Predictors of Post-Stroke Depression: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
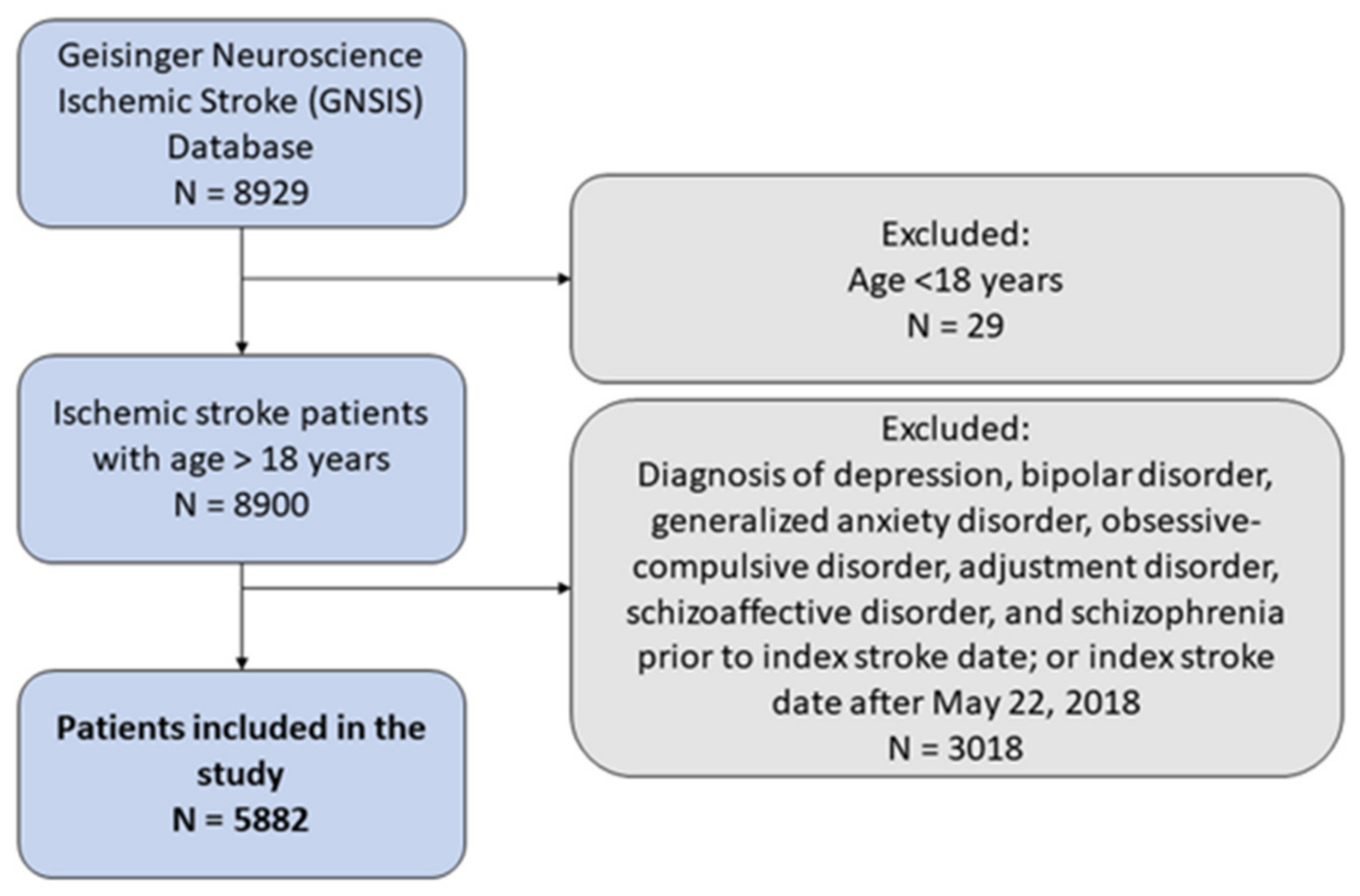
2.2. Cohort Inclusion/Exclusion Criteria
2.3. Assessment Measures or Recorded Variables
2.4. Outcome Definition and Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Sample Characteristics
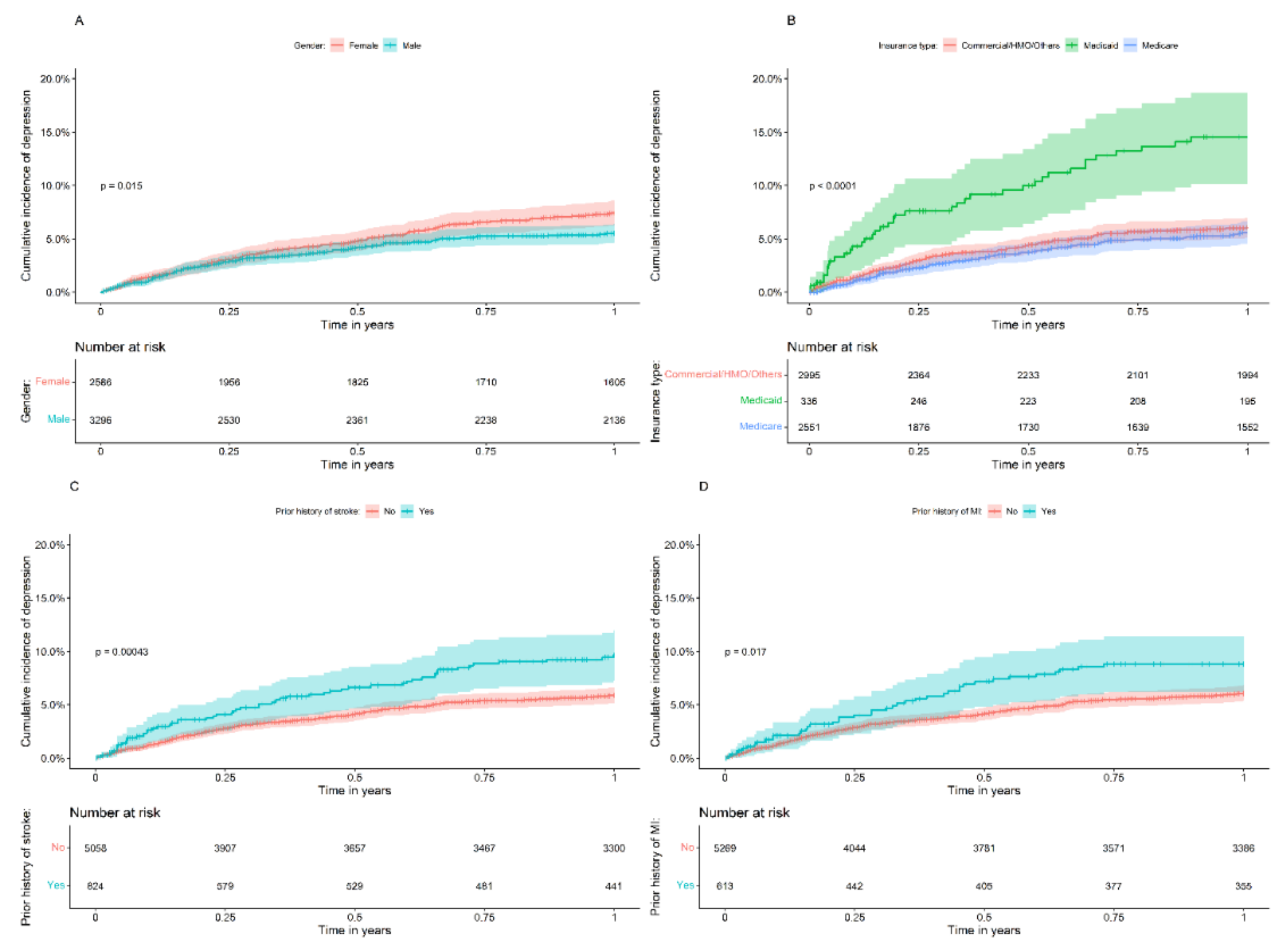
3.2. One-Year Follow-Up Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koton, S.; Rexrode, K.M. Trends in Stroke Incidence in the United States: Will Women Overtake Men? Neurology 2017, 89, 982–983. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.; Chan, L.; Flynn, S. A Systematic Review of the Incidence, Prevalence, Costs, and Activity and Work Limitations of Amputation, Osteoarthritis, Rheumatoid Arthritis, Back Pain, Multiple Sclerosis, Spinal Cord Injury, Stroke, and Traumatic Brain Injury in the United States: A 2019 Update. Arch. Phys. Med. Rehabil. 2021, 102, 115–131. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Akaho, R.; Deguchi, I.; Kigawa, H.; Nishimura, K. Obsessive-Compulsive Disorder Following Cerebrovascular Accident: A Case Report and Literature Review. J. Stroke Cerebrovasc. Dis. 2019, 28, e17–e21. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.Y.Y.; Whiteley, W.N.; Dennis, M.S.; Mead, G.E.; Carson, A.J. Anxiety after Stroke the Importance of Subtyping. Stroke 2018, 49, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Stangeland, H.; Orgeta, V.; Bell, V. Review: Poststroke Psychosis: A Systematic Review. J. Neurol. Neurosurg. Psychiatry 2018, 89, 879. [Google Scholar] [CrossRef] [PubMed]
- McMurtray, A.; Tseng, B.; Diaz, N.; Chung, J.; Mehta, B.; Saito, E. Acute Psychosis Associated with Subcortical Stroke: Comparison between Basal Ganglia and Mid-Brain Lesions. Case Rep. Neurol. Med. 2014, 2014, 428425. [Google Scholar] [CrossRef] [PubMed]
- Skajaa, N.; Adelborg, K.; Horváth-Puhó, E.; Rothman, K.J.; Henderson, V.W.; Thygesen, L.C.; Sørensen, H.T. Stroke and Risk of Mental Disorders Compared With Matched General Population and Myocardial Infarction Comparators. Stroke 2022, 53, 2287–2298. [Google Scholar] [CrossRef]
