 2019, 9, S398. [Google Scholar] [CrossRef]
- Chung, C.; Ha, S.; Kang, H.; Lee, J.; Um, S.M.; Yan, H.; Yoo, Y.E.; Yoo, T.; Jung, H.; Lee, D.; et al. Early Correction of N-Methyl-D-Aspartate Receptor Function Improves Autistic-like Social Behaviors in Adult Shank2-/- Mice. Biol. Psychiatry 2019, 85, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, J.; Liang, G.; Gao, Y.; Jin, S.Y.; Hu, J.; Yang, X.; Lao, J.; Chen, J.; Luo, Z.C.; et al. Upregulated NMDAR-mediated GABAergic transmission underlies autistic-like deficits in Htr3a knockout mice. Theranostics 2021, 11, 9296–9310. [Google Scholar] [CrossRef]
- Kalueff, A.V. The Rights and Wrongs of Zebrafish: Behavioral Phenotyping of Zebrafish, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–327. [Google Scholar]
- Celebi-Birand, D.; Erbaba, B.; Ozdemir, A.T.; Hulusi Kafaligonul, H.; Adams, M. Zebrafish Aging Models and Possible Interventions. In Recent Advances in Zebrafish Researches; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Schmidt, R.; Strähle, U.; Scholpp, S. Neurogenesis in zebrafish–from embryo to adult. Neural Dev. 2013, 8, 3. [Google Scholar] [CrossRef]
- Nunes, A.R.; Carreira, L.; Anbalagan, S.; Blechman, J.; Levkowitz, G.; Oliveira, R.F. Perceptual mechanisms of social affiliation in zebrafish. Sci. Rep. 2020, 10, 3642. [Google Scholar] [CrossRef]
- Adams, M.M.; Kafaligonul, H. Zebrafish-A Model Organism for Studying the Neurobiological Mechanisms Underlying Cognitive Brain Aging and Use of Potential Interventions. Front. Cell Dev. Biol. 2018, 6, 135. [Google Scholar] [CrossRef]
- Andersson, M.Å.; Ek, F.; Olsson, R. Using visual lateralization to model learning and memory in zebrafish larvae. Sci. Rep. 2015, 5, 8667. [Google Scholar] [CrossRef]
- Sovrano, V.; Andrew, R. Eye use during viewing a reflection: Behavioural lateralisation in zebrafish larvae. Behav. Brain Res. 2006, 167, 226–231. [Google Scholar] [CrossRef]
- May, Z.; Morrill, A.; Holcombe, A.; Johnston, T.; Gallup, J.; Fouad, K.; Schalomon, M.; Hamilton, T.J. Object recognition memory in zebrafish. Behav. Brain Res. 2016, 296, 199–210. [Google Scholar] [CrossRef]
- Lucon-Xiccato, T.; Dadda, M. Assessing memory in zebrafish using the one-trial test. Behav. Process. 2014, 106, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Magyary, I. Floating novel object recognition in adult zebrafish: A pilot study. Cogn. Process. 2019, 20, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Balmus, I.; Ciobica, A.; Negură, A.; Negura, L.; Anton, E. Basic aspects in selecting a suitable transgenic rodent model for Alzheimer’s disease. Psychiatr. Danub. 2015, 27, 338–345. [Google Scholar] [PubMed]
- Halladay, A.K.; Amaral, D.; Aschner, M.; Bolivar, V.J.; Bowman, A.; DiCicco-Bloom, E.; Hyman, S.L.; Keller, F.; Lein, P.; Pessah, I.; et al. Animal models of autism spectrum disorders: Information for neurotoxicologists. Neurotoxicology 2009, 30, 811–821. [Google Scholar] [CrossRef]
- Moon-Fanelli, A.A.; Dodman, N.H.; Famula, T.R.; Cottam, N. Characteristics of compulsive tail chasing and associated risk factors in Bull Terriers. J. Am. Vet. Med. Assoc. 2011, 238, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Bálint, A.; Eleod, H.; Magyari, L.; Kis, A.; Gácsi, M. Differences in dogs’ event-related potentials in response to human and dog vocal stimuli; a non-invasive study. R. Soc. Open Sci. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Falcão, M.A.P.; de Souza, L.S.; Dolabella, S.S.; Guimarães, A.G.; Walker, C.I.B. Zebrafish as an alternative method for determining the embryo toxicity of plant products: A systematic review. Environ. Sci. Pollut. Res. Int. 2018, 25, 35015–35026. [Google Scholar] [CrossRef]
