Real-World Implementation of Precision Psychiatry: A Systematic Review of Barriers and Facilitators
Abstract
1. Introduction
Aims
2. METHODS
2.1. Search Strategy and Selection Criteria
2.2. Level of Evidence
2.3. Data Extraction and Analysis
- ▪
- Characteristics of the model: This construct addresses logistical and practical features of the model which may impact upon implementation, as well as more conceptual components of the model and the corresponding strength, accuracy and transparency of the evidence upon which the model is based.
- ▪
- Inner setting: Whilst the inner and outer setting constructs are closely linked and are considered inter-dependent in many respects, this construct refers largely to features of local infrastructure within which the model will be implemented.
- ▪
- Outer setting: Closely intertwined with the inner setting, the outer setting largely takes into consideration the wider system and the external organizations who exist outside of the inner setting, and as such this construct typically addresses the economic, social, cultural and political contexts within which the model is being implemented.
- ▪
- Characteristics of the individuals: This construct considers the individuals involved in the implementation process at the ground-level, including both those involved in the delivery of clinical care and those in receipt of this care. As such, in this current study, we divided this construct into two independent groups of stakeholders due to their unique needs and perspectives: (i) health and social care staff involved in the delivery of care and (ii) service users and their families/caregivers. These constructs address the attitudes, opinions, previous experiences, skills/knowledge, concerns, needs and potential impact of precision psychiatry models on these key stakeholder groups.
- ▪
- Implementation process: Finally, this process construct relates to factors which may affect the actual procedure and operations of implementation, including uptake of and adherence to the process.
3. Results
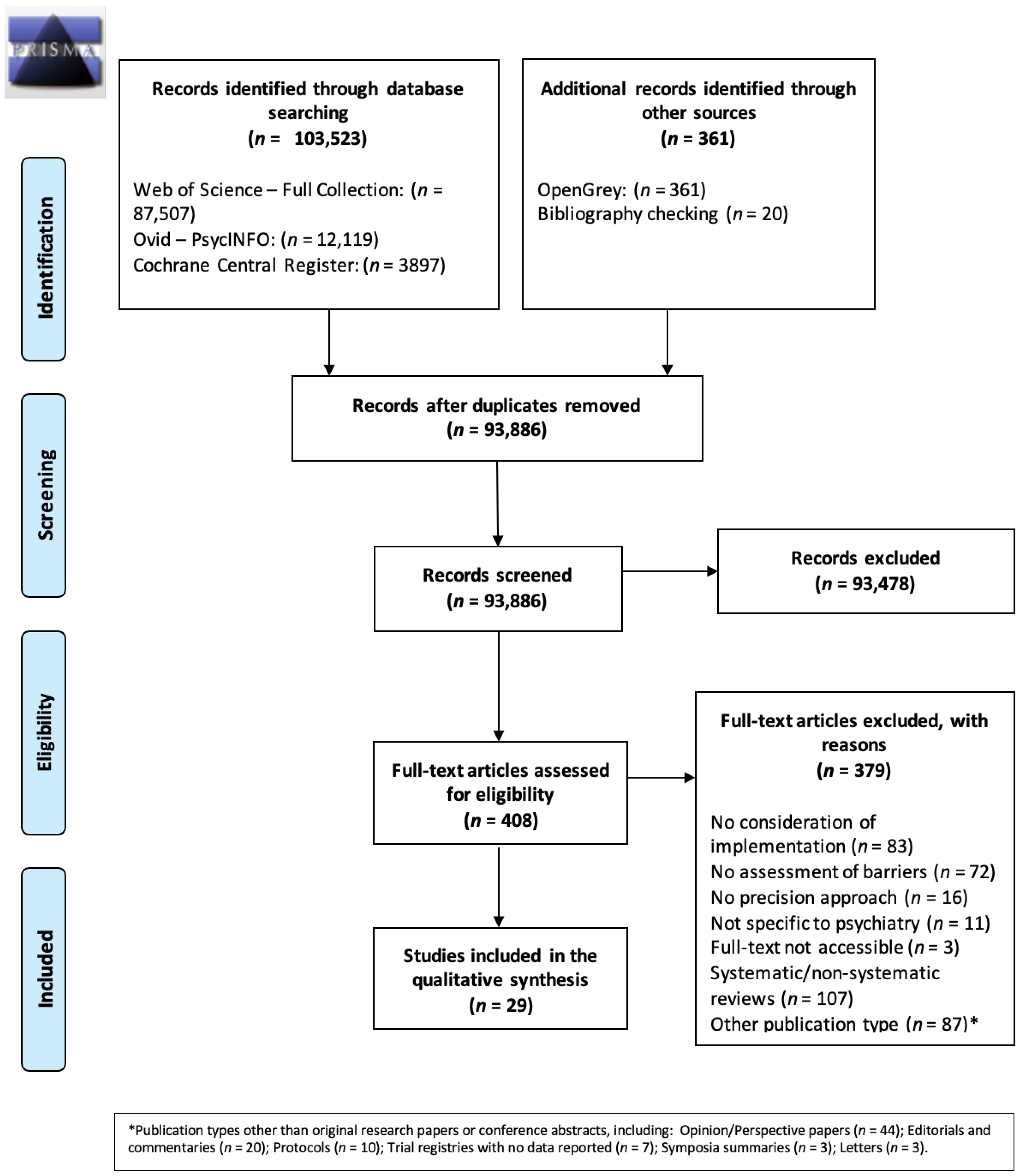
3.1. Literature Search
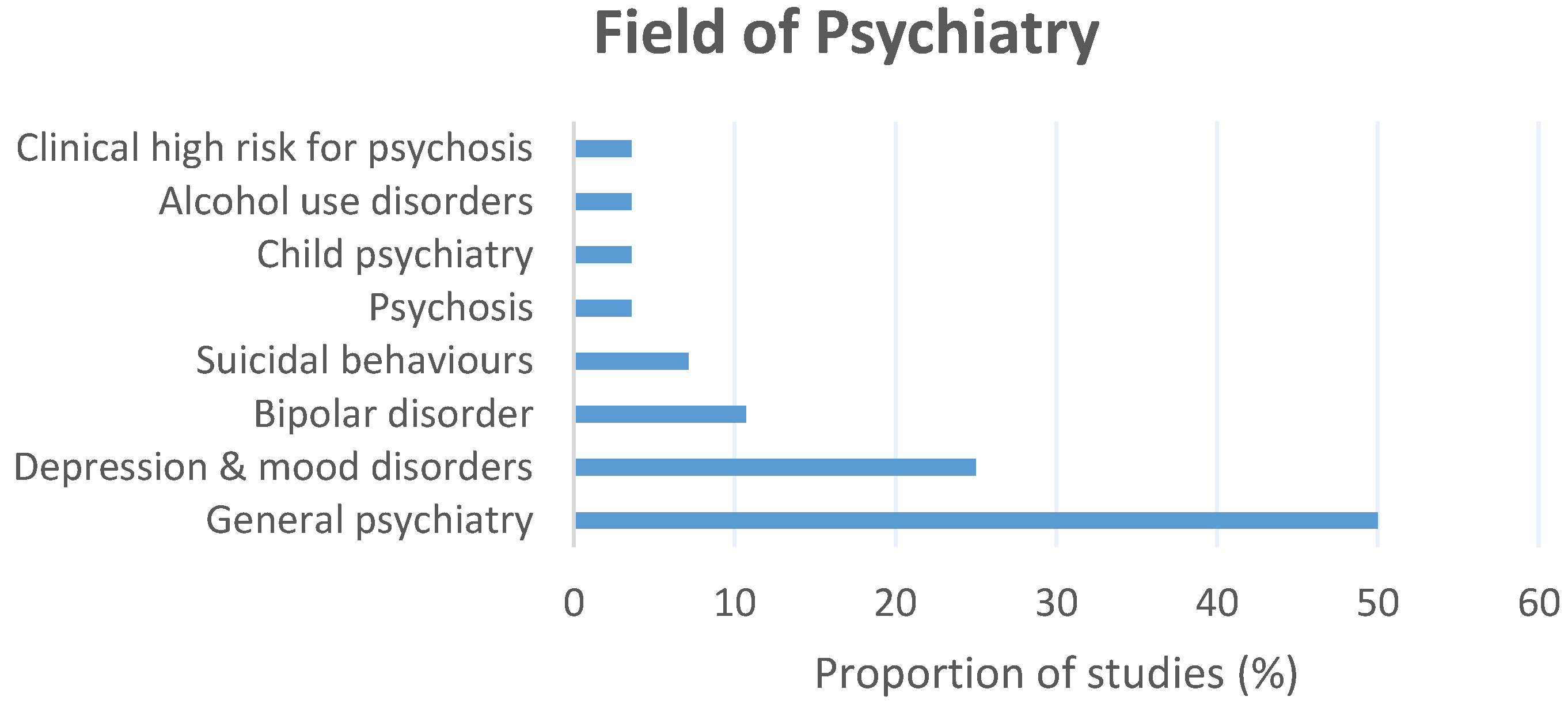
3.2. Description of the Included Studies
3.3. Level of Evidence Summary
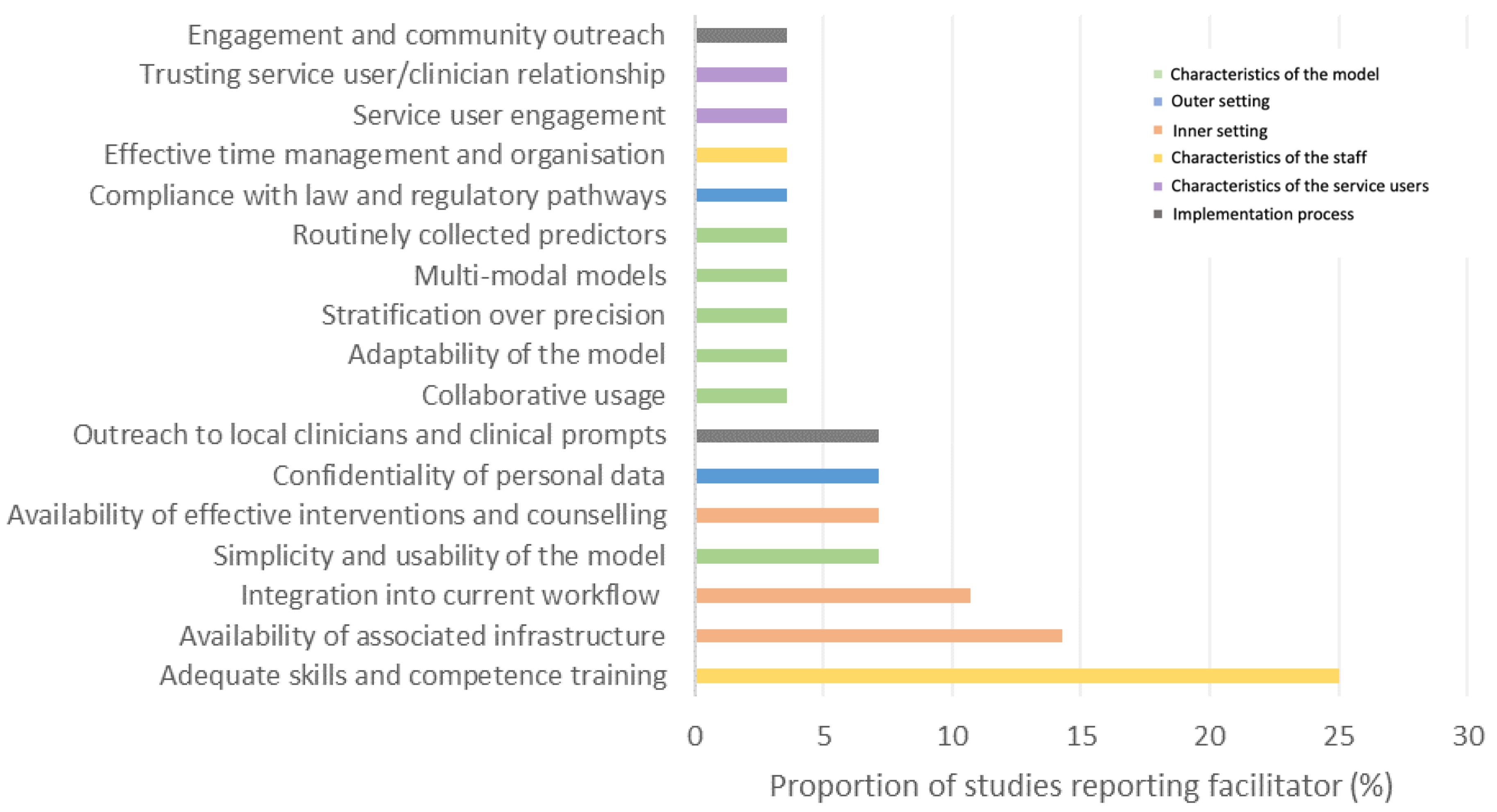
3.4. Factors Affecting the Implementation of Precision Psychiatry
3.4.1. Characteristics of the Model
Barriers
- Cost and time investments
- 2.
