Efficacy of Non-Invasive Brain Stimulation for Refractory Obsessive-Compulsive Disorder: A Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy and Eligibility Criteria
- Patients with a diagnosis of refractory OCD. Refractory OCD is defined as the failed adequate trials of anti-OCD drugs such as SSRIs.
- NIBS (including rTMS, TBS, tDCS) as a way of treatment for OCD.
- No change in the original medication regimen during the experiment.
- Trials that are randomized controlled trials, with a parallel or crossover design are used.
- Experiments that involve at least 10 stimulation sessions.
- The Yale-Brown Obsessive Compulsive Scale (Y-BOCS) change score or clinical response rate is available.
2.2. Data Extraction
2.3. Outcome Measures
2.4. Data Analysis
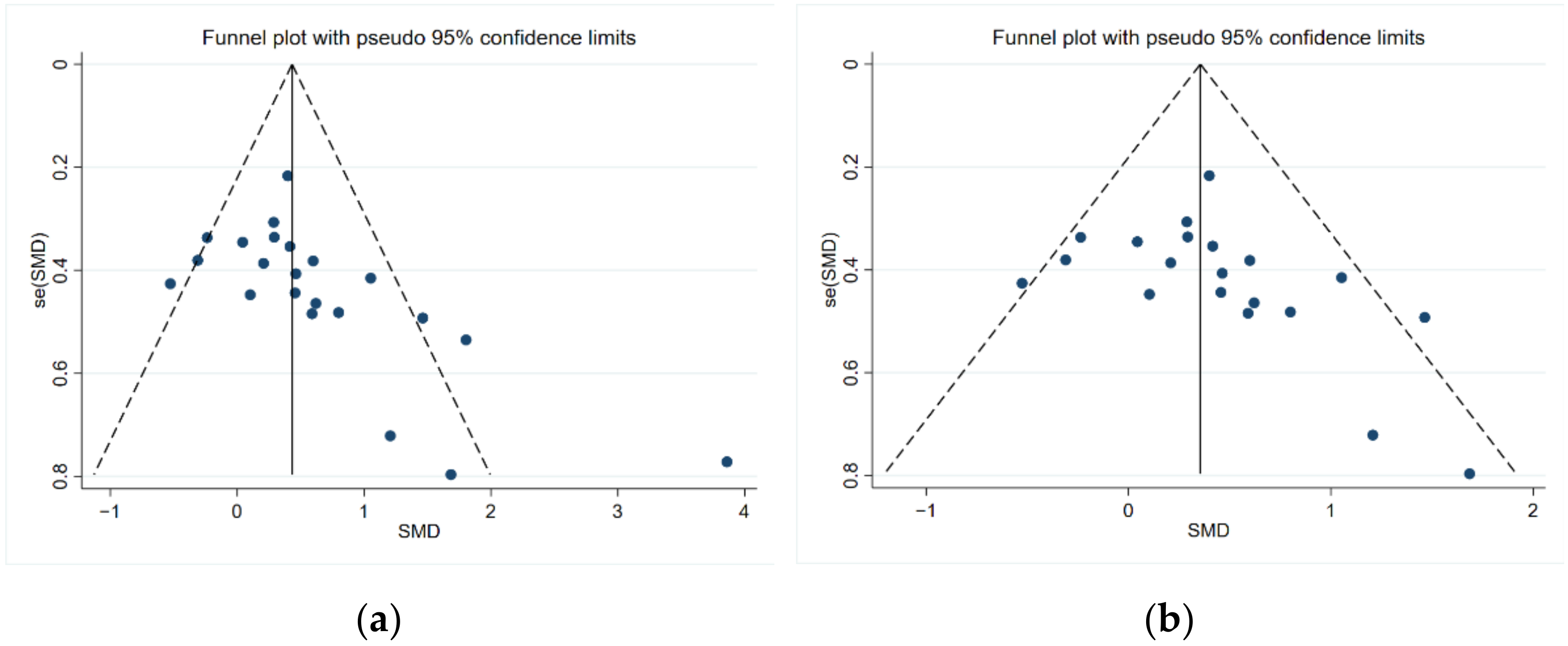
2.5. Risk of Bias Assessment
3. Results
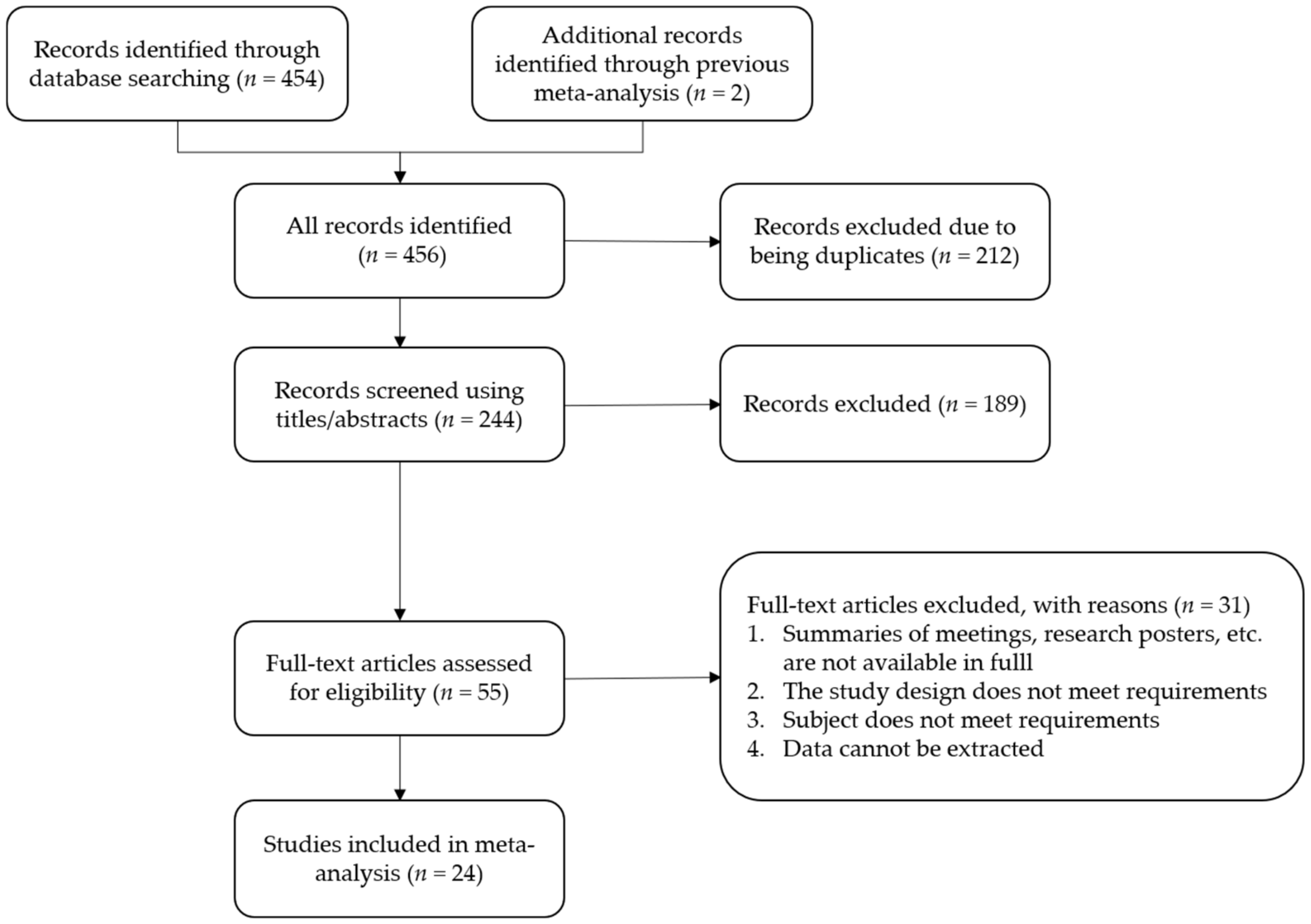
3.1. Study Selection and Characteristics
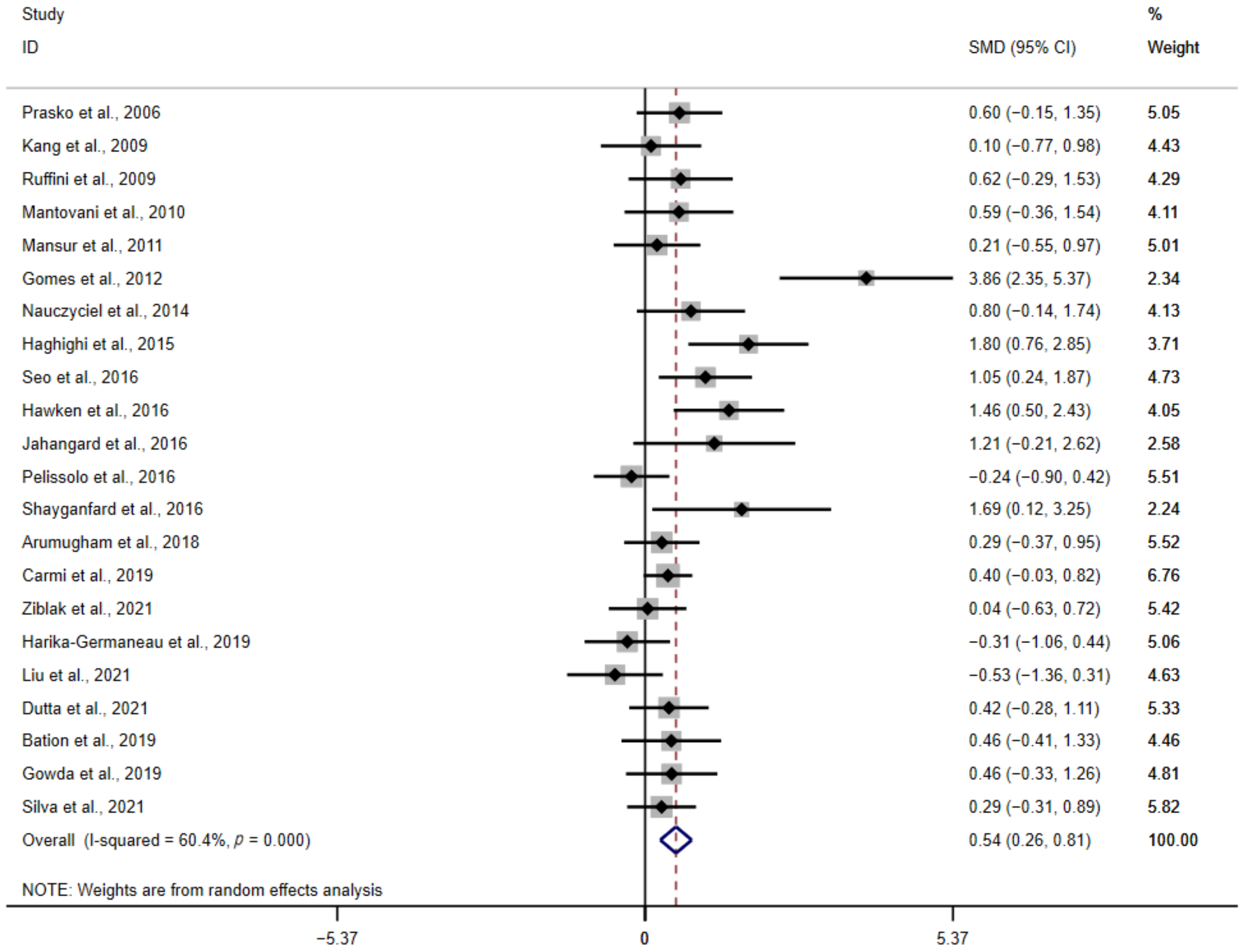
3.2. Analysis of the Primary Outcome
NIBS versus Sham Treatment
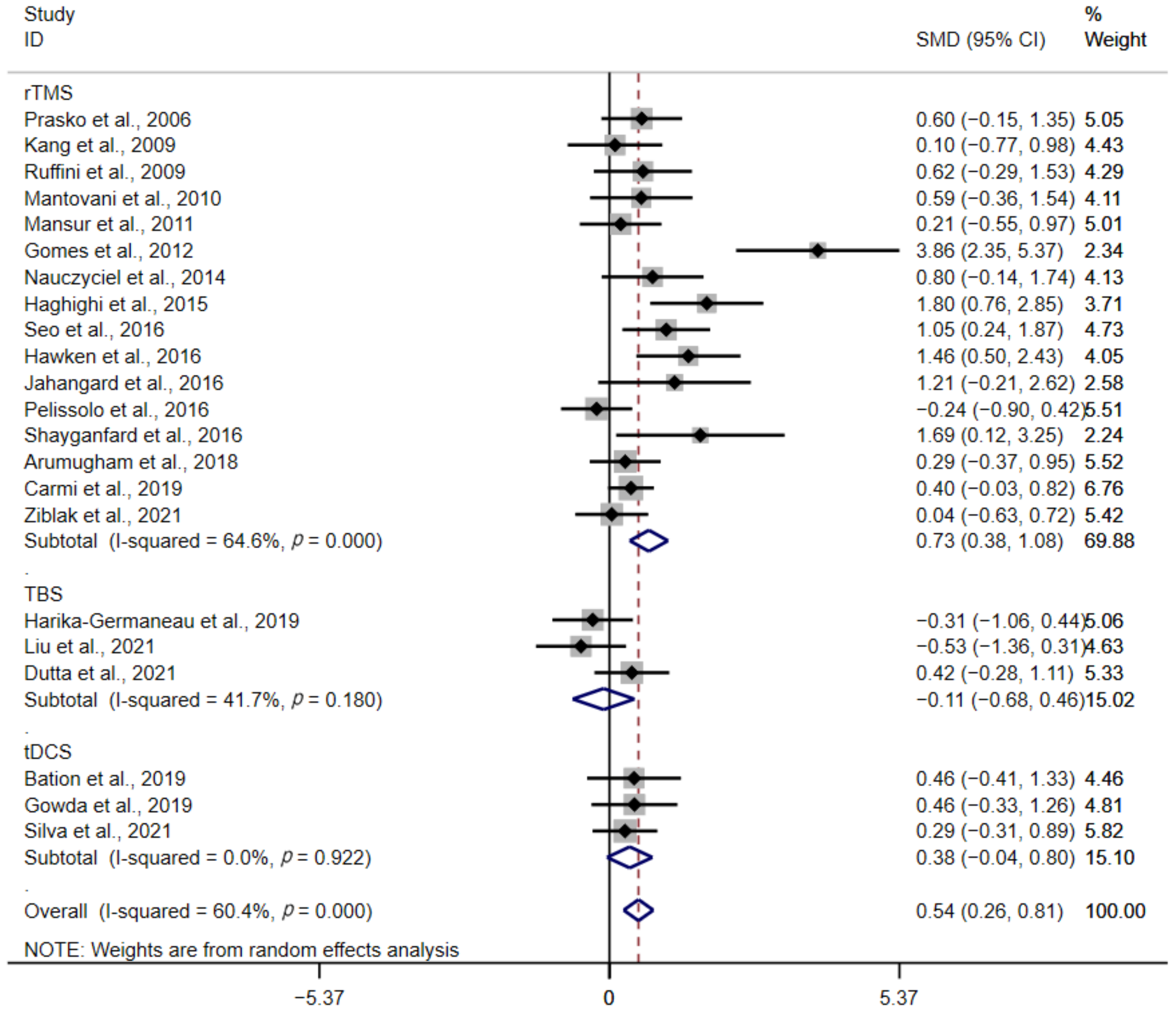
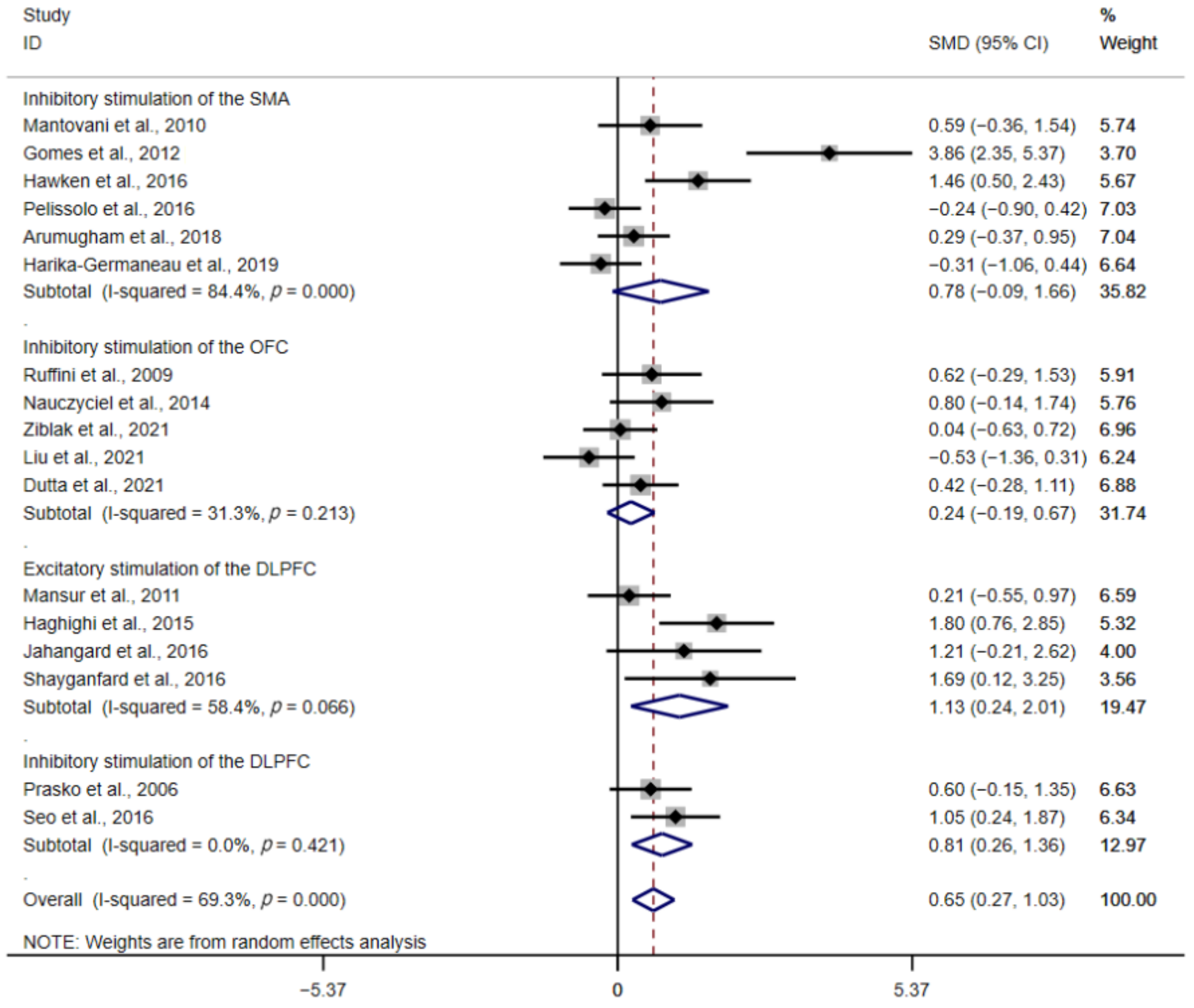
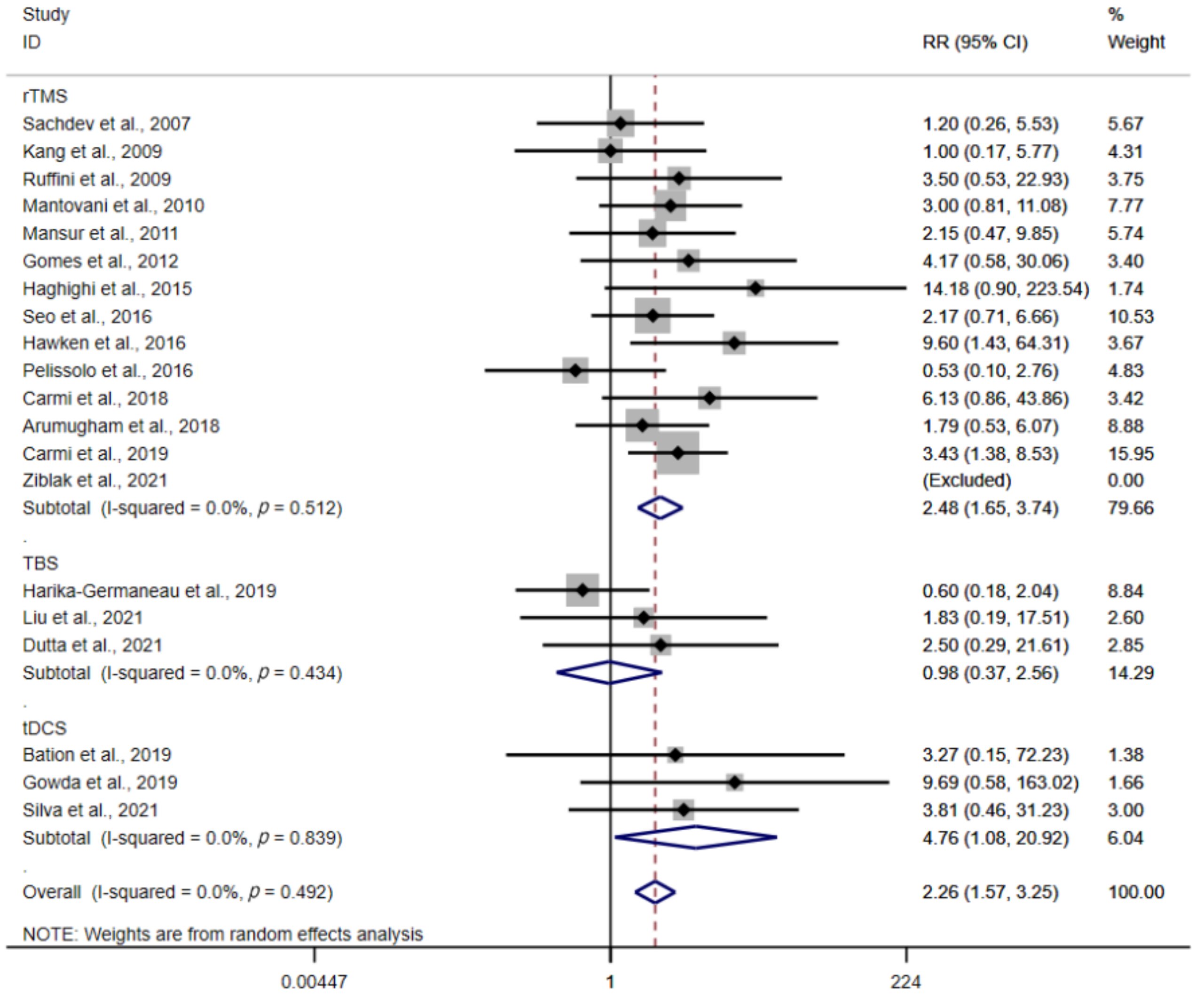
3.3. Subgroup Analysis
3.3.1. Type of Stimulation
3.3.2. Stimulation Protocols
3.4. Response Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Washington, DC, USA, 2013; p. 235. [Google Scholar]
- Ruscio, A.M.; Stein, D.J.; Chiu, W.T.; Kessler, R.C. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol. Psychiatry 2010, 15, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Kessler, R.C.; Petukhova, M.; Sampson, N.A.; Zaslavsky, A.M.; Wittchen, H.U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int. J. Methods Psychiatr. Res. 2012, 21, 169–184. [Google Scholar] [CrossRef]
- Fullana, M.A.; Mataix-Cols, D.; Caspi, A.; Harrington, H.; Grisham, J.R.; Moffitt, T.E.; Poulton, R. Obsessions and compulsions in the community: Prevalence, interference, help-seeking, developmental stability, and co-occurring psychiatric conditions. Am. J. Psychiatry 2009, 166, 329–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, A.C.; Diniz, J.B.; Fossaluza, V.; Torres, A.R.; Fontenelle, L.F.; De Mathis, A.S.; da Conceicao Rosario, M.; Miguel, E.C.; Shavitt, R.G. Clinical correlates of social adjustment in patients with obsessive-compulsive disorder. J. Psychiatr. Res. 2012, 46, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.M.; Mattheisen, M.; Mors, O.; Schendel, D.E.; Mortensen, P.B.; Plessen, K.J. Mortality Among Persons With Obsessive-Compulsive Disorder in Denmark. JAMA Psychiatry 2016, 73, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Del Casale, A.; Sorice, S.; Padovano, A.; Simmaco, M.; Ferracuti, S.; Lamis, D.A.; Rapinesi, C.; Sani, G.; Girardi, P.; Kotzalidis, G.D.; et al. Psychopharmacological Treatment of Obsessive-Compulsive Disorder (OCD). Curr. Neuropharmacol. 2019, 17, 710–736. [Google Scholar] [CrossRef] [PubMed]
- Ost, L.G.; Havnen, A.; Hansen, B.; Kvale, G. Cognitive behavioral treatments of obsessive-compulsive disorder. A systematic review and meta-analysis of studies published 1993–2014. Clin. Psychol. Rev. 2015, 40, 156–169. [Google Scholar] [CrossRef]
