Association between Individual Norepinephrine Transporter (NET) Availability and Response to Pharmacological Therapy in Adults with Attention-Deficit/Hyperactivity Disorder (ADHD)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Design and Measurements
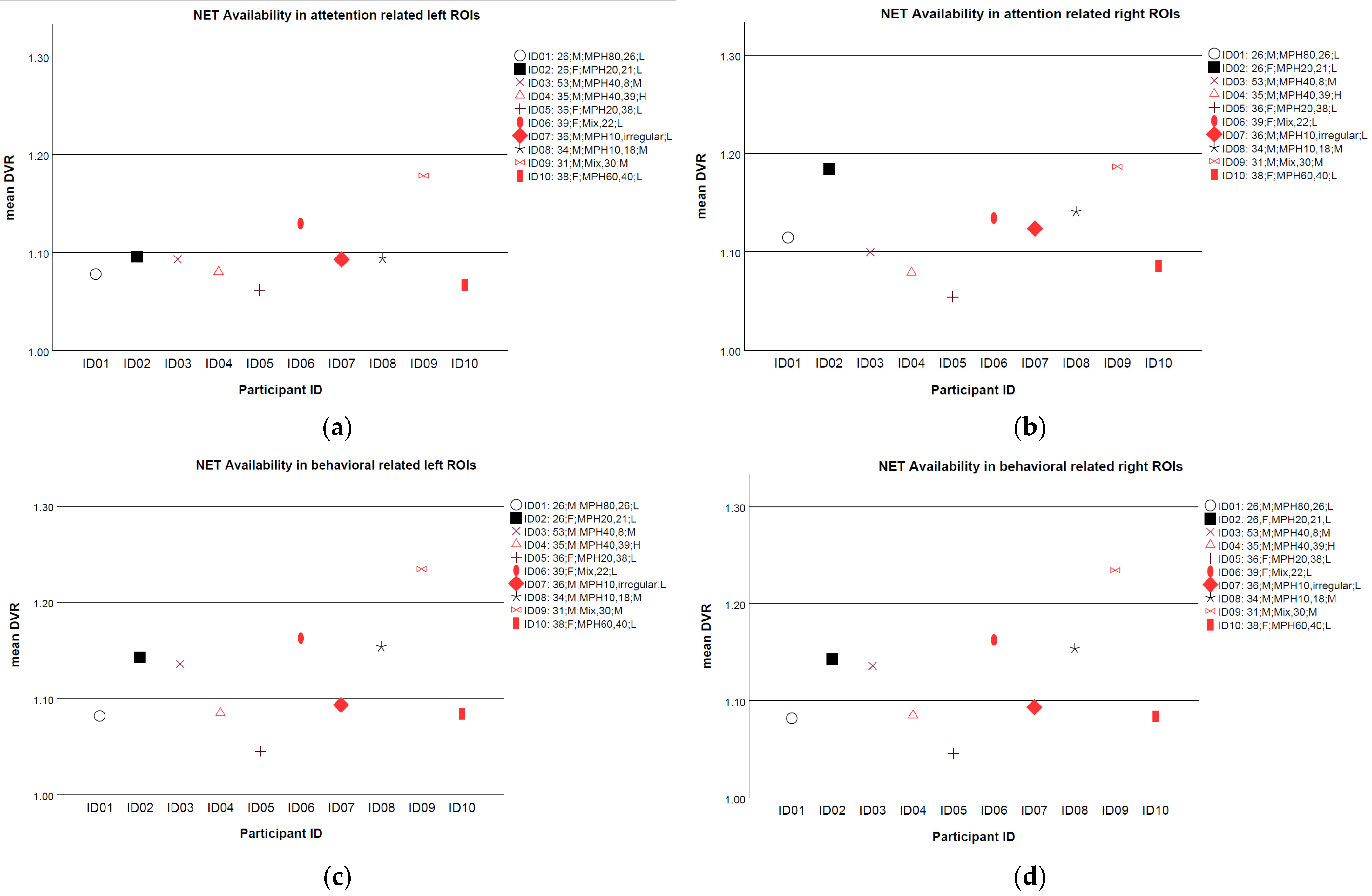
2.3. Initial NET Availability
2.4. Definition of Therapy Response, Health Limitations and Social Limitations
2.5. Statistical Analysis
3. Results
3.1. Description of Sample
3.1.1. Case Description
- ID 01
- ID 02
- ID 03
- ID 04
- ID 05
- ID 06
- ID 07
- ID 08
- ID 09
- ID 10
3.1.2. Case Summary
3.2. Association of NET Availability with Health or Social Limitations and Improvement Due to Treatment at the Group Level
3.3. Association of NET Availability with Health or Social Limitations and Improvement Due to Treatment at the Individual Level
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faraone, S.V.; Asherson, P.; Banaschewski, T.; Biederman, J.; Buitelaar, J.K.; Ramos-Quiroga, J.A.; Rohde, L.A.; Sonuga-Barke, E.J.S.; Tannock, R.; Franke, B. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Primers 2015, 1, 15020. [Google Scholar] [CrossRef] [PubMed]
- Biederman, J.; Petty, C.R.; Woodworth, K.Y.; Lomedico, A.; Hyder, L.L.; Faraone, S.V. Adult outcome of attention-deficit/hyperactivity disorder: A controlled 16-year follow-up study. J. Clin. Psychiatry 2012, 73, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Childress, A.C.; Berry, S.A. Pharmacotherapy of attention-deficit hyperactivity disorder in adolescents. Drugs 2012, 72, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Halmøy, A.; Fasmer, O.B.; Gillberg, C.; Haavik, J. Occupational outcome in adult ADHD: Impact of symptom profile, comorbid psychiatric problems, and treatment: A cross-sectional study of 414 clinically diagnosed adult ADHD patients. J. Atten. Disord. 2009, 13, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Faraone, S.V.; Perlis, R.H.; Doyle, A.E.; Smoller, J.W.; Goralnick, J.J.; Holmgren, M.A.; Sklar, P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol. Psychiatry 2005, 57, 1313–1323. [Google Scholar] [CrossRef] [Green Version]
- Fusar-Poli, P.; Rubia, K.; Rossi, G.; Sartori, G.; Balottin, U. Striatal dopamine transporter alterations in ADHD: Pathophysiology or adaptation to psychostimulants? A meta-analysis. Am. J. Psychiatry 2012, 169, 264–272. [Google Scholar] [CrossRef]
- Ulke, C.; Rullmann, M.; Huang, J.; Luthardt, J.; Becker, G.-A.; Patt, M.; Meyer, P.M.; Tiepolt, S.; Hesse, S.; Sabri, O.; et al. Adult attention-deficit/hyperactivity disorder is associated with reduced norepinephrine transporter availability in right attention networks: A (S,S)-O-11Cmethylreboxetine positron emission tomography study. Transl. Psychiatry 2019, 9, 301. [Google Scholar] [CrossRef]