- Strungaru, S.-A.; Robea, M.A.; Plavan, G.; Todirascu-Ciornea, E.; Ciobica, A.; Nicoara, M. Acute exposure to methylmercury chloride induces fast changes in swimming performance, cognitive processes and oxidative stress of zebrafish (Danio rerio) as reference model for fish community. J. Trace Elem. Med. Biol. 2018, 47, 115–123. [Google Scholar] [CrossRef]
- Strungaru, S.-A.; Plavan, G.; Ciobica, A.; Nicoara, M.; Robea, M.A.; Solcan, C.; Todirascu-Ciornea, E.; Petrovici, A. Acute exposure to gold induces fast changes in social behavior and oxidative stress of zebrafish (Danio rerio). J. Trace Elem. Med. Biol. 2018, 50, 249–256. [Google Scholar] [CrossRef]
- Frye, R.E.; Cakir, J.; Rose, S.; Delhey, L.; Bennuri, S.C.; Tippett, M.; Palmer, R.F.; Austin, C.; Curtin, P.; Arora, M. Early life metal exposure dysregulates cellular bioenergetics in children with regressive autism spectrum disorder. Transl. Psychiatry 2020, 10, 223. [Google Scholar] [CrossRef]
- Sulaiman, R.; Wang, M.; Ren, X. Exposure to Aluminum, Cadmium, and Mercury and Autism Spectrum Disorder in Children: A Systematic Review and Meta-Analysis. Chem. Res. Toxicol. 2020, 33, 2699–2718. [Google Scholar] [CrossRef] [PubMed]
- Golding, J.; Rai, D.; Gregory, S.; Ellis, G.; Emond, A.; Iles-Caven, Y.; Hibbeln, J.; Taylor, C. Prenatal mercury exposure and features of autism: A prospective population study. Mol. Autism 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Hvolgaard Mikkelsen, S.; Obel, C.; Olsen, J.; Niclasen, J.; Bech, B.H. Maternal Caffeine Consumption during Pregnancy and Behavioral Disorders in 11-Year-Old Offspring: A Danish National Birth Cohort Study. J. Pediatr. 2017, 189, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Patti, M.A.; Li, N.; Eliot, M.; Newschaffer, C.; Yolton, K.; Khoury, J.; Chen, A.; Lanphear, B.P.; Lyall, K.; Hertz-Picciotto, I.; et al. Association between self-reported caffeine intake during pregnancy and social responsiveness scores in childhood: The EARLI and HOME studies. PLoS ONE 2021, 16, e0245079. [Google Scholar] [CrossRef] [PubMed]
- Patti, M.; Li, N.; Newschaffer, C.; Yolton, K.; Chen, A.; Lanphear, B.; Lyall, K.; Braun, J. Prenatal Caffeine Exposure and Social Responsiveness Scores in Early Childhood. Environ. Epidemiol. 2019, 3, 305. [Google Scholar] [CrossRef]
- Christensen, Z.P.; Freedman, E.G.; Foxe, J.J. Caffeine exposure in utero is associated with structural brain alterations and deleterious neurocognitive outcomes in 9–10 year old children. Neuropharmacology 2021, 186, 108479. [Google Scholar] [CrossRef]
- Qian, J.; Chen, Q.; Ward, S.M.; Duan, E.; Zhang, Y. Impacts of Caffeine during Pregnancy. Trends Endocrinol. Metab. 2020, 31, 218–227. [Google Scholar] [CrossRef]
- James, D.M.; Kozol, R.A.; Kajiwara, Y.; Wahl, A.L.; Storrs, E.C.; Buxbaum, J.D.; Klein, M.; Moshiree, B.; Dallman, J.E. Intestinal dysmotility in a zebrafish (Danio rerio) shank3a;shank3b mutant model of autism. Mol. Autism 2019, 10, 3. [Google Scholar] [CrossRef]
- Banono, N.S.; Gawel, K.; De Witte, L.; Esguerra, C.V. Zebrafish Larvae Carrying a Splice Variant Mutation in cacna1d: A New Model for Schizophrenia-Like Behaviours? Mol. Neurobiol. 2021, 58, 877–894. [Google Scholar] [CrossRef]
- Xie, J.; Jusuf, P.R.; Bui, B.V.; Dudczig, S.; Sztal, T.E.; Goodbourn, P.T. Altered Visual Function in a Larval Zebrafish Knockout of Neurodevelopmental Risk Gene pdzk1. Investig. Ophthalmol. Vis. Sci. 2021, 62, 29. [Google Scholar] [CrossRef]