- Herbst, N.; Voderholzer, U.; Thiel, N.; Schaub, R.; Knaevelsrud, C.; Stracke, S.; Hertenstein, E.; Nissen, C.; Kulz, A.K. No talking, just writing! Efficacy of an Internet-based cognitive behavioral therapy with exposure and response prevention in obsessive compulsive disorder. Psychother. Psychosom. 2014, 83, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Uhre, C.F.; Uhre, V.F.; Lonfeldt, N.N.; Pretzmann, L.; Vangkilde, S.; Plessen, K.J.; Gluud, C.; Jakobsen, J.C.; Pagsberg, A.K. Systematic Review and Meta-Analysis: Cognitive-Behavioral Therapy for Obsessive-Compulsive Disorder in Children and Adolescents. J. Am. Acad. Child Adolesc. Psychiatry 2020, 59, 64–77. [Google Scholar] [CrossRef] [Green Version]
- Atmaca, M. Treatment-refractory obsessive compulsive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 70, 127–133. [Google Scholar] [CrossRef]
- Kuo, M.F.; Paulus, W.; Nitsche, M.A. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. Neuroimage 2014, 85 Pt 3, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Klomjai, W.; Katz, R.; Lackmy-Vallee, A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann. Phys. Rehabil. Med. 2015, 58, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.-P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Edwards, M.J.; Rounis, E.; Bhatia, K.P.; Rothwell, J.C. Theta burst stimulation of the human motor cortex. Neuron 2005, 45, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Suppa, A.; Huang, Y.Z.; Funke, K.; Ridding, M.C.; Cheeran, B.; Di Lazzaro, V.; Ziemann, U.; Rothwell, J.C. Ten Years of Theta Burst Stimulation in Humans: Established Knowledge, Unknowns and Prospects. Brain Stimul. 2016, 9, 323–335. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527 Pt 3, 633–639. [Google Scholar] [CrossRef]
- van den Heuvel, O.A.; van Wingen, G.; Soriano-Mas, C.; Alonso, P.; Chamberlain, S.R.; Nakamae, T.; Denys, D.; Goudriaan, A.E.; Veltman, D.J. Brain circuitry of compulsivity. Eur. Neuropsychopharmacol. 2016, 26, 810–827. [Google Scholar] [CrossRef] [Green Version]
- Fineberg, N.A.; Chamberlain, S.R.; Hollander, E.; Boulougouris, V.; Robbins, T.W. Translational approaches to obsessive-compulsive disorder: From animal models to clinical treatment. Br. J. Pharm. 2011, 164, 1044–1061. [Google Scholar] [CrossRef] [Green Version]
- Milad, M.R.; Rauch, S.L. Obsessive-compulsive disorder: Beyond segregated cortico-striatal pathways. Trends Cogn. Sci. 2012, 16, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Del Casale, A.; Kotzalidis, G.D.; Rapinesi, C.; Serata, D.; Ambrosi, E.; Simonetti, A.; Pompili, M.; Ferracuti, S.; Tatarelli, R.; Girardi, P. Functional neuroimaging in obsessive-compulsive disorder. Neuropsychobiology 2011, 64, 61–85. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; Version 6.3; John Wiley & Sons: Hoboken, NJ, USA, 2022; Available online: https://training.cochrane.org/handbook (accessed on 10 February 2022).
