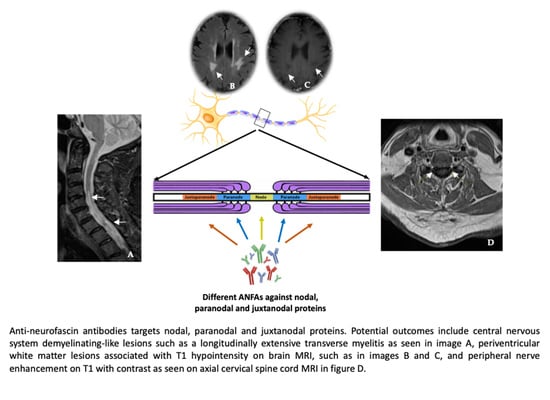
Anti-Neurofascin Antibodies Associated with White Matter Diseases of the Central Nervous System: A Red Flag or a Red Herring?
Abstract
:1. Introduction
2. Materials and Methods
3. Case I
4. Case II
5. Case III
6. Case IV
7. Case V
8. Case VI
| Patient I | Patient II | Patient III | Patient IV | Patient V | Patient VI | |
|---|---|---|---|---|---|---|
| Age, y/Sex | 60/F | 55/F | 60/F | 64/F | 70/M | 30/M |
| Initial symptoms | PNS | CNS | CNS | CNS | CNS | PNS |
| Age at CNS onset, y | 60 | 53 | Unclear | 62 | 43 | 30 |
| Age at PNS onset, y | 57 | No PNS involvement | No PNS involvement | No PNS involvement | Uncertain | 27 |
| Temporal gap between CNS and PNS onsets, y | 3 | Not applicable | Not applicable | Not applicable | Uncertain | 3 |
| Clinical presentation | 1st event: Acute onset sensorimotor symptoms in all limbs 2nd event: Acute onset sensorimotor symptoms in all limbs | Lhermitte’s sign, L foot weakness, fall, L foot purplish discoloration | Parkinsonism | BL numbness in a stocking distribution, gait imbalance | R leg tingling and weakness, gait imbalance, L leg tingling | 1st event: BL legs tingling extending to the abdomen, leg weakness, falls 2nd event: BL arms and legs paresthesia and weakness |
| EDSS | 2017: 7 2020: 9 | 5 | 2 | 5.5 | 6 | 2 |
| Serum ANFA (Western Blot) | Anti-NF155 IgM | Anti-NF140 IgG | Anti-NF155 IgG | Anti-NF155 IgG | Anti-NF155 IgM Anti-NF155 IgG Anti-NF140 IgG | Anti-NF155 IgM |
| CSF cell count and differentials | 1st event: No pleocytosis 2nd event: ND | 1st CSF: No pleocytosis 2nd CSF: No pleocytosis, high neutrophils & eosinophils | No pleocytosis | No pleocytosis | ND | 1st event: No pleocytosis 2nd event: No pleocytosis |
| CSF protein, mg/dL | 1st event: 50 2nd event: ND | 1st CSF: 34 2nd CSF: 30 | 43 | 32 | ND | 1st event: 26 2nd event: 26 |
| CSF IgG index and synthesis | ND | Normal | Normal | Elevated | ND | 1st and 2nd event: Increased |
| CSF-restricted OCB | ND | 1 | 0 | 5 | ND | 1st and 2nd event: 10 & 5 |
