Abstract
Many studies have focused on navigation, spatial skills, and the olfactory system in comparative models, including those concerning the relationship between them and physical activity. Although the results are often in contrast with each other, it is assumed that physical activity can affect cognition in different ways—both indirectly and through a certain influence on some brain structures. In contrast, there is little research that focuses on the relationship between spatial abilities and olfactory abilities in humans. This research aimed to evaluate and compare the performance in working memory tasks of athletes and non-athletes who require good visual–spatial navigation, olfactory–spatial navigation, and olfactory–semantic skills. The study involved 236 participants (83 athletes) between the ages of 18 and 40. All subjects were matched by age or sex. The standard Corsi Block Tapping Test (CBTT) was administrated to investigate the visual-spatial memory. Olfactory–spatial navigation and olfactory–semantic skills were assessed with two modified versions of CBTT: Olfactory CBTT (OCBTT) and Semantic–Olfactory CBTT (SOCBTT) respectively. The results show differences between the CORSI conditions in direction of a poor performance for athletes. A gender effect in favor of men was also found, particularly in the classic version of the CBTT. Both groups performed better in the classic version of the CBTT than OCBTT and SOCBTT. The mean of SOCBTT results is markedly lower, perhaps due to the different information processing systems needed to perform this kind of task. It is possible to explain how sports practice can affect tasks that require spatial skills and olfactory perception differently, thus supporting new hypotheses and opening new scientific horizons.
1. Introduction
1.1. Human Spatial Representation and Navigation
In humans, spatial navigation is generally regulated by three types of spatial knowledge. It is possible to distinguish between: landmarks, i.e., fixed salient features or points of reference in the environment; route knowledge, i.e., sequences of locations as experienced by the navigator, and thus associated with an egocentric reference system; survey knowledge that includes information about the general structures of routes and the spatial relationships between different sites and associated with an allocentric reference system; graph knowledge, a network of maps and places that consists of a topological connection between various locations [1,2,3,4]. Moreover, there are three strategies used in space navigation: egocentric, allocentric, and beacon. The allocentric representation (world-focused) refers to landmarks external to the navigator, while the egocentric type of representation (body-focused) implies a reference to the current body position of the individual. Beacon concerns navigation to one or more objects and requires the memory of the object itself and the ability to distinguish it from other objects and characteristics; therefore, it could be considered as an object that indicates a nearby target location or the target itself [2,5].
A neural network underlying space navigation in humans is based both on specific cells activated in response to specific spatial positions—mainly present in the hippocampal cells that respond to the sight of landmarks. The hippocampus blends spatial and visual characteristics with the context to calculate flexible representations that are similar to maps of space [6]. The hippocampus, in addition to being involved in the organization and expression of memories, is essential for space navigation [7].
In particular, the hippocampus and entorhinal cortex could contain map-like spatial codes, while the parahippocampal and retrosplenial cortex could provide the inputs that allow for cognitive maps to connect to fixed environmental reference points [8,9].
Jacobs et al. [10] have identified cells in humans with a similar activity to grid cells, which are believed to be responsible, in animals, for numerous spatial behaviors. These are mainly distributed in the entorhinal and cingulate cortices and the hippocampus.
In addition, right-lateralized brain activities have been identified in spatial navigation tasks. This allowed for us to hypothesize that gamma oscillations in cerebral electrical activity in the right neocortex (in particular, in the temporal, parietal, and occipital cortices) play an important role in human space navigation [11].
Furthermore, human space navigation would also seem to be influenced by age and sex [12,13,14].
1.2. Spatial Navigation and Olfaction in Humans
Jacobs [15] hypothesizes that the primary function of olfaction was navigation, thanks to its ability to map odorants in space, as well as to discriminate them.
In the animal kingdom, the sense of smell still plays a vital role in navigation and spatial orientation. A hippocampal region associated with spatial orientation and an olfactory–hippocampal projection is preserved in some groups of animals, such as rodents, in which the olfactory system is connected to the hippocampus through the entorhinal cortex [16]. With regard to the spatial organization, young rats are better at using the sense of smell but are less efficient at using visual information as opposed to older rats, although there is still an interaction between different sensory modalities [17]. Seabirds are able to use olfactory cues to navigate even in very large spaces, as well as to search for food [18].