- Verdelho, A.; Hénon, H.; Lebert, F.; Pasquier, F.; Leys, D. Depressive Symptoms after Stroke and Relationship with Dementia: A Three-Year Follow-up Study. Neurology 2004, 62, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Kootker, J.A.; Van Mierlo, M.L.; Hendriks, J.C.; Sparidans, J.; Rasquin, S.M.; De Kort, P.L.; Visser-Meily, J.M.; Geurts, A.C. Risk Factors for Symptoms of Depression and Anxiety One Year Poststroke: A Longitudinal Study. Arch. Phys. Med. Rehabil. 2016, 97, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Ojagbemi, A.; Akpa, O.; Elugbadebo, F.; Owolabi, M.; Ovbiagele, B. Depression after Stroke in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. Behav. Neurol. 2017, 2017, 4160259. [Google Scholar] [CrossRef]
- Syed, M.J.; Farooq, S.; Siddiqui, S.; Awan, S.; Wasay, M. Depression and the Use of Selective Serotonin Reuptake Inhibitors in Patients with Acute Intracerebral Hemorrhage. Cureus 2019, 11, e5975. [Google Scholar] [CrossRef]
- Vicentini, J.E.; Weiler, M.; Regina, S.; Almeida, M.; Machado De Campos, B.; Lenise, V.; Li, M. Depression and Anxiety Symptoms Are Associated to Disruption of Default Mode Network in Subacute Ischemic Stroke. Brain Imaging Behav. 2016, 11, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Ladwig, S.; Zhou, Z.; Xu, Y.; Wang, X.; Chow, C.K.; Werheid, K.; Hackett, M.L. Comparison of Treatment Rates of Depression After Stroke Versus Myocardial Infarction. Psychosom. Med. 2018, 80, 754–763. [Google Scholar] [CrossRef]
- Hackett, M.L.; Pickles, K. Part I: Frequency of Depression after Stroke: An Updated Systematic Review and Meta-Analysis of Observational Studies. Int. J. Stroke 2014, 9, 1017–1025. [Google Scholar] [CrossRef]
- Shi, Y.Z.; Xiang, Y.T.; Yang, Y.; Zhang, N.; Wang, S.; Ungvari, G.S.; Chiu, H.F.K.; Tang, W.K.; Wang, Y.L.; Zhao, X.Q.; et al. Depression after Minor Stroke: Prevalence and Predictors. J. Psychosom. Res. 2015, 79, 143–147. [Google Scholar] [CrossRef]
- Park, G.-Y.; Oh, C.H.; Lee, S.-J.; Pae, C.-U. The Association between the Severity of Poststroke Depression and Clinical Outcomes after First-Onset Stroke in Korean Patients. Gen. Hosp. Psychiatry 2015, 37, 245–250. [Google Scholar] [CrossRef]
- Limampai, P.; Wongsrithep, W.; Kuptniratsaikul, V. Depression after Stroke at 12-Month Follow-up: A Multicenter Study. Int. J. Neurosci. 2017, 127, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Delgado Guajardo, V.; Terroni, L.; De Freitas, M.; Sobreiro, M.; Irene, M.; Zerbini, S.; Tinone, G.; Scaff, M.; Iosifescu, D.V.; Souza De Lucia, M.C.; et al. The Influence of Depressive Symptoms on Quality of Life after Stroke: A Prospective Study. J. Stroke Cerebrovasc. Dis. 2015, 24, 201–209. [Google Scholar] [CrossRef]