- Prasko, J.; Paskova, B.; Zalesky, R.; Novak, T.; Kopecek, M.; Bares, M.; Horacek, J. The effect of repetitive transcranial magnetic stimulation (rTMS) on symptoms in obsessive compulsive disorder. A randomized, double blind, sham controlled study. Neuro Endocrinol. Lett. 2006, 27, 327–332. [Google Scholar] [PubMed]
- Sachdev, P.S.; Loo, C.K.; Mitchell, P.B.; McFarquhar, T.F.; Malhi, G.S. Repetitive transcranial magnetic stimulation for the treatment of obsessive compulsive disorder: A double-blind controlled investigation. Psychol. Med. 2007, 37, 1645–1649. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.I.; Kim, C.H.; Namkoong, K.; Lee, C.I.; Kim, S.J. A randomized controlled study of sequentially applied repetitive transcranial magnetic stimulation in obsessive-compulsive disorder. J. Clin. Psychiatry 2009, 70, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, C.; Locatelli, M.; Lucca, A.; Benedetti, F.; Insacco, C.; Smeraldi, E. Augmentation effect of repetitive transcranial magnetic stimulation over the orbitofrontal cortex in drug-resistant obsessive-compulsive disorder patients: A controlled investigation. Prim. Care Companion J. Clin. Psychiatry 2009, 11, 226–230. [Google Scholar] [CrossRef]
- Mantovani, A.; Simpson, H.B.; Fallon, B.A.; Rossi, S.; Lisanby, S.H. Randomized sham-controlled trial of repetitive transcranial magnetic stimulation in treatment-resistant obsessive-compulsive disorder. Int. J. Neuropsychopharmacol. 2010, 13, 217–227. [Google Scholar] [CrossRef]
- Mansur, C.G.; Myczkowki, M.L.; De Barros Cabral, S.; Sartorelli, M.D.C.B.; Bellini, B.B.; Dias, Á.M.; Bernik, M.A.; Marcolin, M.A. Placebo effect after prefrontal magnetic stimulation in the treatment of resistant obsessive-compulsive disorder: A randomized controlled trial. Int. J. Neuropsychopharmacol. 2011, 14, 1389–1397. [Google Scholar] [CrossRef]
- Gomes, P.V.O.; Brasil-Neto, J.P.; Allam, N.; de Souza, E.R. A Randomized, double- blind trial of repetitive transcranial magnetic stimulation in obsessive- compulsive disorder with three-month follow-up. J. Neuropsychiatry Clin. Neurosci. 2012, 24, 437–443. [Google Scholar] [CrossRef]
- Nauczyciel, C.; Le Jeune, F.; Naudet, F.; Douabin, S.; Esquevin, A.; Vérin, M.; Dondaine, T.; Robert, G.; Drapier, D.; Millet, B. Repetitive transcranial magnetic stimulation over the orbitofrontal cortex for obsessive-compulsive disorder: A double-blind, crossover study. Transl. Psychiatry 2014, 4, e436. [Google Scholar] [CrossRef]
- Haghighi, M.; Shayganfard, M.; Jahangard, L.; Ahmadpanah, M.; Bajoghli, H.; Pirdehghan, A.; Holsboer-Trachsler, E.; Brand, S. Repetitive Transcranial Magnetic Stimulation (rTMS) improves symptoms and reduces clinical illness in patients suffering from OCD--Results from a single-blind, randomized clinical trial with sham cross-over condition. J. Psychiatr. Res. 2015, 68, 238–244. [Google Scholar] [CrossRef]