However, few studies focus on the role of smell in human spatial navigation. For example, Bao et al. [19] explain how odor information can be assembled into spatially navigable cognitive maps, optimizing orientation, and pathfinding to an odor source. Dahmani et al. [20] show that particular structures of the human hippocampus (specifically the fimbria–fornix volume) are connected to both navigational learning and olfactory identification. Dahmani et al. [21] focus on how olfactory identification covaries with spatial memory and how the thickness of the left medial orbitofrontal cortex and the volume of the right hippocampus predict both olfactory identification and spatial memory. Hamburger and Knauff [22] show how humans are able to expand their cognitive map of the environment with olfactory landmarks used in spatial orientation.
Jacobs et al. [23] show that humans can use the olfactory modality to map and reorient themselves in a previously learned place. Goodrich-Hunsaker et al. [24] found that the hippocampus is essential for associative odor-place memory and spatial recognition memory, supporting the hypothesis that associative odor–place memory is mediated by the hippocampus in both rodents and humans.
This highlights that there is a lack of information regarding the connection between the olfactory modality, orientation, and spatial navigation in humans.
1.3. Spatial Navigation in Athletes and Non-Athletes
Spatial representation and navigation represent one of the many cognitive abilities available, and therefore they can influence human life in many contexts. In the recent literature, many studies focus on the relationship between physical activity and its impact on human cognitive abilities—including representation and space navigation—even if there is no unanimous agreement regarding the benefits of physical activity on the cognitive system [25,26].
Although a specific and clear link has not yet been identified, it is assumed that physical exercise may increase cognition indirectly, for example, by improving health and reducing chronic diseases that have a certain impact on neurocognitive functions [27,28]. Furthermore, the benefits associated with physical activity may vary based on genetic or diet-related factors [29].
Some studies report a strong influence of physical activity on brain functions—such as learning, memory, executive functions— and cognitive decline [30,31], as well as an increased volume of gray and white matter regions [32] in the hippocampus. High levels of aerobic exercise appear to be associated with a more extensive right and left hippocampus, and larger hippocampus and higher fitness levels seem to be correlated with improved spatial memory performance [33,34].
Moreover, increased physical activity appears to be linked to greater hippocampal and basal ganglia volume, greater white matter integrity in preadolescents, and greater volumes of the prefrontal cortex and basal ganglia, as well as the hippocampus, in older adults [29].
In addition, physical activity has a more significant effect on cognitive functioning and the future incidence of cognitive decline among subjects carrying at least one copy of the APOE ε4 allele [35]. It could represent an advantage in reducing the risk of Alzheimer’s disease, other forms of dementia (excluding vascular dementia), and cognitive decline [36].
There are not many studies that specifically focus on the relationship between spatial ability and athletes, I and results are often in contrast with what has been previously reported. For example, Cynthia et al. [37] reported that the spatial ability of athletes and non-athletes is not significantly different from each other, concluding that exercise may or may not increase the spatial capacity of both groups. However, specific sport skills did not further affect the spatial abilities of the athletes. Jansen and Lehmann [38] examined visual–spatial cognition in athletes and non-athletes through an object-based mental rotation task, showing that all participants had greater accuracy for the rotation of human figures than objects and only one class of the athletes considered demonstrated a better mental rotation performance than non-athletes.
1.4. Visual–Spatial Memory and Olfaction
The literature shows that olfaction may be related to tasks that require the use of visual–spatial memory [39,40,41].
Furthermore, some researchers hypothesized that olfactory identification and visuospatial memory would be linked by overlapping brain areas, which include the left orbitofrontal cortex and the right hippocampus [21]. Moreover, the visual–spatial component is also extremely present in the olfactory perception, where, from an evolutionary point of view, the further development of ancestral olfactory abilities is linked to the location of the olfactory marker. Evolution has favored the development of vision, leading to a decline in the olfactory sense in the human being [42].
Recently, research has focused on the link between olfactory stimuli and cognitive and physical performance in athletes [23,43,44].
For this reason, this study aimed to examine and compare the performance of athletic subjects with non-athletic subjects in working memory tasks that require good visual–spatial navigation skills, olfactory–spatial navigation skills, and olfactory–semantics skills.
2. Materials and Methods
The Corsi Block Tapping Test (CBTT) associated with olfactory components was used to investigate how sense of smell can be related to tasks requiring the use of visuo-spatial memory, and whether this component could be gender-dependent. For this reason, the possibility of a gender difference was also considered.
The study took place at the Laboratory of Cognitive and Psychophysiological Olfactory Processes (INSPIRE) at the University of Salento, Lecce. The experimental procedure of the study was approved by the Ethical Committee (IRB) of the DiSTeBA, University of Salento. All participants signed a written informed consent form before inclusion in the study.