| Brain MRI | 1st event: Nonspecific multifocal T2/ FLAIR WML 2nd event: Nonspecific multifocal T2/ FLAIR WML | Periventricular and juxtacortical lesion, dorsal medullary lesion extending to cervicomedullary junction | Multifocal T2/ FLAIR WML | 1st MRI: Subcortical, periventricular, anterior temporal lobe, medullary lesions 2nd MRI: Stable | Periventricular, anterior cervico- medullary junction lesion | Periventricular and R frontal deep white matter lesions |
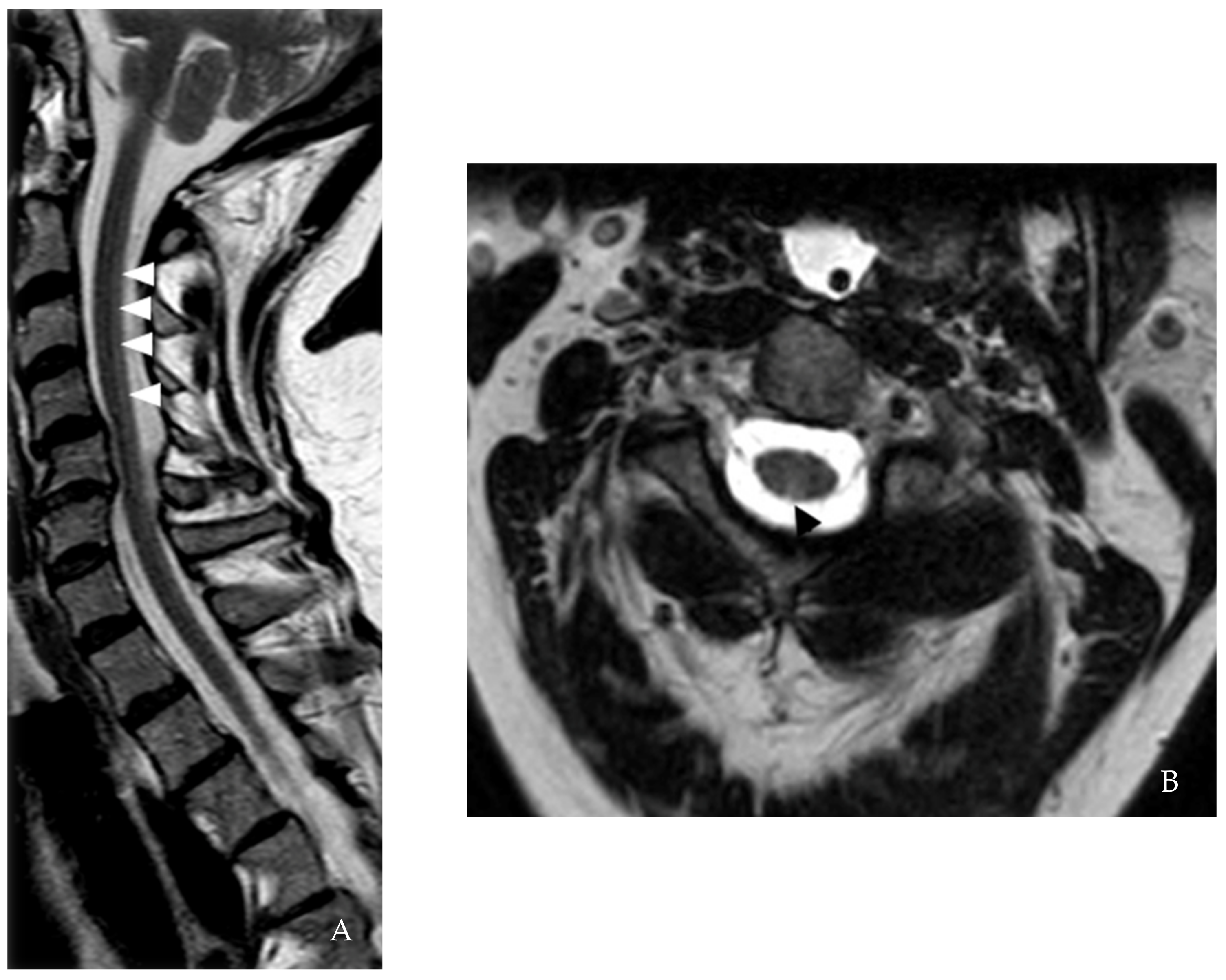
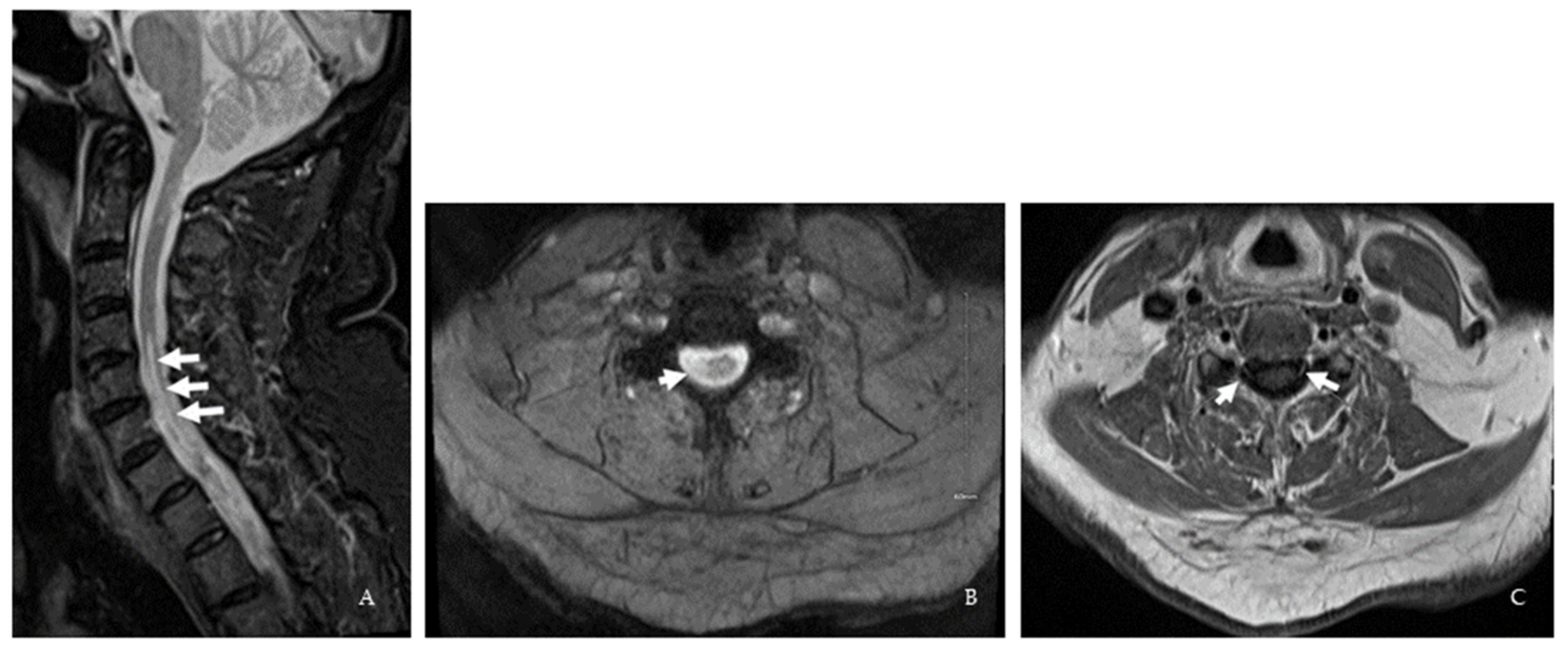
| Spinal cord MRI | 1st event: Cervical spine degenerative joint Disease 2nd event: C2-5 dorsal column T2 hyperintensity | C2-3 and T4 T2/ FLAIR lesions | ND | 1st MRI: C3, C5-6, C7-T1, and T8-9 lesions 2nd MRI: New C3 Gd-enhancing lesion | C2, C4-C7 lesion and cervical nerve root enhancement in the central cervical canal | 1st MRI: Age-related L4-5 changes 2nd MRI: C1 contrast-enhancing lesion 3rd MRI: C2 and C5 lesions |
| MRI Dissemination in Space by 2017 McDonald criteria | Unfulfilled | Fulfilled | Unfulfilled | Fulfilled | Fulfilled | Fulfilled |
| Treatment | IVIG | Ocrelizumab | Not started | Ocrelizumab | Not started | Ocrelizumab |
9. Discussion
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oudejans, E.; Luchicchi, A.; Strijbis, E.M.M.; Geurts, J.J.G.; van Dam, A.M. Is MS affecting the CNS only? Lessons from clinic to myelin pathophysiology. Neurol. Neuroimmunol. Neuroinflamm. 2020, 8, e914. [Google Scholar] [CrossRef] [PubMed]
- Docampo, M.J.; Lutterotti, A.; Sospedra, M.; Martin, R. Mechanistic and Biomarker Studies to Demonstrate Immune Tolerance in Multiple Sclerosis. Front. Immunol. 2022, 12, 787498. [Google Scholar] [CrossRef] [PubMed]
- Sherman, D.L.; Tait, S.; Melrose, S.; Johnson, R.; Zonta, B.; Court, F.A.; Macklin, W.B.; Meek, S.; Smith, A.J.; Cottrell, D.F.; et al. Neurofascins Are Required to Establish Axonal Domains for Saltatory Conduction. Neuron 2005, 48, 737–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kriebel, M.; Wuchter, J.; Trinks, S.; Volkmer, H. Neurofascin: A switch between neuronal plasticity and stability. Int. J. Biochem. Cell Biol. 2012, 44, 694–697. [Google Scholar] [CrossRef]