- Seo, H.J.; Jung, Y.E.; Lim, H.K.; Um, Y.H.; Lee, C.U.; Chae, J.H. Adjunctive low-frequency repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex in patients with treatment-resistant obsessive-compulsive disorder: A randomized controlled trial. Clin. Psychopharmacol. Neurosci. 2016, 14, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Hawken, E.R.; Dilkov, D.; Kaludiev, E.; Simek, S.; Zhang, F.; Milev, R. Transcranial magnetic stimulation of the supplementary motor area in the treatment of obsessive-compulsive disorder: A multi-site study. Int. J. Mol. Sci. 2016, 17, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahangard, L.; Haghighi, M.; Shyayganfard, M.; Ahmadpanah, M.; Sadeghi Bahmani, D.; Bajoghli, H.; Holsboer-Trachsler, E.; Brand, S. Repetitive Transcranial Magnetic Stimulation Improved Symptoms of Obsessive-Compulsive Disorder, but Also Cognitive Performance: Results from a Randomized Clinical Trial with a Cross-Over Design and Sham Condition. Neuropsychobiology 2016, 73, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Pelissolo, A.; Harika-Germaneau, G.; Rachid, F.; Gaudeau-Bosma, C.; Tanguy, M.L.; BenAdhira, R.; Bouaziz, N.; Popa, T.; Wassouf, I.; Saba, G.; et al. Repetitive transcranial magnetic stimulation to supplementary motor area in refractory obsessive-compulsive disorder treatment: A sham-controlled trial. Int. J. Neuropsychopharmacol. 2016, 19, pyw025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shayganfard, M.; Jahangard, L.; Nazaribadie, M.; Haghighi, M.; Ahmadpanah, M.; Sadeghi Bahmani, D.; Bajoghli, H.; Holsboer-Trachsler, E.; Brand, S. Repetitive Transcranial Magnetic Stimulation Improved Symptoms of Obsessive-Compulsive Disorders but Not Executive Functions: Results from a Randomized Clinical Trial with Crossover Design and Sham Condition. Neuropsychobiology 2016, 74, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Carmi, L.; Alyagon, U.; Barnea-Ygael, N.; Zohar, J.; Dar, R.; Zangen, A. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018, 11, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Arumugham, S.S.; Subhasini, V.S.; Madhuri, H.N.; Vinay, B.; Ravi, M.; Sharma, E.; Thirthalli, J.; Reddy, Y.J. Augmentation Effect of Low-Frequency Repetitive Transcranial Magnetic Stimulation over Presupplementary Motor Area in Obsessive-Compulsive Disorder: A Randomized Controlled Trial. J. ECT 2018, 34, 253–257. [Google Scholar] [CrossRef]
- Carmi, L.; Tendler, A.; Bystritsky, A.; Hollander, E.; Blumberger, D.M.; Daskalakis, J.; Ward, H.; Lapidus, K.; Goodman, W.; Casuto, L.; et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: A prospective multicenter randomized double-blind placebo-controlled trial. Am. J. Psychiatry 2019, 176, 931–938. [Google Scholar] [CrossRef]
- Zıblak, A.; Tumkaya, S.; Kashyap, H. Transcraniial magnetic stimulation over orbitofrontal cortex in obsessive compulsive disorder: A double-blind placebo-controlled trial. J. Obs.-Compuls. Relat. Disord. 2021, 31, 100687. [Google Scholar] [CrossRef]
- Harika-Germaneau, G.; Rachid, F.; Chatard, A.; Lafay-Chebassier, C.; Solinas, M.; Thirioux, B.; Millet, B.; Langbour, N.; Jaafari, N. Continuous theta burst stimulation over the supplementary motor area in refractory obsessive-compulsive disorder treatment: A randomized sham-controlled trial. Brain Stimul. 2019, 12, 1565–1571. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Shao, H.; Liao, J.; Yang, D.; Ma, M.; Yang, J. Continuous theta-burst stimulation over the right orbitofrontal cortex in treatment-resistant obsessive-compulsive disorder treatment: A randomized sham-controlled trial. Int. J. Gen. Med. 2021, 14, 3109–3118. [Google Scholar] [CrossRef]