2.1. Participants
The research involved 236 volunteer subjects (aged between 18 and 40 years), who were recruited, assessed, and selected without allergies or evident respiratory/olfactory alterations. They were divided as follows: 83 athletic subjects (group 1), enrolled in the department of Motor Science (54 men; mean age 22.4 ± 5.5) and 153 non-athletic subjects (group 2), enrolled in the department of psychology (61 men; mean age 22.6 ± 4.3). The athletes were recruited from students recognized as student-athletes, belonging to different competitive sports categories (including football, judo, martial arts, horse riding, swimming, basketball, and boxing). A specific sport was not chosen for this study because, as already highlighted in the introduction, specific sport skills did not further affect the spatial abilities of the athletes [37,38]. The control group included non-athlete subjects, who did not follow competitive activities or participate in sports activities for more than two hours a week.
2.2. Olfactory Stimuli
Olfactory Stimuli were purchased as pure odorants by Sigma-Aldrich Products.
Table 1 shows the chemicals used and the common names associated with the odorants.

Table 1.
Name of the chemical/odorant used and common parfum name associated to the odorant.
The odorants were taken pure with a 5 mL syringe, and, for each square of tissue paper, 3 mL of odorant was instilled. Each set of odorants was placed in a transparent envelope numbered with the number associated with the Corsi block tapping test.
2.3. Assessment
The CBTT [45,46] was used to examine visual–spatial memory. It consisted of nine blocks arranged irregularly on a 23 × 28 cm board [47]. Each cube was numbered on the side facing the examiner to simplify the execution.
The CBTT was administered according to three different sessions and modalities: (1) classical version of the CBTT; (2) Olfactory CBTT (OCBTT); and (3) Semantic-Olfactory CBTT (SOCBTT).
In the classical version (CBTT) [48], the examiner (E) showed the subject (S) a spatial sequence by touching the numbered cubes at a regular interval of about 2 s.
The S was seated in front of the E. The E told the S to “touch the cubes that I touch, immediately after me” and touched cube number 1 with their index finger, before returning it to the table placed between himself/herself and the S each time. The S’s task was to touch the same cube with the dominant hand. This continued, in order, for all cubes up to number 9. If the subject did not make any mistakes, the test is carried out, otherwise the preliminary test was repeated up to a maximum of 3 times.
The E touched the index sequences of cubes and progressively increased the length (from 2 to 10 cubes) at the rate of one cube every 2 s, returning the index finger to the table after of each touch. As soon as the demonstration of the sequence finished, the E asked the S to reproduce it by noting the cubes in the same order. Three sequences were presented for each series. If the S correctly reproduced at least 2 out of 3 sequences, the next series was examined [45] (see Video S1 CBTT).
The OCBTT is an experimental and innovative version of the CBTT. In OCBTT, squares of paper wet with specific odorants were placed on the cubes. These squared papers were smelled according to sequences and methods described for the standard CBTT (see Figure 1 and Video S2 OCBTT).
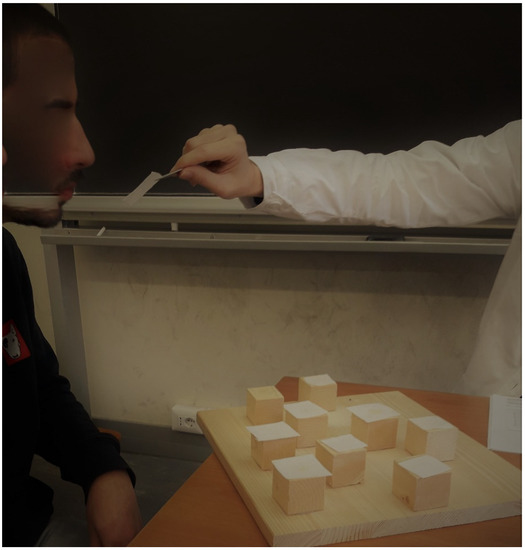
Figure 1.
Example of the Olfactory Corsi Block Tapping Test (OCBTT).
The position of the blocks, the same for all subjects, was described in the Table 1.
The individual blocks of tissue paper with odorants were placed on the individual blocks and were subsequently picked up by the experimenter with long tweezers. At the end of each sequence, the odorant paper square, pseudo-randomly chosen, was extracted from a plastic pocket, and administered to the subjects. The subjects had to link the cube to the odorant they had just smelled during the sequence’s administration. In this version, the test was considered complete after three consecutive errors (see Videos S1 and S2).
In the SOCBTT, the examiner read the complete list of odorants used only once. Then the subjects performed the same task as the OCBTT, but, at the end of each sequence, the subject had to name the recognized odorants during the administration of the sequence and identify their relative position on the cubes (see Video S3 SOCBTT).