- Kooij, J.J.S.; Bijlenga, D.; Salerno, L.; Jaeschke, R.; Bitter, I.; Balázs, J.; Thome, J.; Dom, G.; Kasper, S.; Nunes Filipe, C.; et al. Updated European Consensus Statement on diagnosis and treatment of adult ADHD. Eur. Psychiatry 2019, 56, 14–34. [Google Scholar] [CrossRef]
- Swanson, J.M.; Volkow, N.D. Psychopharmacology: Concepts and opinions about the use of stimulant medications. J. Child Psychol. Psychiatry 2009, 50, 180–193. [Google Scholar] [CrossRef] [Green Version]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Gatley, S.J.; Logan, J.; Ding, Y.S.; Hitzemann, R.; Pappas, N. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am. J. Psychiatry 1998, 155, 1325–1331. [Google Scholar] [CrossRef] [Green Version]
- Upadhyaya, H.P.; Desaiah, D.; Schuh, K.J.; Bymaster, F.P.; Kallman, M.J.; Clarke, D.O.; Durell, T.M.; Trzepacz, P.T.; Calligaro, D.O.; Nisenbaum, E.S.; et al. A review of the abuse potential assessment of atomoxetine: A nonstimulant medication for attention-deficit/hyperactivity disorder. Psychopharmacology 2013, 226, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Eshleman, A.J.; Carmolli, M.; Cumbay, M.; Martens, C.R.; Neve, K.A.; Janowsky, A. Characteristics of drug interactions with recombinant biogenic amine transporters expressed in the same cell type. J. Pharmacol. Exp. Ther. 1999, 289, 877–885. [Google Scholar] [PubMed]
- Hannestad, J.; Gallezot, J.-D.; Planeta-Wilson, B.; Lin, S.-F.; Williams, W.A.; Van Dyck, C.H.; Malison, R.T.; Carson, R.E.; Ding, Y.-S. Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol. Psychiatry 2010, 68, 854–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.-S.; Naganawa, M.; Gallezot, J.-D.; Nabulsi, N.; Lin, S.-F.; Ropchan, J.; Weinzimmer, D.; McCarthy, T.J.; Carson, R.E.; Huang, Y.; et al. Clinical doses of atomoxetine significantly occupy both norepinephrine and serotonin transports: Implications on treatment of depression and ADHD. Neuroimage 2014, 86, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Logan, J.; Wang, G.; Telang, F.; Fowler, J.S.; Alexoff, D.; Zabroski, J.; Jayne, M.; Hubbard, B.; King, P.; Carter, P.; et al. Imaging the norepinephrine transporter in humans with (S,S)-11CO-methyl reboxetine and PET: Problems and progress. Nucl. Med. Biol. 2007, 34, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Dillo, W.; Göke, A.; Prox-Vagedes, V.; Szycik, G.R.; Roy, M.; Donnerstag, F.; Emrich, H.M.; Ohlmeier, M.D. Neuronal correlates of ADHD in adults with evidence for compensation strategies--a functional MRI study with a Go/No-Go paradigm. Ger. Med. Sci. 2010, 8, Doc09. [Google Scholar] [CrossRef]
- Monden, Y.; Dan, H.; Nagashima, M.; Dan, I.; Tsuzuki, D.; Kyutoku, Y.; Gunji, Y.; Yamagata, T.; Watanabe, E.; Momoi, M.Y. Right prefrontal activation as a neuro-functional biomarker for monitoring acute effects of methylphenidate in ADHD children: An fNIRS study. Neuroimage Clin. 2012, 1, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Nagashima, M.; Monden, Y.; Dan, I.; Dan, H.; Tsuzuki, D.; Mizutani, T.; Kyutoku, Y.; Gunji, Y.; Momoi, M.Y.; Watanabe, E.; et al. Neuropharmacological effect of methylphenidate on attention network in children with attention deficit hyperactivity disorder during oddball paradigms as assessed using functional near-infrared spectroscopy. Neurophotonics 2014, 1, 15001. [Google Scholar] [CrossRef] [Green Version]