- Moro, E.; Ozhan-Kizil, G.; Mongera, A.; Beis, D.; Wierzbicki, C.; Young, R.M.; Bournele, D.; Domenichini, A.; Valdivia, L.E.; Argenton, F.; et al. In vivo Wnt signaling tracing through a transgenic biosensor fish reveals novel activity domains. Dev. Biol. 2012, 366, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lei, L.; Tian, L.; Hou, F.; Roper, C.; Ge, X.; Zhao, Y.; Chen, Y.; Dong, Q.; Tanguay, R.L.; et al. Developmental and behavioral alterations in zebrafish embryonically exposed to valproic acid (VPA), An aquatic model for autism. Neurotoxicol. Teratol. 2018, 66, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, C.; Fahnestock, M. The valproic acid-induced rodent model of autism. Exp. Neurol. 2018, 299, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Chaliha, D.; Albrecht, M.; Vaccarezza, M.; Takechi, R.; Lam, V.; Al-Salami, H.; Mamo, J. A Systematic Review of the Valproic-Acid-Induced Rodent Model of Autism. Dev. Neurosci. 2020, 42, 12–48. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, Y.H.; Yun, J.S.; Lee, C.J. Valproic acid decreases the proliferation of telencephalic cells in zebrafish larvae. Neurotoxicol. Teratol. 2013, 39, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Peng, X.; Hu, C.; Li, C.; Li, Q.; Xu, X. Developmental profiling of ASD-related shank3 transcripts and their differential regulation by valproic acid in zebrafish. Dev. Genes Evol. 2016, 226, 389–400. [Google Scholar] [CrossRef]
- Lee, S.; Chun, H.S.; Lee, J.; Park, H.J.; Kim, K.T.; Kim, C.H.; Yoon, S.; Kim, W. Plausibility of the zebrafish embryos/larvae as an alternative animal model for autism: A comparison study of transcriptome changes. PLoS ONE 2018, 13, e0203543. [Google Scholar] [CrossRef]
- Dwivedi, S.; Medishetti, R.; Rani, R.; Sevilimedu, A.; Kulkarni, P.; Yogeeswari, P. Larval zebrafish model for studying the effects of valproic acid on neurodevelopment: An approach towards modeling autism. J. Pharmacol. Toxicol. Methods 2019, 95, 56–65. [Google Scholar] [CrossRef]
- Ornoy, A. Valproic acid in pregnancy: How much are we endangering the embryo and fetus? Reprod. Toxicol. 2009, 28, 1–10. [Google Scholar] [CrossRef]
- Sison, M.; Gerlai, R. Behavioral performance altering effects of MK-801 in zebrafish (Danio rerio). Behav. Brain Res. 2011, 220, 331–337. [Google Scholar] [CrossRef]
- Chen, J.; Patel, R.; Friedman, T.C.; Jones, K.S. The Behavioral and Pharmacological Actions of NMDA Receptor Antagonism are Conserved in Zebrafish Larvae. Int. J. Comp. Psychol. 2010, 23, 82–90. [Google Scholar] [PubMed]
- Svoboda, J.; Stankova, A.; Entlerova, M.; Stuchlik, A. Acute administration of MK-801 in an animal model of psychosis in rats interferes with cognitively demanding forms of behavioral flexibility on a rotating arena. Front. Behav. Neurosci. 2015, 9, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rung, J.P.; Carlsson, A.; Rydén Markinhuhta, K.; Carlsson, M.L. (+)-MK-801 induced social withdrawal in rats; a model for negative symptoms of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Berg, E.L.; Copping, N.A.; Rivera, J.K.; Pride, M.C.; Careaga, M.; Bauman, M.D.; Berman, R.F.; Lein, P.J.; Harony-Nicolas, H.; Buxbaum, J.D.; et al. Developmental social communication deficits in the Shank3 rat model of phelan-mcdermid syndrome and autism spectrum disorder. Autism Res. 2018, 11, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Delling, J.P.; Boeckers, T.M. Comparison of SHANK3 deficiency in animal models: Phenotypes, treatment strategies, and translational implications. J. Neurodevelop. Disord. 2021, 13, 55. [Google Scholar] [CrossRef]