The tests’ sequence was alternated for each subject to avoid a primacy and habituation effect linked to olfactory stimulation.
2.4. Statistical Analysis
SPSS software was used for statistical data analysis. A GLM repeated measures was performed, considering the three variants of the Corsi Test as within condition (3 levels: CBTT; OCBTT; SOCBTT) and the SPORT as between condition (athletes, non-athletes), also considering sex and smoking as covariate.
3. Results
The descriptive analysis for each version of the test in each group showed the following results:
CBTT (group 1: mean 5.30; SD 0.852; group 2: mean 5.574; SD 1.061), OCBTT (group 1: mean 3.37; SD 2.105/group 2: mean 4.61; SD 2.191), SOCBTT (group 1: mean 0.51; SD 1.713/group 2: mean 3.22; SD 3.056).
A GLM repeated-measures showed, for the variable SPORT (athletes/non athletes) on TEST (3 Levels), a significant value on CBTT (p = 0.000; F = 16.055 part η2 = 0.075), on OBTT (p = 0.000; F = 16.331; part η2 = 0.076) and on SOBTT (p = 0.000; F = 52.204; part η2 = 0.209). Moreover, the results highlighted a significant effect for smoke in SOBCTT (p = 0.002; F = 10.252; part η2 = 0.049) and for sex in CBTT (p = 0.000; F = 18.154; part η2 = 0.084) and in SOBCTT (p = 0.006; F = 7.87; part η2 = 0.038) (Figure 2 and Figure 3). Multivariate test highlighted significant value for Factor 1 (p = 0.000; F = 113.92; part η2 = 0.586); significant interaction between Factor 1 and smoke (p = 0.003; F = 6.144; part η2 = 0.141) and significant interaction between Factor1 and SPORT (p = 0.000; F= 16.396; part η2 = 0.141) (Figure 4 and Figure 5).
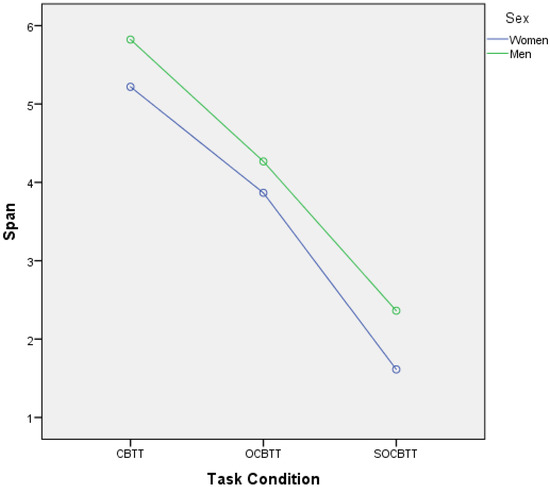
Figure 2.
Gender difference and span expressed in the different task conditions (i.e., CBTT; OCBTT and SOCBTT).
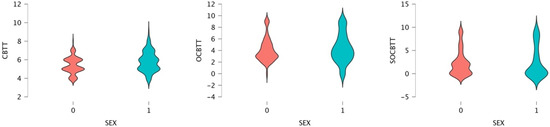
Figure 3.
Violin Plot for gender difference and Span expressed in CBTT, OCBTT and SOCBTT.
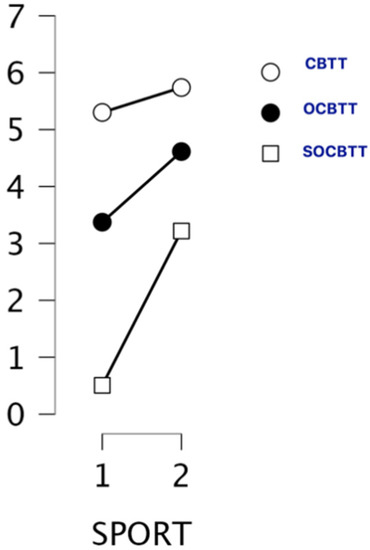
Figure 4.
Span and sport conditions expressed through the different task conditions (i.e., CBTT; OCBTT and SOCBTT).
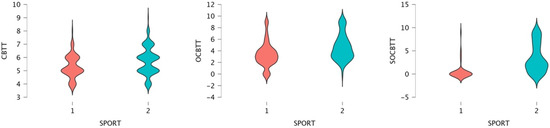
Figure 5.
Violin plot for the span and sport conditions (1 = athletes; 2 = non-athletes) through the CBTT, OCBTT and SOCBTT.