- Zhang, A.; Desmazieres, A.; Zonta, B.; Melrose, S.; Campbell, G.; Mahad, D.; Li, Q.; Sherman, D.L.; Reynolds, R.; Brophy, P.J. Neurofascin 140 Is an Embryonic Neuronal Neurofascin Isoform That Promotes the Assembly of the Node of Ranvier. J. Neurosci. 2015, 35, 2246–2254. [Google Scholar] [CrossRef] [Green Version]
- Howell, O.W.; Palser, A.; Polito, A.; Melrose, S.; Zonta, B.; Scheiermann, C.; Vora, A.J.; Brophy, P.J.; Reynolds, R. Disruption of neurofascin localization reveals early changes preceding demyelination and remyelination in multiple sclerosis. Brain 2006, 129, 3173–3185. [Google Scholar] [CrossRef]
- Mathey, E.K.; Derfuss, T.; Storch, M.K.; Williams, K.R.; Hales, K.; Woolley, D.R.; Al-Hayani, A.; Davies, S.N.; Rasband, M.; Olsson, T.; et al. Neurofascin as a novel target for autoantibody-mediated axonal injury. J. Exp. Med. 2007, 204, 2363–2372. [Google Scholar] [CrossRef] [Green Version]
- Ng, J.K.M.; Malotka, J.; Kawakami, N.; Derfuss, T.; Khademi, M.; Olsson, T.; Linington, C.; Odaka, M.; Tackenberg, B.; Prüss, H.; et al. Neurofascin as a target for autoantibodies in peripheral neuropathies. Neurology 2012, 79, 2241–2248. [Google Scholar] [CrossRef] [Green Version]
- Devaux, J.J.; Miura, Y.; Fukami, Y.; Inoue, T.; Manso, C.; Belghazi, M.; Sekiguchi, K.; Kokubun, N.; Ichikawa, H.; Wong, A.H.Y.; et al. Neurofascin-155 IgG4 in chronic inflammatory demyelinating polyneuropathy. Neurology 2016, 86, 800–807. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, N.; Yamasaki, R.; Yonekawa, T.; Matsushita, T.; Kusunoki, S.; Nagayama, S.; Fukuda, Y.; Ogata, H.; Matsuse, D.; Murai, H.; et al. Anti-neurofascin antibody in patients with combined central and peripheral demyelination. Neurology 2013, 81, 714–722. [Google Scholar] [CrossRef]
- Ogata, H.; Matsuse, D.; Yamasaki, R.; Kawamura, N.; Matsushita, T.; Yonekawa, T.; Hirotani, M.; Murai, H.; Kira, J.-I. A nationwide survey of combined central and peripheral demyelination in Japan. J. Neurol. Neurosurg. Psychiatry 2016, 87, 29–36. [Google Scholar] [CrossRef]