- Bernier, R.; Golzio, C.; Xiong, B.; Stessman, H.A.; Coe, B.P.; Penn, O.; Witherspoon, K.; Gerdts, J.; Baker, C.; Silfhout, A.T.V.; et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell 2014, 158, 263–276. [Google Scholar] [CrossRef]
- Cloney, K.; Steele, S.L.; Stoyek, M.R.; Croll, R.P.; Smith, F.M.; Prykhozhij, S.V.; Brown, M.M.; Midgen, C.; Blake, K.; Berman, J.N. Etiology and functional validation of gastrointestinal motility dysfunction in a zebrafish model of CHARGE syndrome. FEBS J. 2018, 285, 2125–2140. [Google Scholar] [CrossRef]
- Weissberg, O.; Elliott, E. The Mechanisms of CHD8 in Neurodevelopment and Autism Spectrum Disorders. Genes 2021, 12, 1133. [Google Scholar] [CrossRef]
- Jiménez, J.A.; Ptacek, T.S.; Tuttle, A.H.; Schmid, R.S.; Moy, S.S.; Simon, J.M.; Zylka, M.J. Chd8 haploinsufficiency impairs early brain development and protein homeostasis later in life. Mol. Autism 2020, 11, 74. [Google Scholar] [CrossRef]
- Jamadagni, P.; Breuer, M.; Schmeisser, K.; Cardinal, T.; Kassa, B.; Parker, J.A.; Pilon, N.; Samarut, E.; Patten, S.A. Chromatin remodeller CHD7 is required for GABAergic neuron development by promoting PAQR3 expression. EMBO Rep. 2021, 22, e50958. [Google Scholar] [CrossRef]
- Golzio, C.; Willer, J.; Talkowski, M.E.; Oh, E.C.; Taniguchi, Y.; Jacquemont, S.; Reymond, A.; Sun, M.; Sawa, A.; Gusella, J.F.; et al. KCTD13 is a major driver of mirrored neuroanatomical phenotypes of the 16p11.2 copy number variant. Nature 2012, 485, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, D.E.; Kasah, S.; Donovan, A.P.A.; Ellegood, J.; Riegman, K.L.H.; Volk, H.A.; McGonnell, I.; Lerch, J.P.; Basson, M.A. Distinct cerebellar foliation anomalies in a CHD7 haploinsufficient mouse model of CHARGE syndrome. Am. J. Med. Genet. Part. C Semin Med. Genet. 2017, 175, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Escamilla, C.O.; Filonova, I.; Walker, A.K.; Xuan, Z.X.R.; Holehonnur, R.; Espinosa, F.; Liu, S.; Thyme, S.B.; López-García, I.A.; Mendoza, D.B.; et al. Kctd13 deletion reduces synaptic transmission via increased RhoA. Nature 2017, 551, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Ponpornpisit, A.; Pirarat, N.; Suthikrai, W.; Binwihok, A. Toxicity Test of Kameng (Eclipta prostrate Linn.) and Kradhuawean (Spilanthes acmella (Linn.) Murr.) to Early Life Stage of Zebrafish (Danio rerio). Thai J. Vet. Med. 2011, 41, 523–527. [Google Scholar]
- Thanh, D.T.H.; Thanh, N.L.; Thang, N.D.; Thi, D. Toxicological and melanin synthesis effects of Polygonum multiflorum root extracts on zebrafish embryos and human melanocytes. Biomed. Res. Ther. 2016, 3, 2015–2017. [Google Scholar] [CrossRef]
- Yumnamcha, T.; Roy, D.; Devi, M.D.; Nongthomba, U. Evaluation of developmental toxicity and apoptotic induction of the aqueous extract of Millettia pachycarpa using zebrafish as model organism. Toxicol. Environ. Chem. 2015, 97, 1368–1381. [Google Scholar] [CrossRef]
- Wang, S.; Liu, K.; Wang, X.; He, Q.; Chen, X. Toxic effects of celastrol on embryonic development of zebrafish (Danio rerio). Drug Chem. Toxicol. 2010, 34, 65–66. [Google Scholar] [CrossRef]
- Peng, W.-H.; Lee, Y.-C.; Chau, Y.-P.; Lu, K.-S.; Kung, H.-N. Short-Term Exposure of Zebrafish Embryos to Arecoline Leads to Retarded Growth, Motor Impairment, and Somite Muscle Fiber Changes. Zebrafish 2015, 12, 58–70. [Google Scholar] [CrossRef] [Green Version]
| Similarities | Differences/ Particularities | Measurable Behaviors | Clinical Relevance | Ref. | ||
|---|---|---|---|---|---|---|
| ASD | Schizophrenia | |||||
| Olfactory stimuli response |