- Dutta, P.; Dhyani, M.; Garg, S.; Tikka, S.K.; Khattri, S.; Mehta, S.; Mishra, J. Efficacy of intensive orbitofrontal continuous Theta Burst Stimulation (iOFcTBS) in Obsessive Compulsive Disorder: A Randomized Placebo Controlled Study. Psychiatry Res. 2021, 298, 113784. [Google Scholar] [CrossRef] [PubMed]
- Bation, R.; Mondino, M.; Le Camus, F.; Saoud, M.; Brunelin, J. Transcranial direct current stimulation in patients with obsessive compulsive disorder: A randomized controlled trial. Eur. Psychiatry 2019, 62, 38–44. [Google Scholar] [CrossRef]
- Gowda, S.M.; Narayanaswamy, J.C.; Hazari, N.; Bose, A.; Chhabra, H.; Balachander, S.; Bhaskarapillai, B.; Shivakumar, V.; Venkatasubramanian, G.; Reddy, Y.C.J. Efficacy of pre-supplementary motor area transcranial direct current stimulation for treatment resistant obsessive compulsive disorder: A randomized, double blinded, sham controlled trial. Brain Stimul. 2019, 12, 922–929. [Google Scholar] [CrossRef]
- Silva, R.M.F.; Brunoni, A.R.; Goerigk, S.; Batistuzzo, M.C.; Costa, D.L.C.; Diniz, J.B.; Padberg, F.; D’Urso, G.; Miguel, E.C.; Shavitt, R.G. Efficacy and safety of transcranial direct current stimulation as an add-on treatment for obsessive-compulsive disorder: A randomized, sham-controlled trial. Neuropsychopharmacology 2021, 46, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Rawji, V.; Ciocca, M.; Zacharia, A.; Soares, D.; Truong, D.; Bikson, M.; Rothwell, J.; Bestmann, S. tDCS changes in motor excitability are specific to orientation of current flow. Brain Stimul. 2018, 11, 289–298. [Google Scholar] [CrossRef]
- Perera, M.P.N.; Mallawaarachchi, S.; Miljevic, A.; Bailey, N.W.; Herring, S.E.; Fitzgerald, P.B. Repetitive Transcranial Magnetic Stimulation for Obsessive-Compulsive Disorder: A Meta-analysis of Randomized, Sham-Controlled Trials. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.-D.; Wang, W.; Wang, G.-M.; Li, D.-Q.; Kuang, L. An updated meta-analysis: Short-term therapeutic effects of repeated transcranial magnetic stimulation in treating obsessive-compulsive disorder. J. Affect. Disord. 2017, 215, 187–196. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef]
- Bation, R.; Poulet, E.; Haesebaert, F.; Saoud, M.; Brunelin, J. Transcranial direct current stimulation in treatment-resistant obsessive-compulsive disorder: An open-label pilot study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 65, 153–157. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, N.; Verma, R. Safety and efficacy of adjunctive transcranial direct current stimulation in treatment-resistant obsessive-compulsive disorder: An open-label trial. Indian J. Psychiatry 2019, 61, 327–334. [Google Scholar] [CrossRef]