- Michielsen, M.; Kleef, D.; Bijlenga, D.; Zwennes, C.; Dijkhuizen, K.; Smulders, J.; Hazewinkel, A.; Beekman, A.T.F.; Kooij, J.J.S. Response and Side Effects Using Stimulant Medication in Older Adults With ADHD: An Observational Archive Study. J. Atten. Disord. 2021, 25, 1712–1719. [Google Scholar] [CrossRef]
- Spencer, T.; Biederman, J.; Wilens, T.; Doyle, R.; Surman, C.; Prince, J.; Mick, E.; Aleardi, M.; Herzig, K.; Faraone, S. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol. Psychiatry 2005, 57, 456–463. [Google Scholar] [CrossRef]
- Strauß, M.; Petroff, D.; Huang, J.; Ulke, C.; Paucke, M.; Bogatsch, H.; Böhme, P.; Hoffmann, K.; Reif, A.; Kittel-Schneider, S.; et al. The “VIP-ADHD trial”: Does brain arousal have prognostic value for predicting response to psychostimulants in adult ADHD patients? Eur. Neuropsychopharmacol. 2021, 43, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Berridge, C.W.; Waterhouse, B.D. The locus coeruleus–noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 2003, 42, 33–84. [Google Scholar] [CrossRef]
- Christiansen, H.; Hirsch, O.; Abdel-Hamid, M.; Kis, B. Conners Skalen zu Aufmerksamkeit und Verhalten für Erwachsene (CAARS). In Deutschsprachige Adaptation der Conners‘ Adult ADHD Rating Scales (CAARS); von Conners, C.K., Erhardt, D., Sparrow, E., Eds.; Hogrefe: Bern, Switzerland, 2014. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G. Manual for the Beck Depression Inventory-II; Psychological Coporration: San Antonio, TX, USA, 1996. [Google Scholar]
- World Health Organization. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Health Care, 2nd ed.; Babor, T.F., Higgins-Biddle, J.C., Saunders, J.B., Monteiro, M.G., Eds.; World Health Organization: Geneva, Switzerland, 2001; Available online: https://apps.who.int/iris/handle/10665/67205 (accessed on 20 May 2022).
- Berman, A.H.; Bergman, H.; Palmstierna, T.; Schlyter, F. The Drug Use Disorders Identification Test (DUDIT); Karolinska Institut: Stocklholm, Sweden, 2002; Available online: https://www.emcdda.europa.eu/system/files/attachments/12173/DUDIT%20Deutsch.pdf (accessed on 20 May 2022).
- Kooij, J.J.S.; Francken, M.H. DIVA 2.0. Diagnostisches Interview für ADHS. bei Erwachsenen (DIVA) DEUTSCH; DIVA Foundation: Den Haag, The Netherlands, 2010. [Google Scholar]
- Löwe, B.; Spitzer, R.L.; Zipfel, S.; Herzog, W. Gesundheitsfragebogen für Patienten (PHQ-D). Manual undTestunterlagen (2 Auflage). Available online: https://www.klinikum.uni-heidelberg.de/fileadmin/Psychosomatische_Klinik/pdf_Material/PHQ_Komplett_Fragebogen1.pdf (accessed on 20 May 2022).