- Poor accuracy and utility of the model
- 3.
- Poor perceived relative advantage of the model
- 4.
- Poor transparency and complexity of the model
- 5.
- Lack of clear guidelines
Facilitators
- 6.
- Simplicity and usability of the model
- 7.
- Collaborative usage
- 8.
- Adaptability of the model
- 9.
- Stratification over precision
- 10.
- Multi-modal models
- 11.
- Routinely collected predictors
3.4.2. Inner Setting
Barriers
- 12.
- Lack of clinical resources
- 13.
- Lack of effective interventions
Facilitators
- 14.
- Availability of associated infrastructure
- 15.
- Integration into current workflow
- 16.
- Availability of effective interventions and counselling
3.4.3. Outer Setting
Barriers
- 17.
- Potential misuse of personal data
- 18.
- Ethics of risk communication
Facilitators
- 19.
- Confidentiality of personal data
- 20.
- Compliance with law and regulatory pathways
3.4.4. Characteristics of the Individuals—Staff
Barriers
- 21.
- Negative staff perceptions of precision medicine
- 22.
- Poor perceived competence in precision medicine
- 23.
- Poor previous experience
- 24.
- Lack of motivation to address mental health in primary care
Facilitators
- 25.
- Adequate skills and competence training
- 26.
- Effective time management and organization
3.4.5. Characteristics of the Individuals—Service Users and Families/Caregivers
Barriers
- 27.
- Potential psychological harm
- 28.
- Potential economic and occupational harm
- 29.
- Potential stigmatization
- 30.
- Skepticism regarding genetics
- 31.
- Negative attitudes towards psychiatry
- 32.
- Resistance to knowing risk scores
- 33.
- Fear of invasive procedures
- 34.
- Weak demand and engagement
Facilitators
- 35.
- Service user engagement
- 36.
- Trusting service user/clinician relationship
3.4.6. Implementation Process
Facilitators
- 37.
- Outreach to local clinicians and clinical prompts
- 38.
- Engagement and community outreach
4. Discussion
4.1. Key Findings
4.2. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hodson, R. Precision medicine. Nature 2016, 537, S49. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Hijazi, Z.; Stahl, D.; Steyerberg, E.W. The Science of Prognosis in Psychiatry. JAMA Psychiatry 2018, 75, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.S.; Williams, L.M.; Steiner, J.; Leboyer, M.; Carvalho, A.F.; Berk, M. The new field of ‘precision psychiatry’. BMC Med. 2017, 15, 80. [Google Scholar] [CrossRef]
- Perna, G.; Grassi, M.; Caldirola, D.; Nemeroff, C.B. The revolution of personalized psychiatry: Will technology make it happen sooner? Psychol. Med. 2018, 48, 705–713. [Google Scholar] [CrossRef]
- Zanardi, R.; Prestifilippo, D.; Fabbri, C.; Colombo, C.; Maron, E.; Serretti, A. Precision psychiatry in clinical practice. Int. J. Psychiatry Clin. Pract. 2020, 25, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Salazar de Pablo, G.S.; Studerus, E.; Vaquerizo-Serrano, J.; Irving, J.; Catalan, A.; Oliver, D.; Baldwin, H.; Danese, A.; Fazel, S.; Steyerberg, E.W.; et al. Implementing Precision Psychiatry: A Systematic Review of Individualized Prediction Models for Clinical Practice. Schizophr. Bull. 2021, 47, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Domchek, S.M.; Eisen, A.; Calzone, K.; Stopfer, J.; Blackwood, A.; Weber, B.L. Application of Breast Cancer Risk Prediction Models in Clinical Practice. J. Clin. Oncol. 2003, 21, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Wilson, P.W.F.; Larson, M.G.; Beiser, A.; Leip, E.P.; D’Agostino, R.B.; Levy, D. Framingham risk score and prediction of lifetime risk for coronary heart disease. Am. J. Cardiol. 2004, 94, 20–24. [Google Scholar] [CrossRef]