- Harika-Germaneau, G.; Heit, D.; Chatard, A.; Thirioux, B.; Langbour, N.; Jaafari, N. Treating refractory obsessive-compulsive disorder with transcranial direct current stimulation: An open label study. Brain Behav. 2020, 10, e01648. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, P.; Tang, Y.; Zhang, C.; Lin, L.; Gao, J.; Wang, Z. Transcranial direct current stimulation improve symptoms and modulates cortical inhibition in obsessive-compulsive disorder: A TMS-EEG study. J. Affect. Disord. 2022, 298, 558–564. [Google Scholar] [CrossRef]
- Thamby, A.; Seshachala, K.; Sharma, L.; Thimmashetty, V.H.; Balachander, S.; Shivakumar, V.; Hazari, N.; Chhabra, H.; Arumugham, S.S.; Ts, J.; et al. Transcranial direct current stimulation for treatment-resistant obsessive-compulsive disorder-A large case series. Asian J. Psychiatr. 2021, 60, 102625. [Google Scholar] [CrossRef] [PubMed]
- Hamada, M.; Hanajima, R.; Terao, Y.; Arai, N.; Furubayashi, T.; Inomata-Terada, S.; Yugeta, A.; Matsumoto, H.; Shirota, Y.; Ugawa, Y. Quadro-pulse stimulation is more effective than paired-pulse stimulation for plasticity induction of the human motor cortex. Clin. Neurophysiol. 2007, 118, 2672–2682. [Google Scholar] [CrossRef] [PubMed]
- Chistyakov, A.V.; Kreinin, B.; Marmor, S.; Kaplan, B.; Khatib, A.; Darawsheh, N.; Koren, D.; Zaaroor, M.; Klein, E. Preliminary assessment of the therapeutic efficacy of continuous theta-burst magnetic stimulation (cTBS) in major depression: A double-blind sham-controlled study. J. Affect. Disord. 2015, 170, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Picó-Pérez, M.; Moreira, P.S.; de Melo Ferreira, V.; Radua, J.; Mataix-Cols, D.; Sousa, N.; Soriano-Mas, C.; Morgado, P. Modality-specific overlaps in brain structure and function in obsessive-compulsive disorder: Multimodal meta-analysis of case-control MRI studies. Neurosci. Biobehav. Rev. 2020, 112, 83–94. [Google Scholar] [CrossRef]
- Loo, C.K.; Sachdev, P.S.; Haindl, W.; Wen, W.; Mitchell, P.B.; Croker, V.M.; Malhi, G.S. High (15 Hz) and low (1 Hz) frequency transcranial magnetic stimulation have different acute effects on regional cerebral blood flow in depressed patients. Psychol. Med. 2003, 33, 997–1006. [Google Scholar] [CrossRef]
- George, M.S.; Stallings, L.E.; Speer, A.M.; Nahas, Z.; Spicer, K.M.; Vincent, D.J.; Bohning, D.E.; Cheng, K.T.; Molloy, M.; Teneback, C.C. Prefrontal repetitive transcranial magnetic stimulation (rTMS) changes relative perfusion locally and remotely. Hum. Psychopharmacol. Clin. Exp. 1999, 14, 161–170. [Google Scholar] [CrossRef]
- De Wit, S.J.; de Vries, F.E.; van der Werf, Y.D.; Cath, D.C.; Heslenfeld, D.J.; Veltman, E.M.; van Balkom, A.J.; Veltman, D.J.; van den Heuvel, O.A. Presupplementary motor area hyperactivity during response inhibition: A candidate endophenotype of obsessive-compulsive disorder. Am. J. Psychiatry 2012, 169, 1100–1108. [Google Scholar] [CrossRef]
- Norman, L.J.; Taylor, S.F.; Liu, Y.; Radua, J.; Chye, Y.; De Wit, S.J.; Huyser, C.; Karahanoglu, F.I.; Luks, T.; Manoach, D.; et al. Error Processing and Inhibitory Control in Obsessive-Compulsive Disorder: A Meta-analysis Using Statistical Parametric Maps. Biol. Psychiatry 2019, 85, 713–725. [Google Scholar] [CrossRef] [Green Version]