Both groups (athletes and non-athletes) performed better on the CBTT (mean scores for athletes 5.30; mean scores for non-athletes 5.74). Both groups showed lower performance on the SOCBTT (mean scores for athletes 0.51; mean scores for non-athletes 3.22).
4. Discussion and Conclusions
The olfactory component can be fundamental if we consider spatial navigation and spatial memory. The subcortical pathways that represent these aspects are connected to hippocampal and entorhinal activation, where, the chemoceptive component is relevant [49]. The recent literature has focused on the link between olfactory stimuli and cognitive and physical performance in athletes [23,43,44], highlighting no specific differences between the types of sport, but intensive or competitive athletic activity can be considered as a non-specific sport clustering factor.
For this reason, this study aimed to examine and compare the performance of athletic subjects with non-athletic subjects in working memory tasks that require good visual–spatial navigation skills, olfactory–spatial navigation skills, and olfactory–semantics skills, using a modified paradigm linked to the classical CBTT. This aspect can be a limit, but also a strength of the work, as it proposes a new task, connected to a classic one, and integrates an olfactory sensescape that could be strongly suitable for (but currently missing from) neuropsychological testing [50].
The main results of this study highlighted significant differences between various levels of the task (CBTT, OCBTT, SOCBTT) for the SPORT variable, and for this reason, lower scores are noted for athletes, which indicates a better performance on tasks for non-athletes. Furthermore, it is possible to observe a positive correlation between the variants of the tasks and the sports practice.
Both groups (athletes and non-athletes) had a better performance in the classic version of the CBTT than the other two variants (i.e., OCBTT and SOCBTT). The mean of the SOCBTT results is markedly lower than the classic variant of the CBTT and the OCBTT, possibly because it may be more difficult to perform the task due to the different information processing systems required.
As reported in the literature [13,14], in this study the gender effect was also confirmed. Men in both groups performed better than women in the classic variant of the CBTT and in the SOCBTT, while no significant difference was found in the OCBTT. More specifically, it is possible to observe that non-athlete men and women perform better in carrying out the task than men and women athletes. The lower performance of athletes was a result, although not hoped for, that was partly present in the literature [51]. The question of whether athletes have greater cognitive performance than controls has been much debated [52].
A limitation of the study was not considering the frequency of use of words connected to odorants, their familiarity, or their pleasantness. These variables may affect memory and recall during the task [50].
It is possible to explain how sports practice can differently effect tasks that require the use of spatial skills and olfactory perception, thus supporting new hypotheses.
This research reveals some prospects. Indeed, it may be appropriate to analyze whether and how sports activity frequency can influence olfactory perception and spatial memory tasks. In future, we will evaluate these variants of the test with a behavioral and psychophysiological analysis in clinical aging (e.g., MCI), where spatial and olfactory abilities are impaired [53], during rehabilitative motor activity, and through the use of an olfactometer also interfaced in EEG.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/brainsci12081108/s1, Video S1: CBTT; Video S2: OCBTT; Video S3: SOCBTT.
Author Contributions
Conceptualization and methodology S.I.; investigation, M.S., G.A., M.L., T.S., F.F.; resources, V.C., S.I.; data curation, S.I.; writing—original draft preparation, S.I., G.A., M.L., writing—review and editing, S.I., D.L. All authors have read and agreed to the published version of the manuscript.