- van Os, J. Personalised psychiatry: No substitute for personal care. Tijdschr Voor Psychiatr. 2018, 60, 199–204. [Google Scholar]
- van Os, J.; Kohne, A.C.J. It is not enough to sing its praises: The very foundations of precision psychiatry may be scientifically unsound and require examination. Psychol. Med. 2021, 51, 1415–1417. [Google Scholar] [CrossRef]
- Köhne, A.C.J.; van Os, J. Precision psychiatry: Promise for the future or rehash of a fossilised foundation? Psychol. Med. 2021, 51, 1409–1411. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, D.C. Barriers and Research Priorities for Implementing Precision Medicine. Pancreas 2019, 48, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Pettitt, D.; Smith, J.; Meadows, N.; Arshad, Z.; Schuh, A.; DiGiusto, D.; Bountra, C.; Holländer, G.; Barker, R.; Brindley, D. Regulatory barriers to the advancement of precision medicine. Expert Rev. Precis. Med. Drug Dev. 2016, 1, 319–329. [Google Scholar] [CrossRef]
- Holden, C.; Bignell, L.; Mukhopadhyay, S.; Jones, C. The public perception of the facilitators and barriers to implementing personalized medicine: A systematic review. Pers. Med. 2019, 16, 409–420. [Google Scholar] [CrossRef]
- Wright, S.J.; Daker-White, G.; Newman, W.; Payne, K. Understanding barriers to the introduction of precision medicine in non-small cell lung cancer: A qualitative interview study. Wellcome Open Res. 2021, 6, 25. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Zhou, B. Barriers and Solutions in Clinical Implementation of Pharmacogenomics for Personalized Medicine. In Pharmacogenomics in Precision Medicine; Cai, W., Liu, Z., Miao, L., Xiang, X., Eds.; Springer: Singapore, 2020; pp. 277–289. [Google Scholar] [CrossRef]
- Chanfreau-Coffinier, C.; Peredo, J.; Russell, M.M.; Yano, E.M.; Hamilton, A.B.; Lerner, B.; Provenzale, D.; Knight, S.J.; Voils, C.I.; Scheuner, M.T. A logic model for precision medicine implementation informed by stakeholder views and implementation science. Genet. Med. 2019, 21, 1139–1154. [Google Scholar] [CrossRef]
- Bzdok, D.; Meyer-Lindenberg, A. Machine Learning for Precision Psychiatry: Opportunities and Challenges. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 2018, 3, 223–230. [Google Scholar] [CrossRef]
- Arango, C.; Dragioti, E.; Solmi, M.; Cortese, S.; Domschke, K.; Murray, R.M.; Jones, P.B.; Uher, R.; Carvalho, A.F.; Reichenberg, A.; et al. Risk and protective factors for mental disorders beyond genetics: An evidence-based atlas. World Psychiatry 2021, 20, 417–436. [Google Scholar] [CrossRef]
- Ball, T.M.; Kalinowski, A.; Williams, L.M. Ethical implementation of precision psychiatry. Pers. Med. Psychiatry 2020, 19–20, 100046. [Google Scholar] [CrossRef]
- Manchia, M.; Pisanu, C.; Squassina, A.; Carpiniello, B. Challenges and Future Prospects of Precision Medicine in Psychiatry. Pharm. Pers. Med. 2020, 13, 127–140. [Google Scholar] [CrossRef]
- Aref-Adib, G.; McCloud, T.; Ross, J.; O’Hanlon, P.; Appleton, V.; Rowe, S.; Murray, E.; Johnson, S.; Lobban, F. Factors affecting implementation of digital health interventions for people with psychosis or bipolar disorder, and their family and friends: A systematic review. Lancet Psychiatry 2019, 6, 257–266. [Google Scholar] [CrossRef]
- Damschroder, L.J.; Aron, D.C.; Keith, R.E.; Kirsh, S.R.; Alexander, J.A.; Lowery, J.C. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement. Sci. 2009, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Mathews, C.A. 21.2 Integrating Pharmacogenetic Testing Into a Child Psychiatry Clinic. J. Am. Acad. Child Adolesc. Psychiatry 2018, 57, S301. [Google Scholar] [CrossRef]