- Cortese, A.; Franciotta, D.; Alfonsi, E.; Visigalli, N.; Zardini, E.; Diamanti, L.; Prunetti, P.; Osera, C.; Gastaldi, M.; Berzero, G.; et al. Combined central and peripheral demyelination: Clinical features, diagnostic findings, and treatment. J. Neurol. Sci. 2016, 363, 182–187. [Google Scholar] [CrossRef]
- Stich, O.; Perera, S.; Berger, B.; Jarius, S.; Wildemann, B.; Baumgartner, A.; Rauer, S. Prevalence of neurofascin-155 antibodies in patients with multiple sclerosis. J. Neurol. Sci. 2016, 364, 29–32. [Google Scholar] [CrossRef]
- Charles, P.; Tait, S.; Faivre-Sarrailh, C.; Barbin, G.; Gunn-Moore, F.; Denisenko-Nehrbass, N.; Guennoc, A.-M.; Girault, J.-A.; Brophy, P.J.; Lubetzki, C. Neurofascin Is a Glial Receptor for the Paranodin/Caspr-Contactin Axonal Complex at the Axoglial Junction. Curr. Biol. 2002, 12, 217–220. [Google Scholar] [CrossRef] [Green Version]
- Koike, H.; Kadoya, M.; Kaida, K.-I.; Ikeda, S.; Kawagashira, Y.; Iijima, M.; Kato, D.; Ogata, H.; Yamasaki, R.; Matsukawa, N.; et al. Paranodal dissection in chronic inflammatory demyelinating polyneuropathy with anti-neurofascin-155 and anti-contactin-1 antibodies. J. Neurol. Neurosurg. Psychiatry 2017, 88, 465–473. [Google Scholar] [CrossRef]
- Brier, M.R.; Everett, E.A.; Bucelli, R.C. An Atypical and Multifactorial Acute Immune Polyradiculopathy: A Case Report. Neurohospitalist 2020, 10, 118–120. [Google Scholar] [CrossRef]
- Burnor, E.; Yang, L.; Zhou, H.; Patterson, K.R.; Quinn, C.; Reilly, M.M.; Rossor, A.; Scherer, S.S.; Lancaster, E. Neurofascin antibodies in autoimmune, genetic, and idiopathic neuropathies. Neurology 2018, 90, e31–e38. [Google Scholar] [CrossRef]
- Delmont, E.; Manso, C.; Querol, L.; Cortese, A.; Berardinelli, A.; Lozza, A.; Belghazi, M.; Malissart, P.; Labauge, P.; Taieb, G.; et al. Autoantibodies to nodal isoforms of neurofascin in chronic inflammatory demyelinating polyneuropathy. Brain 2017, 40, 1851–1858. [Google Scholar] [CrossRef] [Green Version]