- Volz, M.; Ladwig, S.; Werheid, K. Neuropsychological Rehabilitation An International Journal Gender Differences in Post-Stroke Depression: A Longitudinal Analysis of Prevalence, Persistence and Predictive Value of Known Risk Factors. Neuropsychol. Rehabil. 2019, 31, 1–17. [Google Scholar] [CrossRef]
- Ellis, C.; Zhao, Y.; Egede, L.E. Depression and Increased Risk of Death in Adults with Stroke. J. Psychosom. Res. 2010, 68, 545–551. [Google Scholar] [CrossRef]
- Ayerbe, L.; Ayis, S.; Crichton, S. The Long-Term Outcomes of Depression up to 10 Years after Stroke; the South London Stroke Register. J. Neurol. Neurosurg. Psychiatry 2014, 85, 514–521. [Google Scholar] [CrossRef]
- Thayabaranathan, T.; Andrew, N.E.; Kilkenny, M.F.; Stolwyk, R.; Thrift, A.G.; Grimley, R.; Johnston, T.; Sundararajan, V.; Lannin, N.A.; Cadilhac, D.A. Factors influencing self-reported anxiety or depression following stroke or TIA using linked registry and hospital data. Qual. Life Res. 2018, 27, 3145–3155. [Google Scholar] [CrossRef] [PubMed]
- Hamano, T.; Li, X.; Lönn, S.L.; Nabika, T.; Shiwaku, K.; Sundquist, J.; Sundquist, K. Depression, Stroke and Gender: Evidence of a Stronger Association in Men. J. Neurol. Neurosurg. Psychiatry 2014, 86, 319–323. [Google Scholar] [CrossRef]
- Lambert, C.; Chaudhary, D.; Olulana, O.; Shahjouei, S.; Avula, V.; Li, J.; Abedi, V.; Zand, R. Sex Disparity in Long-Term Stroke Recurrence and Mortality in a Rural Population in the United States. Ther. Adv. Neurol. Disord. 2020, 13, 175628642097189. [Google Scholar] [CrossRef]
- Chaudhary, D.; Khan, A.; Shahjouei, S.; Gupta, M.; Lambert, C.; Avula, V.; Schirmer, C.M.; Holland, N.; Griessenauer, C.J.; Azarpazhooh, M.R.; et al. Trends in Ischemic Stroke Risk Factors and Outcomes in a Rural Population in the United States. Stroke 2021, 52, AP699. [Google Scholar] [CrossRef]
- Chaudhary, D.; Khan, A.; Gupta, M.; Hu, Y.; Li, J.; Abedi, V.; Zand, R. Obesity and Mortality after the First Ischemic Stroke: Is Obesity Paradox Real? PLoS ONE 2021, 16, e0246877. [Google Scholar] [CrossRef]