- Oliver, D.; Spada, G.; Colling, C.; Broadbent, M.; Baldwin, H.; Patel, R.; Stewart, R.; Stahl, D.; Dobson, R.; McGuire, P.; et al. Real-world implementation of precision psychiatry: Transdiagnostic risk calculator for the automatic detection of individuals at-risk of psychosis. Schizophr. Res. 2020, 227, 52–60. [Google Scholar] [CrossRef]
- Reger, G.M.; McClure, M.L.; Ruskin, D.; Bricker-Carter, S.P.; Reger, M.A. Integrating Predictive Modeling Into Mental Health Care: An Example in Suicide Prevention. Psychiatr. Serv. 2019, 70, 71–74. [Google Scholar] [CrossRef]
- Dunbar, L.; Butler, R.; Wheeler, A.; Pulford, J.; Miles, W.; Sheridan, J. Clinician experiences of employing the AmpliChip? CYP450 test in routine psychiatric practice. J. Psychopharmacol. 2012, 26, 390–397. [Google Scholar] [CrossRef]
- Hoop, J.G.; Lapid, M.I.; Paulson, R.M.; Roberts, L.W. Clinical and Ethical Considerations in Pharmacogenetic Testing: Views of Physicians in 3 “Early Adopting” Departments of Psychiatry. J. Clin. Psychiatry 2010, 71, 745–753. [Google Scholar] [CrossRef]
- Moreno-Peral, P.; Conejo-Cerón, S.; Fernández, A.; Martín-Pérez, C.; Fernández-Alonso, C.; Rodríguez-Bayón, A.; Ballesta-Rodríguez, M.I.; Aiarzagüena, J.M.; Montón-Franco, C.; King, M.; et al. Family physicians’ views on participating in prevention of major depression. The predictD-EVAL qualitative study. PLoS ONE 2019, 14, e0217621. [Google Scholar] [CrossRef]
- Walden, L.M.; Brandl, E.J.; Changasi, A.; Sturgess, J.E.; Soibel, A.; Notario, J.F.D.; Cheema, S.; Braganza, N.; Marshe, V.S.; Freeman, N.; et al. Physicians’ opinions following pharmacogenetic testing for psychotropic medication. Psychiatry Res. 2015, 229, 913–918. [Google Scholar] [CrossRef]
- Bellón, J.Á.; Moreno-Peral, P.; Moreno-Küstner, B.; Motrico, E.; Aiarzaguena, J.M.; Fernandez, A.; Fernandez-Alonso, C.; Montón-Franco, C.; Rodríguez-Bayón, A.; Ballesta-Rodríguez, M.I.; et al. Patients’ Opinions about Knowing Their Risk for Depression and What to Do about It. The PredictD-Qualitative Study. PLoS ONE 2014, 9, e92008. [Google Scholar] [CrossRef]
- Brown, L.A.; Benhamou, K.; May, A.M.; Mu, W.; Berk, R. Machine Learning Algorithms in Suicide Prevention: Clinician Interpretations as Barriers to Implementation. J. Clin. Psychiatry 2020, 81. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.W.; Chua, B.Y.; Subramaniam, M.; Suen, E.L.K.; Lee, J. Clinicians’ perceptions of pharmacogenomics use in psychiatry. Pharmacogenomics 2017, 18, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Doraiswamy, P.M.; Blease, C.; Bodner, K. Artificial intelligence and the future of psychiatry: Insights from a global physician survey. Artif. Intell. Med. 2020, 102, 101753. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.A.; Cho, M.K. Interest, rationale, and potential clinical applications of genetic testing for mood disorders: A survey of stakeholders. J. Affect. Disord. 2013, 145, 240–245. [Google Scholar] [CrossRef][Green Version]
- Evanoff, B.; Bierut, L. Achieving The Promise of Translational Genomics In Psychiatric Care. Eur. Neuropsychopharmacol. 2017, 27, S370–S371. [Google Scholar] [CrossRef]
- Finn, C.T.; Wilcox, M.A.; Korf, B.R.; Blacker, D.; Racette, S.R.; Sklar, P.; Smoller, J.W. Psychiatric Genetics: A Survey of Psychiatrists’ Knowledge, Opinions, and Practice Patterns. J. Clin. Psychiatry 2005, 66, 821–830. [Google Scholar] [CrossRef]