- Cortese, A.; Lombardi, R.; Briani, C.; Callegari, I.; Benedetti, L.; Manganelli, F.; Luigetti, M.; Ferrari, S.; Clerici, A.M.; Marfia, G.A.; et al. Antibodies to neurofascin, contactin-1, and contactin-associated protein 1 in CIDP. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e639. [Google Scholar] [CrossRef] [Green Version]
- Doppler, K.; Stengel, H.; Appeltshauser, L.; Grosskreutz, J.; Ng, J.K.M.; Meinl, E.; Sommer, C. Neurofascin-155 IgM autoantibodies in patients with inflammatory neuropathies. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1145–1151. [Google Scholar] [CrossRef]
- Bergamaschi, R.; Tonietti, S.; Franciotta, D.; Candeloro, E.; Tavazzi, E.; Piccolo, G.; Romani, A.; Cosi, V. Oligoclonal bands in Devic’s neuromyelitis optica and multiple sclerosis: Differences in repeated cerebrospinal fluid examinations. Mult. Scler. J. 2004, 10, 2–4. [Google Scholar] [CrossRef]
- Magliozzi, R.; Mazziotti, V.; Montibeller, L.; Pisani, A.I.; Marastoni, D.; Tamanti, A.; Rossi, S.; Crescenzo, F.; Calabrese, M. Cerebrospinal Fluid IgM Levels in Association with Inflammatory Pathways in Multiple Sclerosis Patients. Original Research. Front. Cell. Neurosci. 2020, 14, 569827. [Google Scholar] [CrossRef]
- Bunschoten, C.; Jacobs, B.C.; Van den Bergh, P.Y.K.; Cornblath, D.R.; van Doorn, P.A. Progress in diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy. Lancet Neurol. 2019, 18, 784–794. [Google Scholar] [CrossRef] [Green Version]
- Eftimov, F.; Lucke, I.M.; Querol, L.A.; Rajabally, Y.A.; Verhamme, C. Diagnostic challenges in chronic inflammatory demyelinating polyradiculoneuropathy. Brain 2020, 143, 3214–3224. [Google Scholar] [CrossRef]
- Shelly, S.; Shouman, K.; Paul, P.; Engelstad, J.; Amrami, K.K.; Spinner, R.J.; Dubey, D.; Campo, R.V.D.; Dyck, P.J.; Klein, C.J. Expanding the Spectrum of Chronic Immune Sensory Polyradiculopathy. Neurology 2021, 96, e2078–e2089. [Google Scholar] [CrossRef]
- Querol, L.; Nogales-Gadea, G.; Rojas-Garcia, R.; Diaz-Manera, J.; Pardo, J.; Ortega-Moreno, A.; Sedano, M.J.; Gallardo, E.; Berciano, J.; Blesa, R.; et al. Neurofascin IgG4 antibodies in CIDP associate with disabling tremor and poor response to IVIg. Neurology 2014, 82, 879–886. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Liu, C.; Li, W.; Zhang, D.; Shan, Y.; Zheng, J.; Shan, J.; Zhao, Y.; Yan, C.; Wang, Q. Clinical and diagnostic features of anti-neurofascin-155 antibody-positive neuropathy in Han Chinese. Ann. Clin. Transl. Neurol. 2022, 9, 695–706. [Google Scholar] [CrossRef]