- Karas, P.J.; Lee, S.; Jimenez-Shahed, J.; Goodman, W.K.; Viswanathan, A.; Sheth, S.A. Deep Brain Stimulation for Obsessive Compulsive Disorder: Evolution of Surgical Stimulation Target Parallels Changing Model of Dysfunctional Brain Circuits. Front. Neurosci. 2018, 12, 998. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wu, W.; Lin, Y.; Wang, J.; Zhou, D.; Guo, J.; Gu, S.; He, M.; Ahmed, S.; Hu, J.; et al. Localization of cerebral functional deficits in patients with obsessive-compulsive disorder: A resting-state fMRI study. J. Affect. Disord. 2012, 138, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Nakao, T.; Nakagawa, A.; Yoshiura, T.; Nakatani, E.; Nabeyama, M.; Yoshizato, C.; Kudoh, A.; Tada, K.; Yoshioka, K.; Kawamoto, M.; et al. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: A functional magnetic resonance imaging study. Biol. Psychiatry 2005, 57, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Maltby, N.; Tolin, D.F.; Worhunsky, P.; O’Keefe, T.M.; Kiehl, K.A. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: An event-related fMRI study. Neuroimage 2005, 24, 495–503. [Google Scholar] [CrossRef]
- Pujol, J.; Soriano-Mas, C.; Alonso, P.; Cardoner, N.; Menchón, J.M.; Deus, J.; Vallejo, J. Mapping structural brain alterations in obsessive-compulsive disorder. Arch. Gen. Psychiatry 2004, 61, 720–730. [Google Scholar] [CrossRef] [Green Version]
- Beucke, J.C.; Sepulcre, J.; Talukdar, T.; Linnman, C.; Zschenderlein, K.; Endrass, T.; Kaufmann, C.; Kathmann, N. Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA Psychiatry 2013, 70, 619–629. [Google Scholar] [CrossRef] [Green Version]
- Barnwell-Menard, J.L.; Li, Q.; Cohen, A.A. Effects of categorization method, regression type, and variable distribution on the inflation of Type-I error rate when categorizing a confounding variable. Stat. Med. 2015, 34, 936–949. [Google Scholar] [CrossRef]
- Purgato, M.; Barbui, C. Dichotomizing rating scale scores in psychiatry: A bad idea? Epidemiol. Psychiatr. Sci. 2013, 22, 17–19. [Google Scholar] [CrossRef] [Green Version]
| Study | Active | Sham | Sessions | Trial Duration | Parameters | Response’s Definition | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Age | N | Age | Location | Frequency (Hz) | % rMT | Pulses per Session | ||||
| rTMS (n = 18) | |||||||||||
| Prasko et al., 2006 [23] | 18 | 28.9 (7.7) | 12 | 33.4 (8.7) | 10 | 2 weeks | L-DLPFC | 1 | 110 | 1800 | NA |
| Sachdev et al., 2007 [24] | 10 | 29.5 (9.9) | 8 | 35.8 (8.2) | 10 | 2 weeks | L-DLPFC | 10 | 110 | 1500 | 40% |
| Kang et al., 2009 [25] | 10 | 28.6 (12.7) | 10 | 26.2 (10.5) | 10 | 2 weeks | R-DLPFC/SMA | 1 | 110/100 | 1200 | 25% |
| Ruffini et al., 2009 [26] | 16 | NA | 7 | NA | 15 | 3 weeks | L-OFC | 1 | 80 | NA | 25% |
| Mantovani et al., 2010 [27] | 9 | 39.7 (8.6) | 9 | 39.4 (10.2) | 20 | 4 weeks | SMA | 1 | 100 | 1200 | 25% |
| Mansur et al., 2011 [28] | 13 | 42.1 (11.9) | 14 | 39.3 (13.9) | 30 | 6 weeks | R-DLPFC | 10 | 110 | 2000 | 30% |
| Gomes et al., 2012 [29] | 12 | 35.5 (7.5) | 10 | 37.5 (5.7) | 10 | 2 weeks | SMA | 1 | 100 | 1200 | 25% |
| Nauczyciel et al., 2014 [30] | 10 | 40.0 (NA) | 9 | 39.0 (NA) | 10 | 1 week | R-OFC | 1 | 120 | 1200 | NA |