Funding
CALIPER Gender Equality in STEM; European Union’s Horizon 2020 Research and Innovation programme under Grant Agreement No. 873134.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of DiSTeBA, University of Salento (protocol code 001 and date of approval 18 January 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author sara.invitto@unisalento.it.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Werner, S.; Krieg-Brueckner, B.; Mallot, H.; Schweizer, K.; Freksa, C. Spatial Cognition: The Role of Landmark, Route, and Survey Knowledge in Human and Robot Navigation. In Informatik ’97 Informatik als Innovationsmotor; Springer: Berlin, Heidelberg, 1997; Available online: http://www.cosy.informatik.uni-bremen.de/spp/SPP_onlines/ProjektD/gi97.pdf (accessed on 1 August 2022). [CrossRef]
- Chan, E.; Baumann, O.; Bellgrove, M.; Mattingley, J. From Objects to Landmarks: The Function of Visual Location Information in Spatial Navigation. Front. Psychol. 2012, 3, 304. [Google Scholar] [CrossRef]
- Chrastil, E.R. Neural Evidence Supports a Novel Framework for Spatial Navigation. Psychon. Bull. Rev. 2013, 20, 208–227. [Google Scholar] [CrossRef] [PubMed]
- Chrastil, E.R.; Warren, W.H. Active and Passive Spatial Learning in Human Navigation: Acquisition of Survey Knowledge. J. Exp. Psychol. Learn. Mem. Cogn. 2013, 39, 1520–1537. [Google Scholar] [CrossRef]
- Ekstrom, A.D.; Isham, E.A. Human Spatial Navigation: Representations across Dimensions and Scales. Curr. Opin. Behav. Sci. 2017, 17, 84–89. [Google Scholar] [CrossRef]
- Ekstrom, A.D.; Kahana, M.J.; Caplan, J.B.; Fields, T.A.; Isham, E.A.; Newman, E.L.; Fried, I. Cellular Networks Underlying Human Spatial Navigation. Nature 2003, 425, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Eichenbaum, H. The Role of the Hippocampus in Navigation Is Memory. J. Neurophysiol. 2017, 117, 1785–1796. [Google Scholar] [CrossRef]
- Epstein, R.A.; Patai, E.Z.; Julian, J.B.; Spiers, H.J. The Cognitive Map in Humans: Spatial Navigation and Beyond. Nat. Neurosci. 2017, 20, 1504–1513. [Google Scholar] [CrossRef]
- Epstein, R.A. Parahippocampal and Retrosplenial Contributions to Human Spatial Navigation. Trends Cogn. Sci. 2008, 12, 388–396. [Google Scholar] [CrossRef]
- Jacobs, J.; Weidemann, C.T.; Miller, J.F.; Solway, A.; Burke, J.F.; Wei, X.-X.; Suthana, N.; Sperling, M.R.; Sharan, A.D.; Fried, I.; et al. Direct Recordings of Grid-like Neuronal Activity in Human Spatial Navigation. Nat. Neurosci. 2013, 16, 1188–1190. [Google Scholar] [CrossRef]
- Jacobs, J.; Korolev, I.O.; Caplan, J.B.; Ekstrom, A.D.; Litt, B.; Baltuch, G.; Fried, I.; Schulze-Bonhage, A.; Madsen, J.R.; Kahana, M.J. Right-Lateralized Brain Oscillations in Human Spatial Navigation. J. Cogn. Neurosci. 2010, 22, 824–836. [Google Scholar] [CrossRef]
- Moffat, S.D.; Elkins, W.; Resnick, S.M. Age Differences in the Neural Systems Supporting Human Allocentric Spatial Navigation. Neurobiol. Aging 2006, 27, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Sneider, J.T.; Hamilton, D.A.; Cohen-Gilbert, J.E.; Crowley, D.J.; Rosso, I.M.; Silveri, M.M. Sex Differences in Spatial Navigation and Perception in Human Adolescents and Emerging Adults. Behav. Process. 2015, 111, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Baizan, C.; Arias, J.L.; Mendez, M. Spatial Memory Assessment Reveals Age-Related Differences in Egocentric and Allocentric Memory Performance. Behav. Brain Res. 2020, 388, 112646. [Google Scholar] [CrossRef]
- Jacobs, L.F. From Chemotaxis to the Cognitive Map: The Function of Olfaction. Proc. Natl. Acad. Sci. USA 2012, 109, 10693–10700. [Google Scholar] [CrossRef] [PubMed]
- Aboitiz, F.; Montiel, J.F. Olfaction, Navigation, and the Origin of Isocortex. Front. Neurosci. 2015, 9, 402. [Google Scholar] [CrossRef]