- Goodspeed, A.; Kostman, N.; Kriete, T.E.; Longtine, J.W.; Smith, S.M.; Marshall, P.; Williams, W.; Clark, C.; Blakeslee, W.W. Leveraging the utility of pharmacogenomics in psychiatry through clinical decision support: A focus group study. Ann. Gen. Psychiatry 2019, 18, 13. [Google Scholar] [CrossRef]
- Henshall, C.; Marzano, L.; Smith, K.; Attenburrow, M.-J.; Puntis, S.; Zlodre, J.; Kelly, K.; Broome, M.R.; Shaw, S.; Barrera, A.; et al. A web-based clinical decision tool to support treatment decision-making in psychiatry: A pilot focus group study with clinicians, patients and carers. BMC Psychiatry 2017, 17, 265. [Google Scholar] [CrossRef]
- Hoop, J.G.; Roberts, L.W.; Hammond, K.A.G.; Cox, N.J. Psychiatrists’ attitudes, knowledge, and experience regarding genetics: A preliminary study. Genet. Med. 2008, 10, 439–449. [Google Scholar] [CrossRef]
- Illes, J.; Lombera, S.; Rosenberg, J.; Arnow, B. In the mind’s eye: Provider and patient attitudes on functional brain imaging. J. Psychiatr. Res. 2008, 43, 107–114. [Google Scholar] [CrossRef]
- Jenkins, S.; Arribas-Ayllon, M. Genetic Counselling for Psychiatric Disorders: Accounts of Psychiatric Health Professionals in the United Kingdom. J. Genet. Couns. 2016, 25, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Laegsgaard, M.M.; Mors, O. Psychiatric genetic testing: Attitudes and intentions among future users and providers. Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2008, 147, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Lucero, K.S.; Chatterjee-Shin, P.; Dermer, S.J. 137 CME on Pharmacogenomics Testing Improves Knowledge, Competence, and Confidence Related to Implementing Testing in Practice. CNS Spectrums 2020, 25, 287. [Google Scholar] [CrossRef]
- Salm, M.; Abbate, K.; Appelbaum, P.; Ottman, R.; Chung, W.; Marder, K.; Leu, C.-S.; Alcalay, R.; Goldman, J.; Curtis, A.M.; et al. Use of Genetic Tests among Neurologists and Psychiatrists: Knowledge, Attitudes, Behaviors, and Needs for Training. J. Genet. Couns. 2014, 23, 156–163. [Google Scholar] [CrossRef]
- Smith, L.B.; Sapers, B.; Reus, V.; Freimer, N.B. Attitudes towards bipolar disorder and predictive genetic testing among patients and providers. J. Med Genet. 1996, 33, 544–549. [Google Scholar] [CrossRef]
- Trippitelli, C.L.; Jamison, K.R.; Folstein, M.F.; Bartko, J.J.; DePaulo, J.R. Pilot Study on Patients’ and Spouses’ Attitudes Toward Potential Genetic Testing for Bipolar Disorder. Am. J. Psychiatry 1998, 155, 899–904. [Google Scholar] [CrossRef]
- Wachtler, C.; Coe, A.; Davidson, S.; Fletcher, S.; Mendoza, A.; Sterling, L.; Gunn, J. Development of a Mobile Clinical Prediction Tool to Estimate Future Depression Severity and Guide Treatment in Primary Care: User-Centered Design. JMIR mHealth uHealth 2018, 6, e95. [Google Scholar] [CrossRef]
- Wilde, A.; Meiser, B.; Mitchell, P.B.; Schofield, P.R. Public interest in predictive genetic testing, including direct-to-consumer testing, for susceptibility to major depression: Preliminary findings. Eur. J. Hum. Genet. 2009, 18, 47–51. [Google Scholar] [CrossRef]
- Williams, E.C.; Young, J.P.; Achtmeyer, C.E.; Hendershot, C.S. Primary Care Providers’ Interest in Using a Genetic Test to Guide Alcohol Use Disorder Treatment. J. Subst. Abuse Treat. 2016, 70, 14–20. [Google Scholar] [CrossRef]
- Zhou, Y.Z.; Wilde, A.; Meiser, B.; Mitchell, P.B.; Barlow-Stewart, K.; Schofield, P.R. Attitudes of medical genetics practitioners and psychiatrists toward communicating with patients about genetic risk for psychiatric disorders. Psychiatr. Genet. 2014, 24, 94–101. [Google Scholar] [CrossRef]