- Montgomery, S.A.; Asberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef] [PubMed]
- De Winter, J.C.F. Using the Student’s t-test with extremely small sample sizes. Pract. Assess. Res. 2013, 18, 10. [Google Scholar] [CrossRef]
- Shaw, M.; Hodgkins, P.; Caci, H.; Young, S.; Kahle, J.; Woods, A.G.; Arnold, L.E. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: Effects of treatment and non-treatment. BMC Med. 2012, 10, 99. [Google Scholar] [CrossRef] [Green Version]
- Faraone, S.V.; Biederman, J.; Spencer, T.; Mick, E.; Murray, K.; Petty, C.; Adamson, J.J.; Monuteaux, M.C. Diagnosing adult attention deficit hyperactivity disorder: Are late onset and subthreshold diagnoses valid? Am. J. Psychiatry 2006, 163, 1720–1729. [Google Scholar] [CrossRef]
- Karam, R.G.; Breda, V.; Picon, F.A.; Rovaris, D.L.; Victor, M.M.; Salgado, C.A.I.; Vitola, E.S.; Silva, K.L.; Guimarães-da-Silva, P.O.; Mota, N.R.; et al. Persistence and remission of ADHD during adulthood: A 7-year clinical follow-up study. Psychol. Med. 2015, 45, 2045–2056. [Google Scholar] [CrossRef] [Green Version]
- De Crescenzo, F.; Cortese, S.; Adamo, N.; Janiri, L. Pharmacological and non-pharmacological treatment of adults with ADHD: A meta-review. Evid. Based Ment. Health 2017, 20, 4–11. [Google Scholar] [CrossRef] [Green Version]
| ID | 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 | Mean | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) a | 26 | 26 | 53 | 35 | 36 | 39 | 26 | 34 | 31 | 38 | 34.3 | 8.2 |
| Sex | M | F | M | M | F | F | M | M | M | F | ||
| Therapy response 1 | N | N | Y | Y | N | Y | Y | N | Y | Y | ||
| Medical therapy dose (mg) | MPH 80 | MPH 20 | MPH 40 | MPH 40 | MPH 20 | ATX 40 LDX 70 | MPH 10 | MPH 10 | MPH 30 ATX 80 LDX 70 | MPH 60 | ||
| Duration (months) b | 26 | 21 | 8 | 39 | 38 | 22 | / 2 | 18 | 30 | 40 | ||
| Other therapy | None | None | None | None | None | None | ST NFB | None | None | None | ||
| Complications | None | MDD AD SD | None | None | None | MDD | MDD AD | None | None | MDD | ||
| Therapy for complication | None | PSYT CITA 40, 23 m | None | None | None | PSYT TMS | PSYT | None | None | PSYT | ||
| Physical diseases | None | None | None | None | None | None | None | None | None | LTHY | ||
| Marital status | ||||||||||||
| Prior | S | S | P | P | S | S | S | P | S | P | ||
| Follow-up | P | S | P | P | P | S | S | P | D | P | ||
| Graduation | ||||||||||||
| Prior | N | N | N | Y | Y | N | N | Y | N | Y | ||
| Follow-up | Y | Y | N | Y | Y | Y | N | Y | Y | Y | ||
| Job status | ||||||||||||
| Prior | STU | UE | E | UE | E | E | STU | E | E | E | ||
| Follow-up | E | E | E | E | E | E | STU | E | E | E | ||
| CAARS DSM-Global (R) | ||||||||||||
| Prior | 33 | 42 | 32 | 36 | 28 | 42 | 44 | 24 | 39 | 45 | 36.5 | 7.1 |
| Follow-up | 34 | 49 | 31 | 8 | 30 | 38 | 41 | 32 | 18 | 25 | 30.6 | 11.6 |
| CAARS DSM-Global (T) | ||||||||||||
| Prior | 76 | 90 | 90 | 84 | 71 | 89 | 90 | 68 | 84 | 90 | 83.2 | 8.5 |
| Follow-up | 77 | 90 | 88 | 46 | 74 | 84 | 86 | 79 | 60 | 67 | 75.1 | 13.9 |
| BDI | ||||||||||||
| Prior | 3 | 7 | 4 | 11 | 10 | 0 | 33 | 1 | 17 | 40 | 12.6 | 13.7 |
| Follow-up | 0 | 36 | 3 | 5 | 17 | 19 | 45 | 2 | 10 | 8 | 14.5 | 15.2 |
| MADRS | ||||||||||||