- Rossier, J.; Schenk, F. Olfactory and/or Visual Cues for Spatial Navigation through Ontogeny: Olfactory Cues Enable the Use of Visual Cues. Behav. Neurosci. 2003, 117, 412–425. [Google Scholar] [CrossRef]
- Gagliardo, A.; Bried, J.; Lambardi, P.; Luschi, P.; Wikelski, M.; Bonadonna, F. Oceanic Navigation in Cory’s Shearwaters: Evidence for a Crucial Role of Olfactory Cues for Homing after Displacement. J. Exp. Biol. 2013, 216, 2798–2805. [Google Scholar] [CrossRef]
- Bao, X.; Gjorgieva, E.; Shanahan, L.K.; Howard, J.D.; Kahnt, T.; Gottfried, J.A. Grid-like Neural Representations Support Olfactory Navigation of a Two-Dimensional Odor Space. Neuron 2019, 102, 1066–1075.e5. [Google Scholar] [CrossRef]
- Dahmani, L.; Courcot, B.; Near, J.; Patel, R.; Amaral, R.S.C.; Chakravarty, M.M.; Bohbot, V.D. Fimbria-Fornix Volume Is Associated with Spatial Memory and Olfactory Identification in Humans. Front. Syst. Neurosci. 2020, 13, 87. [Google Scholar] [CrossRef]
- Dahmani, L.; Patel, R.M.; Yang, Y.; Chakravarty, M.M.; Fellows, L.K.; Bohbot, V.D. An Intrinsic Association between Olfactory Identification and Spatial Memory in Humans. Nat. Commun. 2018, 9, 4162. [Google Scholar] [CrossRef]
- Hamburger, K.; Knauff, M. Odors Can Serve as Landmarks in Human Wayfinding. Cogn. Sci. 2019, 43, e12798. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, L.F.; Arter, J.; Cook, A.; Sulloway, F.J. Olfactory Orientation and Navigation in Humans. PLoS ONE 2015, 10, e0129387. [Google Scholar] [CrossRef] [PubMed]
- Goodrich-Hunsaker, N.J.; Gilbert, P.E.; Hopkins, R.O. The Role of the Human Hippocampus in Odor-Place Associative Memory. Chem. Senses 2009, 34, 513–521. [Google Scholar] [CrossRef] [PubMed]
- van Uffelen, J.G.Z.; Chin, A.; Paw, M.J.M.; Hopman-Rock, M.; van Mechelen, W. The Effects of Exercise on Cognition in Older Adults with and without Cognitive Decline: A Systematic Review. Clin. J. Sport Med. 2008, 18, 486–500. [Google Scholar] [CrossRef]
- Sabia, S.; Dugravot, A.; Dartigues, J.-F.; Abell, J.; Elbaz, A.; Kivimäki, M.; Singh-Manoux, A. Physical Activity, Cognitive Decline, and Risk of Dementia: 28 Year Follow-up of Whitehall II Cohort Study. BMJ 2017, 357, j2709. [Google Scholar] [CrossRef]
- Bherer, L.; Erickson, K.I.; Liu-Ambrose, T. A Review of the Effects of Physical Activity and Exercise on Cognitive and Brain Functions in Older Adults. J. Aging Res. 2013, 2013, 657508. [Google Scholar] [CrossRef]
- Brasure, M.; Desai, P.; Davila, H.; Nelson, V.A.; Calvert, C.; Jutkowitz, E.; Butler, M.; Fink, H.A.; Ratner, E.; Hemmy, L.S.; et al. Physical Activity Interventions in Preventing Cognitive Decline and Alzheimer-Type Dementia: A Systematic Review. Ann. Intern. Med. 2018, 168, 30–38. [Google Scholar] [CrossRef]
- Erickson, K.I.; Hillman, C.H.; Kramer, A.F. Physical Activity, Brain, and Cognition. Curr. Opin. Behav. Sci. 2015, 4, 27–32. [Google Scholar] [CrossRef]
- Lista, I.; Sorrentino, G. Biological Mechanisms of Physical Activity in Preventing Cognitive Decline. Cell. Mol. Neurobiol. 2010, 30, 493–503. [Google Scholar] [CrossRef]
- Park, H.; Park, J.H.; Na, H.R.; Hiroyuki, S.; Kim, G.M.; Jung, M.K.; Kim, W.K.; Park, K.W. Combined Intervention of Physical Activity, Aerobic Exercise, and Cognitive Exercise Intervention to Prevent Cognitive Decline for Patients with Mild Cognitive Impairment: A Randomized Controlled Clinical Study. J. Clin. Med. 2019, 8, E940. [Google Scholar] [CrossRef]
- Colcombe, S.J.; Erickson, K.I.; Scalf, P.E.; Kim, J.S.; Prakash, R.; McAuley, E.; Elavsky, S.; Marquez, D.X.; Hu, L.; Kramer, A.F. Aerobic Exercise Training Increases Brain Volume in Aging Humans. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1166–1170. [Google Scholar] [CrossRef]