- Kadoya, M.; Kaida, K.; Koike, H.; Takazaki, H.; Ogata, H.; Moriguchi, K.; Shimizu, J.; Nagata, E.; Takizawa, S.; Chiba, A.; et al. IgG4 anti-neurofascin155 antibodies in chronic inflammatory demyelinating polyradiculoneuropathy: Clinical significance and diagnostic utility of a conventional assay. J. Neuroimmunol. 2016, 301, 16–22. [Google Scholar] [CrossRef]
- Uncini, A.; Susuki, K.; Yuki, N. Nodo-paranodopathy: Beyond the demyelinating and axonal classification in anti-ganglioside antibody-mediated neuropathies. Clin. Neurophysiol. 2013, 124, 1928–1934. [Google Scholar] [CrossRef]
- Devaux, J.J. Antibodies to Gliomedin Cause Peripheral Demyelinating Neuropathy and the Dismantling of the Nodes of Ranvier. Am. J. Pathol. 2012, 181, 1402–1413. [Google Scholar] [CrossRef]
- Manso, C.; Querol, L.; Lleixà, C.; Poncelet, M.; Mekaouche, M.; Vallat, J.-M.; Illa, I.; Devaux, J.J. Anti–neurofascin-155 IgG4 antibodies prevent paranodal complex formation in vivo. J. Clin. Investig. 2019, 129, 2222–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Test | Infectious | Autoimmune | Paraneoplastic Antibody Panel | Inflammatory | Hematology | Nutritional | Miscellaneous | Positive Findings |
|---|---|---|---|---|---|---|---|---|
| Case I | HIV, hepatitis, M. pneunoniae IgM, Lyme & WNV serologies | None | Negative, except for findings in the “Positive” column | ACE | SIF, SPEP | Vitamin B12, MMA, homocysteine | None | M. pneumoniae IgM, VGKC titer = 0.13; ref: ≤0.02 nmol/L |
| Case II | Hepatitis B, HTLV-1 & 2, Lyme, WNV & syphilis serologies | ANA, ANCA, anti-ds DNA, anti-TPO, AQP-4, RF & MOG IgG | Negative | ACE, ESR | C3-C4, SPEP, SIF | Amino acids, VLCFA, copper, ferritin, MMA, vitamins B1/B6/B12/D | HgbA1c, TSH | APLA IgM (repeat test negative) |