- Caeiro, L.; Ferro, J.M.; Santos, C.O.; Figueira, M.L. Depression in Acute Stroke. J. Psychiatry Neurosci. 2006, 31, 377–383. [Google Scholar] [PubMed]
- Poynter, B.; Shuman Hon, M.; Diaz-Granados, N.; Kapral, M.; Grace, S.L.; Stewart, D.E. Sex Differences in the Prevalence of Post-Stroke Depression: A Systematic Review. Psychosomatics 2009, 50, 563–569. [Google Scholar] [CrossRef]
- Nys, G.M.S.; Van Zandvoort, M.J.E.; Van Der Worp, H.B.; De Haan, E.H.F.; De Kort, P.L.M.; Jansen, B.P.W.; Kappelle, L.J. Early Cognitive Impairment Predicts Long-Term Depressive Symptoms and Quality of Life after Stroke. J. Neurol. Sci. 2006, 247, 149–156. [Google Scholar] [CrossRef]
- Leentjens, A.F.G.; Aben, I.; Lodder, J.; Verhey, F.R.J. General and Disease-Specific Risk Factors for Depression after Ischemic Stroke: A Two-Step Cox Regression Analysis. Int. Psychogeriatr. 2006, 18, 739–748. [Google Scholar] [CrossRef]
- Paradiso, S.; Robinson, R.G. Gender Differences in Poststroke Depression. J. Neuropsychiatry Clin. Neurosc. 1998, 10, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Andersen, G.; Vestergaard, K.; Ingemann-Nielsen, M.; Lauritzen, L. Risk Factors for Post-Stroke Depression. Acta Psychiatr. Scand. 1995, 92, 193–198. [Google Scholar] [CrossRef]
- Haley, W.E.; Roth, D.L.; Kissela, B.; Perkins, M.; Howard, G. Quality of Life after Stroke: A Prospective Longitudinal Study. Qual. Life Res. 2011, 20, 799–806. [Google Scholar] [CrossRef]
- Hurn, P.D.; Brass, L.M. Estrogen and Stroke: A Balanced Analysis. Stroke 2003, 34, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Xiao, L.; Jin, K.; Shao, B. Estrogen Administration Attenuates Post-Stroke Depression by Enhancing CREB/BDNF/TrkB Signaling in the Rat Hippocampus. Exp. Ther. Med. 2021, 21, 433. [Google Scholar] [CrossRef]
- Shors, T.J.; Leuner, B. Estrogen-Mediated Effects on Depression and Memory Formation in Females. J. Affect. Disord. 2003, 74, 85. [Google Scholar] [CrossRef]
- Medeiros, G.C.; Roy, D.; Kontos, N.; Beach, S.R. Post-Stroke Depression: A 2020 Updated Review. Gen. Hosp. Psychiatry 2020, 66, 70–80. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Xia, Y.; Jiang, S.; Xu, P.; Tao, C.; Sun, W.; Liu, X. Thyroid Function Affects the Risk of Post-Stroke Depression in Patients With Acute Lacunar Stroke. Front. Neurol. 2022, 13, 792843. [Google Scholar] [CrossRef]