- Erickson, K.I.; Prakash, R.S.; Voss, M.W.; Chaddock, L.; Hu, L.; Morris, K.S.; White, S.M.; Wójcicki, T.R.; McAuley, E.; Kramer, A.F. Aerobic Fitness Is Associated with Hippocampal Volume in Elderly Humans. Hippocampus 2009, 19, 1030–1039. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise Training Increases Size of Hippocampus and Improves Memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Brini, S.; Sohrabi, H.R.; Peiffer, J.J.; Karrasch, M.; Hämäläinen, H.; Martins, R.N.; Fairchild, T.J. Physical Activity in Preventing Alzheimer’s Disease and Cognitive Decline: A Narrative Review. Sports Med. 2018, 48, 29–44. [Google Scholar] [CrossRef]
- Guure, C.B.; Ibrahim, N.A.; Adam, M.B.; Said, S.M. Impact of Physical Activity on Cognitive Decline, Dementia, and Its Subtypes: Meta-Analysis of Prospective Studies. BioMed Res. Int. 2017, 2017, 9016924. [Google Scholar] [CrossRef]
- Cynthia, J.; Lubis, L. Vitriana Spatial Ability Differences in Athletes and Non-Athletes. AMJ 2016, 3, 533–537. [Google Scholar] [CrossRef]
- Jansen, P.; Lehmann, J. Mental Rotation Performance in Soccer Players and Gymnasts in an Object-Based Mental Rotation Task. Adv. Cogn. Psychol. 2013, 9, 92–98. [Google Scholar] [CrossRef]
- Simpon, W.F.; Coady, R.C.; Osowski, E.E.; Bode, D.S. The Effect of Aromatherapy on Exercise Performance. Kinesiol. On-Line. 2001, 9. [Google Scholar]
- Marchand, S.; Arsenault, P. Odors Modulate Pain PerceptionA Gender-Specific Effect. Physiol. Behav. 2002, 76, 251–256. [Google Scholar] [CrossRef]
- Barker, S.; Grayhem, P.; Koon, J.; Perkins, J.; Whalen, A.; Raudenbush, B. Improved Performance on Clerical Tasks Associated with Administration of Peppermint Odor. Percept. Mot. Ski. 2003, 97, 1007–1010. [Google Scholar] [CrossRef]
- Gamble, C. The Cambridge Encyclopedia of Human Evolution. Int. J. Osteoarchaeol. 1994, 4, 265–266. [Google Scholar] [CrossRef]
- Raudenbush, B.; Corley, N.; Eppich, W. Enhancing Athletic Performance through the Administration of Peppermint Odor. J. Sport Exerc. Psychol. 2001, 23, 156–160. [Google Scholar] [CrossRef]
- Basevitch, I.; Thompson, B.; Braun, R.; Razon, S.; Arsal, G.; Tokac, U.; Filho, E.M.; Nascimento, T.; Tenenbaum, G. Olfactory Effects on Attention Allocation and Perception of Exertion. Sport Psychol. 2011, 25, 144–158. [Google Scholar] [CrossRef]
- Corsi, P.M. Human Memory and the Medial Temporal Region of the Brain. Available online: https://escholarship.mcgill.ca/concern/theses/05741s554 (accessed on 1 August 2022).
- Orsini, A.; Grossi, D.; Capitani, E.; Laiacona, M.; Papagno, C.; Vallar, G. Verbal and Spatial Immediate Memory Span: Normative Data from 1355 Adults and 1112 Children. Ital. J. Neurol. Sci. 1987, 8, 537–548. [Google Scholar] [CrossRef]
- Milner, B. Interhemispheric Differences in the Localization of Psychological Processes in Man. Br. Med. Bull. 1971, 27, 272–277. [Google Scholar] [CrossRef]
- Bianchi, A.; Dai Prà, M. Twenty Years after Spinnler and Tognoni: New Instruments in the Italian Neuropsychologist’s Toolbox. Neurol. Sci. 2008, 29, 209–217. [Google Scholar] [CrossRef]
- Bannerman, D.; Yee, B.; Lemaire, M.; Wilbrecht, L.; Jarrard, L.; Iversen, S.; Rawlins, J.; Good, M. The Role of the Entorhinal Cortex in Two Forms of Spatial Learning and Memory. Exp. Brain Res. 2001, 141, 281–303. [Google Scholar] [CrossRef]
- Invitto, S.; Grasso, A. Chemosensory Perception: A Review on Electrophysiological Methods in “Cognitive Neuro-Olfactometry”. Chemosensors 2019, 7, 45. [Google Scholar] [CrossRef]
- Bowman, J.K.; Boone, R.T.; Goldman, S.; Auerbach, A. The Athletic Intelligence Quotient and Performance Outcomes in Professional Baseball. Front. Psychol. 2021, 12, 629827. [Google Scholar] [CrossRef]
- Wilson, R.S.; David, G.K.; Murphy, S.C.; Angilletta, M.J.; Niehaus, A.C.; Hunter, A.H.; Smith, M.D. Skill Not Athleticism Predicts Individual Variation in Match Performance of Soccer Players. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170953. [Google Scholar] [CrossRef]
- Invitto, S.; Piraino, G.; Ciccarese, V.; Carmillo, L.; Caggiula, M.; Trianni, G.; Nicolardi, G.; Di Nuovo, S.; Balconi, M. Potential Role of OERP as Early Marker of Mild Cognitive Impairment. Front. Aging Neurosci. 2018, 10, 272. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).