| Case III | ANA, ANCA, AQP-4, & MOG IgG, celiac screen | Negative | CRP | SIF, C3-C4, SPEP, | Ferritin, folic acid, vitamin B12, VLCFA | None | ||
| Case IV | HIV & Lyme serologies | ANA, AQP-4, & MOG IgG, lupus anticoagulant | Negative, except for findings in the “Positive” column | CRP, ESR | None | Copper, vitamins B12/D/E, zinc | None | CPK: 306 U/L (ref: 38-234). P/Q-type calcium channel antibody |
| Case V | HTLV-1 & 2 | AQP-4 & MOG IgG | Negative | None | None | Copper, folate, MMA, vitamins B12/D/E | None | None |
| Case VI | 1st event: Syphilis serology 2nd event: Hepatitis, HIV & Lyme serologies | 2nd event: ANCA, AQP-4 & MOG IgG | 2nd event: Negative | 2nd event: ACE | 2nd event: Igs, SPEP, | 1st event: Copper, iron panel, MMA, vitamins A/B6/ B12/D, zinc 2nd Event: Copper, homocysteine, vitamins A/B12/E | 1st event: HgbA1c, PTH 2nd event: HgbA1c, TSH | 1st event: Hypovitaminosis B1 2nd event: Hypovitaminosis D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, N.; Shirani, A.; Arcot Jayagopal, L.; Piccione, E.; Hartman, E.; Zabad, R.K. Anti-Neurofascin Antibodies Associated with White Matter Diseases of the Central Nervous System: A Red Flag or a Red Herring? Brain Sci. 2022, 12, 1124. https://doi.org/10.3390/brainsci12091124
Gupta N, Shirani A, Arcot Jayagopal L, Piccione E, Hartman E, Zabad RK. Anti-Neurofascin Antibodies Associated with White Matter Diseases of the Central Nervous System: A Red Flag or a Red Herring? Brain Sciences. 2022; 12(9):1124. https://doi.org/10.3390/brainsci12091124
Chicago/Turabian StyleGupta, Navnika, Afsaneh Shirani, Lakshman Arcot Jayagopal, Ezequiel Piccione, Elizabeth Hartman, and Rana Khalil Zabad. 2022. "Anti-Neurofascin Antibodies Associated with White Matter Diseases of the Central Nervous System: A Red Flag or a Red Herring?" Brain Sciences 12, no. 9: 1124. https://doi.org/10.3390/brainsci12091124
APA StyleGupta, N., Shirani, A., Arcot Jayagopal, L., Piccione, E., Hartman, E., & Zabad, R. K. (2022). Anti-Neurofascin Antibodies Associated with White Matter Diseases of the Central Nervous System: A Red Flag or a Red Herring? Brain Sciences, 12(9), 1124. https://doi.org/10.3390/brainsci12091124