- McCarthy, M.J.; Sucharew, H.J.; Alwell, K.; Moomaw, C.J.; Woo, D.; Flaherty, M.L.; Khatri, P.; Ferioli, S.; Adeoye, O.; Kleindorfer, D.O.; et al. Age, Subjective Stress, and Depression after Ischemic Stroke. J. Behav. Med. 2016, 39, 55–64. [Google Scholar] [CrossRef]
- Fang, J.; Cheng, Q. Etiological Mechanisms of Post-Stroke Depression: A Review. Neurol. Res. 2009, 31, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Towfighi, A.; Ovbiagele, B.; El Husseini, N.; Hackett, M.L.; Jorge, R.E.; Kissela, B.M.; Mitchell, P.H.; Skolarus, L.E.; Whooley, M.A.; Williams, L.S. Poststroke Depression: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2017, 48, e30–e43. [Google Scholar] [CrossRef]
- Aben, I.; Verhey, F.; Strik, J.; Lousberg, R.; Lodder, J.; Honig, A. A Comparative Study into the One Year Cumulative Incidence of Depression after Stroke and Myocardial Infarction. J. Neurol. Neurosurg. Psychiatry 2003, 74, 581–585. [Google Scholar] [CrossRef][Green Version]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef]
- Allah Haghgoo, H.; Saed Pazuki, E.; Hosseini, A.S.; Rassafiani, M. Depression, Activities of Daily Living and Quality of Life in Patients with Stroke. J. Neurol. Sci. 2013, 328, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, N.; Black, S.E.; Lawrence, J.; Szekely, C.; Szalai, J.P. The Sunnybrook Stroke Study. Stroke 1998, 29, 618–624. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Sheth, B.; Gill, J.; Yadegarfar, M.; Stubbs, B.; Yadegarfar, M.; Meader, N. Prevalence and Predictors of Post-Stroke Mood Disorders: A Meta-Analysis and Meta-Regression of Depression, Anxiety and Adjustment Disorder. Gen. Hosp. Psychiatry 2017, 47, 48–60. [Google Scholar] [CrossRef]
- Fluharty, M.; Taylor, A.E.; Grabski, M.; Munafò, M.R. The Association of Cigarette Smoking with Depression and Anxiety: A Systematic Review. Nicotine Tob. Res. 2017, 19, 3–13. [Google Scholar] [CrossRef]
- Breslau, N.; Peterson, E.L.; Schultz, L.R.; Chilcoat, H.D.; Andreski, P. Major Depression and Stages of Smoking: A Longitudinal Investigation. Arch. Gen. Psychiatry 1998, 55, 161–166. [Google Scholar] [CrossRef]
- Volkow, N.D. The Reality of Comorbidity: Depression and Drug Abuse. Biol. Psychiatry 2004, 56, 714–717. [Google Scholar] [CrossRef]