|
| Chemical or socially driven olfactory response | Hypersensibility to olfactory stimuli | Circuitry desensibilisation of response to olfactory stimuli | [27,28,29,35,36,37] |
| Visual stimuli response |
|
|
| Atypical brain activation in visual detection tasks (focus to local detail, such as contrast and color) | Delays in visual perception, visual distortions | [35,36,37,38,39,40,41,42,43] |
| Descending motor and premotor pathways | Preserved descending motor and premotor pathways, such as reticulospinal tract, projections from the midbrain and cerebellum to brainstem targets | Locomotor activity | Behavioral hallmarks—repetitive movement and impaired motor response to external stimuli | Increased motor activity, motor glitches (tics, stereotypies), anxiety-related locomotor behaviour changes | [44,45,46] | |
| Spatial memory | Spatial memory pathways involving lateral pallium, similar to mammals | Lateral pallium homologous hippocampus (medial pallium-derived) | Short-term and long-term learning and memory | Episodic memory and working memory deficits | Memory deficits and decreased cognitive performance | [47,48,49,50] |
| Neurogenesis and synapse plasticity | Neuronal cell type differentiation modulated by both neurogenic genes and proneural genes |
| Conditioned place preference, dark/light transition, social decision making, aggressivity | Specific changes in learning and memory formation | [6,49,50,51] | |
| Related to stimuli response | Related to perceiving fear | |||||
| Neuromodulatory pathways | All key neuromodulator systems are highly preserved amongst vertebrates | Inhibitory avoidance, e-flat mirror test, perseverative behaviour | Limbic system impairments | [6,49,52,53] | ||
| Neuroendocrine modulation |
| |||||
| Affective behaviour | Modulated by amygdala and habenula (a group of nuclei in the epithalamus) | More complex neurotransmitter-modulated affective display | ||||
| Social behavior and sociability |
| More complex hormonal patterns in stress response in mammals | Sociability behaviors starting with embryo movement | Typical social behavior phenotypes | [54,55,56,57,58] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curpăn, A.S.; Balmus, I.-M.; Dobrin, R.P.; Ciobica, A.; Chele, G.E.; Gorgan, D.L.; Boloș, A. A Mini-Review Regarding the Modalities to Study Neurodevelopmental Disorders-Like Impairments in Zebrafish—Focussing on Neurobehavioural and Psychological Responses. Brain Sci. 2022, 12, 1147. https://doi.org/10.3390/brainsci12091147
Curpăn AS, Balmus I-M, Dobrin RP, Ciobica A, Chele GE, Gorgan DL, Boloș A. A Mini-Review Regarding the Modalities to Study Neurodevelopmental Disorders-Like Impairments in Zebrafish—Focussing on Neurobehavioural and Psychological Responses. Brain Sciences. 2022; 12(9):1147. https://doi.org/10.3390/brainsci12091147
Chicago/Turabian StyleCurpăn, Alexandrina S., Ioana-Miruna Balmus, Romeo P. Dobrin, Alin Ciobica, Gabriela E. Chele, Dragos Lucian Gorgan, and Alexandra Boloș. 2022. "A Mini-Review Regarding the Modalities to Study Neurodevelopmental Disorders-Like Impairments in Zebrafish—Focussing on Neurobehavioural and Psychological Responses" Brain Sciences 12, no. 9: 1147. https://doi.org/10.3390/brainsci12091147
APA StyleCurpăn, A. S., Balmus, I.-M., Dobrin, R. P., Ciobica, A., Chele, G. E., Gorgan, D. L., & Boloș, A. (2022). A Mini-Review Regarding the Modalities to Study Neurodevelopmental Disorders-Like Impairments in Zebrafish—Focussing on Neurobehavioural and Psychological Responses. Brain Sciences, 12(9), 1147. https://doi.org/10.3390/brainsci12091147