| Prior | 11 | 7 | 7 | 21 | 15 | 8 | 19 | 7 | 6 | 15 | 11.6 | 5.5 |
| Follow-up | 2 | 24 | 7 | 0 | 12 | 23 | 27 | 15 | 10 | 4 | 9.2 | 7.9 |
| AUDIT | ||||||||||||
| Prior | 3 | 1 | 1 | 5 | 4 | 4 | 12 | 6 | 7 | 3 | 4.6 | 3.2 |
| Follow-up | 2 | 1 | 1 | 4 | 8 | 3 | 6 | 4 | 4 | 1 | 3.4 | 2.3 |
| DUDIT | ||||||||||||
| Prior | 0 | 0 | 0 | 4 | 3 | 0 | 0 | 8 | 5 | 0 | 2.0 | 2.9 |
| Follow-up | 0 | 0 | 0 | 0 | 7 | 0 | 2 | 5 | 0 | 0 | 1.4 | 2.5 |
| DIVA-C | ||||||||||||
| Prior | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ||
| Follow-up | ||||||||||||
| Work/Education | L | M | M | M | None | M | H | M | M | None | ||
| Relationship | M | M | M | M | M | None | None | M | L | L | ||
| Social contacts | L | None | L | H | M | L | L | H | H | None | ||
| Hobby | M | L | M | H | L | M | None | L | M | L | ||
| Self-confidence | H | None | None | H | M | M | None | M | M | None | ||
| Overall | M | L | M | H | L | L | L | M | M | L | ||
| PHQ-D | ||||||||||||
| Prior | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ||
| Follow-up | ||||||||||||
| Depressive syndrome | 3 | 22 | 5 | 5 | 11 | 15 | 25 | 3 | 9 | 8 | 10.6 | 7.8 |
| Panic syndrome | N | N | N | N | N | N | Y | N | N | N | ||
| Other anxiety syndrome | 0 | 14 | 1 | 1 | 1 | 12 | 14 | 1 | 0 | 1 | 4.5 | 6.1 |
| Somatic syndrome | 1 | 17 | 1 | 9 | 14 | 9 | 16 | 1 | 2 | 12 | 8.2 | 6.5 |
| Psychosocial stressors | 1 | 12 | 0 | 7 | 8 | 9 | 18 | 1 | 5 | 8 | 6.9 | 5.5 |
| Bulimia nervosa | N | N | N | N | N | N | N | N | N | N | ||
| Binge-eating disorder | N | N | N | N | N | N | Y | N | N | N | ||
| Alcohol syndrome | N | N | N | N | Y | N | N | N | N | N |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Mauche, N.; Rullmann, M.; Ulke, C.; Becker, G.-A.; Patt, M.; Zientek, F.; Hesse, S.; Sabri, O.; Strauß, M. Association between Individual Norepinephrine Transporter (NET) Availability and Response to Pharmacological Therapy in Adults with Attention-Deficit/Hyperactivity Disorder (ADHD). Brain Sci. 2022, 12, 965. https://doi.org/10.3390/brainsci12080965
Huang J, Mauche N, Rullmann M, Ulke C, Becker G-A, Patt M, Zientek F, Hesse S, Sabri O, Strauß M. Association between Individual Norepinephrine Transporter (NET) Availability and Response to Pharmacological Therapy in Adults with Attention-Deficit/Hyperactivity Disorder (ADHD). Brain Sciences. 2022; 12(8):965. https://doi.org/10.3390/brainsci12080965
Chicago/Turabian StyleHuang, Jue, Nicole Mauche, Michael Rullmann, Christine Ulke, Georg-Alexander Becker, Marianne Patt, Franziska Zientek, Swen Hesse, Osama Sabri, and Maria Strauß. 2022. "Association between Individual Norepinephrine Transporter (NET) Availability and Response to Pharmacological Therapy in Adults with Attention-Deficit/Hyperactivity Disorder (ADHD)" Brain Sciences 12, no. 8: 965. https://doi.org/10.3390/brainsci12080965
APA StyleHuang, J., Mauche, N., Rullmann, M., Ulke, C., Becker, G.-A., Patt, M., Zientek, F., Hesse, S., Sabri, O., & Strauß, M. (2022). Association between Individual Norepinephrine Transporter (NET) Availability and Response to Pharmacological Therapy in Adults with Attention-Deficit/Hyperactivity Disorder (ADHD). Brain Sciences, 12(8), 965. https://doi.org/10.3390/brainsci12080965