- Davis, L.; Uezato, A.; Newell, J.M.; Frazier, E. Major Depression and Comorbid Substance Use Disorders. Curr. Opin. Psychiatry 2008, 21, 14–18. [Google Scholar] [CrossRef]
- Tian, D.; Qu, Z.; Wang, X.; Guo, J.; Xu, F.; Zhang, X.; Chan, C.L. The Role of Basic Health Insurance on Depression: An Epidemiological Cohort Study of a Randomized Community Sample in Northwest China. BMC Psychiatry 2012, 12, 151. [Google Scholar] [CrossRef]
- Fry, C.E.; Sommers, B.D. Effect of Medicaid Expansion on Health Insurance Coverage and Access to Care among Adults with Depression. Psychiatr. Serv. 2018, 69, 1146–1152. [Google Scholar] [CrossRef]
- Baicker, K.; Allen, H.L.; Wright, B.J.; Taubman, S.L.; Finkelstein, A.N. The Effect of Medicaid on Management of Depression: Evidence from the Oregon Health Insurance Experiment. Milbank Q. 2018, 96, 29–56. [Google Scholar] [CrossRef]
- Sareen, J.; Afifi, T.O.; McMillan, K.A.; Asmundson, G.J.G. Relationship between Household Income and Mental Disorders: Findings from a Population-Based Longitudinal Study. Arch. Gen. Psychiatry 2011, 68, 419–427. [Google Scholar] [CrossRef]
- Khedr, E.M.; Abdelrahman, A.A.; Desoky, T.; Zaki, A.F.; Gamea, A. Post-Stroke Depression: Frequency, Risk Factors, and Impact on Quality of Life among 103 Stroke Patients—Hospital-Based Study. Egypt. J. Neurol. Psychiatry Neurosurg. 2020, 56, 66. [Google Scholar] [CrossRef]
| Variable | Overall | No Depression at One-Year Follow-Up | Depression within One Year Post-Stroke | p-Value |
|---|---|---|---|---|
| Number of patients, n | 5882 | 3741 | 294 | |
| Gender: male, n (%) | 3296 (56.0) | 2136 (57.1) | 145 (49.3) | 0.011 |
| Age at ischemic stroke diagnosis in years, median [IQR] | 72.00 [61.70, 81.20] | 71.00 [61.10, 80.10] | 67.20 [57.60, 77.88] | 0.001 |
| Atrial fibrillation, n (%) | 1274 (21.7) | 759 (20.3) | 51 (17.3) | 0.256 |
| Hypertension, n (%) | 4261 (72.4) | 2763 (73.9) | 215 (73.1) | 0.838 |
| Myocardial infarction, n (%) | 613 (10.4) | 355 (9.5) | 41 (13.9) | 0.018 |
| Diabetes, n (%) | 1762 (30.0) | 1134 (30.3) | 90 (30.6) | 0.967 |
| Dyslipidemia, n (%) | 3387 (57.6) | 2288 (61.2) | 175 (59.5) | 0.623 |
| Congestive heart failure, n (%) | 708 (12.0) | 364 (9.7) | 35 (11.9) | 0.271 |
| Hypercoagulable states, n (%) | 76 (1.3) | 49 (1.3) | 5 (1.7) | 0.766 |
| Chronic liver disease, n (%) | 107 (1.8) | 67 (1.8) | 4 (1.4) | 0.756 |