| Haghighi et al., 2015 [31] | 10 | 34.9 (5.9) | 11 | 36.6 (4.0) | 10 | 2 weeks | L-DLPFC | 20 | 100 | 750 | 35% |
| Seo et al., 2016 [32] | 14 | 34.6 (9.8) | 13 | 36.3 (12.5) | 15 | 3 weeks | R-DLPFC | 1 | 100 | 1200 | 25% |
| Hawken et al., 2016 [33] | 10 | 33.0 (10.0) | 12 | 34.0 (14.0) | 25 | 6 weeks | SMA | 1 | 110 | NA | 25% |
| Jahangard et al., 2016 [34] | 5 | 32.4 (9.0) | 5 | 33.8 (5.8) | 10 | 2 weeks | B-DLPFC | 20 | 100 | 750 | NA |
| Pelissolo et al., 2016 [35] | 20 | 39.1 (10.4) | 16 | 42.3 (10.6) | 20 | 4 weeks | SMA | 1 | 100 | 1500 | 25% |
| Shayganfard et al., 2016 [36] | 5 | 33.8 (9.6) | 5 | 33.2 (7.9) | 10 | 2 weeks | B-DLPFC | 20 | 100 | 750 | NA |
| Carmi et al., 2018 [37] | 16 | 36.0 (NA) | 14 | 35.0 (NA) | 25 | 5 weeks | mPFC | 20 | 100 | 2000 | 30% |
| Arumugham et al., 2018 [38] | 19 | 27.7 (7.9) | 17 | 30.7 (10.4) | 18 | 3 weeks | SMA | 1 | 100 | 1200 | 35% |
| Carmi et al., 2019 [39] | 47 | 41.1 (12.0) | 47 | 36.5 (11.4) | 29 | 6 weeks | ACC/mPFC | 20 | 100 | 2000 | 30% |
| Ziblak et al., 2021 [40] | 19 | 41.5 (10.2) | 15 | 36.5 (13.7) | 20 | 2 weeks | R-OFC | 1 | 110 | 1000 | 35% |
| TBS (n = 3) | |||||||||||
| Harika-Germaneau et al., 2019 [41] | 14 | 46.3 (10.1) | 14 | 48.2 (12.9) | 30 | 6 weeks | SMA | 50 (cTBS) | 70 | 600 | 25% |
| Liu et al., 2021 [42] | 12 | 28.2 (9.8) | 11 | 31.0 (7.5) | 10 | 2 weeks | R-OFC | 50 (cTBS) | 80 | 600 | 25% |
| Dutta et al., 2021 [43] | 18 | 30.5 (12.4) | 15 | 28.3 (7.4) | 10 | 1 week | L-OFC | 50 (cTBS) | 80 | 600 | 35% |
| tDCS Study (n = 3) | Active | Sham | Sessions | Trial Duration | Parameters | Response’s Definition | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Age | N | Age | Anode Location | Cathode Location | Current | Session Duration | ||||
| Bation et al., 2019 [44] | 10 | 44.8 (19.9) | 11 | 41.2 (11.9) | 10 | 1 week | cerebellum | OFC | 2 mA | 20 min | 35% |
| Gowda et al., 2019 [45] | 12 | 30.83 (5.9) | 13 | 25.9 (5.2) | 10 | 1 week | SMA | supra-orbital area | 2 mA | 20 min | 35% |
| Silva et al., 2021 [46] | 22 | 38.4 (11.0) | 21 | 36.9 (12.2) | 20 | 4 weeks | deltoid | SMA | 2 mA | 30 min | 35% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Fang, Y. Efficacy of Non-Invasive Brain Stimulation for Refractory Obsessive-Compulsive Disorder: A Meta-Analysis of Randomized Controlled Trials. Brain Sci. 2022, 12, 943. https://doi.org/10.3390/brainsci12070943
Zhou S, Fang Y. Efficacy of Non-Invasive Brain Stimulation for Refractory Obsessive-Compulsive Disorder: A Meta-Analysis of Randomized Controlled Trials. Brain Sciences. 2022; 12(7):943. https://doi.org/10.3390/brainsci12070943
Chicago/Turabian StyleZhou, Shu, and Yan Fang. 2022. "Efficacy of Non-Invasive Brain Stimulation for Refractory Obsessive-Compulsive Disorder: A Meta-Analysis of Randomized Controlled Trials" Brain Sciences 12, no. 7: 943. https://doi.org/10.3390/brainsci12070943
APA StyleZhou, S., & Fang, Y. (2022). Efficacy of Non-Invasive Brain Stimulation for Refractory Obsessive-Compulsive Disorder: A Meta-Analysis of Randomized Controlled Trials. Brain Sciences, 12(7), 943. https://doi.org/10.3390/brainsci12070943