| Chronic lung disease, n (%) | 1028 (17.5) | 657 (17.6) | 49 (16.7) | 0.757 |
| Rheumatic diseases, n (%) | 201 (3.4) | 125 (3.3) | 14 (4.8) | 0.263 |
| Chronic kidney disease, n (%) | 928 (15.8) | 554 (14.8) | 40 (13.6) | 0.635 |
| Neoplasm, n (%) | 850 (14.5) | 532 (14.2) | 43 (14.6) | 0.917 |
| Perivascular disease, n (%) | 834 (14.2) | 548 (14.6) | 44 (15.0) | 0.950 |
| Ever-smoker, n (%) | 2013 (34.2) | 1397 (37.3) | 123 (41.8) | 0.142 |
| Drug abuse or dependence, n (%) | 103 (1.8) | 51 (1.4) | 10 (3.4) | 0.012 |
| Insurance, n (%) | <0.001 | |||
| Commercial/HMO/others | 2995 (50.9) | 1994 (53.3) | 149 (50.7) | |
| Medicaid | 336 (5.7) | 195 (5.2) | 39 (13.3) | |
| Medicare | 2551 (43.4) | 1552 (41.5) | 106 (36.1) | |
| Stroke history, n (%) | 824 (14.0) | 441 (11.8) | 58 (19.7) | <0.001 |
| Variable | Hazard Ratio (HR) | 95% Confidence Interval (CI) | p-Value |
|---|---|---|---|
| Gender: female | 1.47 | 1.18–1.85 | 0.001 |
| Insurance type: | |||
| commercial/HMO/others | Reference | ||
| Medicaid | 2.16 | 1.5–3.12 | <0.001 |
| Medicare | 0.95 | 0.73–1.23 | 0.692 |
| History of prior stroke | 1.58 | 1.18–2.11 | 0.002 |
| History of drug abuse/dependence | 1.66 | 0.87–3.16 | 0.122 |
| Prior history of myocardial infarction | 1.47 | 1.05–2.06 | 0.025 |
| Ever-smoker | 1.21 | 0.95–1.53 | 0.118 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhary, D.; Friedenberg, I.; Sharma, V.; Sharma, P.; Abedi, V.; Zand, R.; Li, J. Predictors of Post-Stroke Depression: A Retrospective Cohort Study. Brain Sci. 2022, 12, 993. https://doi.org/10.3390/brainsci12080993
Chaudhary D, Friedenberg I, Sharma V, Sharma P, Abedi V, Zand R, Li J. Predictors of Post-Stroke Depression: A Retrospective Cohort Study. Brain Sciences. 2022; 12(8):993. https://doi.org/10.3390/brainsci12080993
Chicago/Turabian StyleChaudhary, Durgesh, Isabel Friedenberg, Vishakha Sharma, Pragyan Sharma, Vida Abedi, Ramin Zand, and Jiang Li. 2022. "Predictors of Post-Stroke Depression: A Retrospective Cohort Study" Brain Sciences 12, no. 8: 993. https://doi.org/10.3390/brainsci12080993
APA StyleChaudhary, D., Friedenberg, I., Sharma, V., Sharma, P., Abedi, V., Zand, R., & Li, J. (2022). Predictors of Post-Stroke Depression: A Retrospective Cohort Study. Brain Sciences, 12(8), 993. https://doi.org/10.3390/brainsci12080993