- Mittal, V.A.; Dean, D.J.; Mittal, J.; Saks, E.R. Ethical, Legal, and Clinical Considerations when Disclosing a High-Risk Syndrome for Psychosis. Bioethics 2015, 29, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Yung, A.R.; Wood, S.J.; Malla, A.; Nelson, B.; McGorry, P.; Shah, J. The reality of at risk mental state services: A response to recent criticisms. Psychol. Med. 2021, 51, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.H.; Link, B.G.; Ben-David, S.; Gill, K.E.; Girgis, R.R.; Brucato, G.; Wonpat-Borja, A.J.; Corcoran, C.M. Stigma related to labels and symptoms in individuals at clinical high-risk for psychosis. Schizophr. Res. 2015, 168, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.H.; Woodberry, K.A.; Link, B.G.; Corcoran, C.M.; Bryant, C.; Shapiro, D.I.; Downing, D.; Girgis, R.R.; Brucato, G.; Huang, D.; et al. Impact of “psychosis risk” identification: Examining predictors of how youth view themselves. Schizophr. Res. 2019, 208, 300–307. [Google Scholar] [CrossRef]
- Anglin, D.M.; Greenspoon, M.I.; Lighty, Q.; Corcoran, C.M.; Yang, L.H. Spontaneous labelling and stigma associated with clinical characteristics of peers ‘at-risk’ for psychosis. Early Interv. Psychiatry 2013, 8, 247–252. [Google Scholar] [CrossRef]
- Yang, L.H.; Anglin, D.M.; WonPat-Borja, A.J.; Opler, M.G.; Greenspoon, M.; Corcoran, C.M. Public Stigma Associated with Psychosis Risk Syndrome in a College Population: Implications for Peer Intervention. Psychiatr. Serv. 2013, 64, 284–288. [Google Scholar] [CrossRef]
- Parrish, E.M.; Kim, N.S.; Woodberry, K.A.; Friedman-Yakoobian, M. Clinical high risk for psychosis: The effects of labelling on public stigma in a undergraduate population. Early Interv. Psychiatry 2019, 13, 874–881. [Google Scholar] [CrossRef]
- Welsh, P.; Tiffin, P.A. Observations of a Small Sample of Adolescents Experiencing an At-Risk Mental State (ARMS) for Psychosis. Schizophr. Bull. 2012, 38, 215–218. [Google Scholar] [CrossRef]
- Byrne, R.; Morrison, A.P. Young people at risk of psychosis: A user-led exploration of interpersonal relationships and communication of psychological difficulties. Early Interv. Psychiatry 2010, 4, 162–168. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Correll, C.U.; Arango, C.; Berk, M.; Patel, V.; Ioannidis, J.P.A. Preventive psychiatry: A blueprint for improving the mental health of young people. World Psychiatry 2021, 20, 200–221. [Google Scholar] [CrossRef]
- Moons, K.G.M.; Wolff, R.F.; Riley, R.D.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S. PROBAST: A Tool to Assess Risk of Bias and Applicability of Prediction Model Studies: Explanation and Elaboration. Ann. Intern. Med. 2019, 170, W1–W33. [Google Scholar] [CrossRef] [PubMed]
- Montemagni, C.; Bellino, S.; Bracale, N.; Bozzatello, P.; Rocca, P. Models Predicting Psychosis in Patients with High Clinical Risk: A Systematic Review. Front. Psychiatry 2020, 11, 223. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.; Betz, L.T.; Schultze-Lutter, F.; Chisholm, K.; Haidl, T.K.; Kambeitz-Ilankovic, L.; Bertolino, A.; Borgwardt, S.; Brambilla, P.; Lencer, R.; et al. Towards clinical application of prediction models for transition to psychosis: A systematic review and external validation study in the PRONIA sample. Neurosci. Biobehav. Rev. 2021, 125, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Rubanovich, C.K.; Cheung, C.; Mandel, J.; Bloss, C.S. Physician preparedness for big genomic data: A review of genomic medicine education initiatives in the United States. Hum. Mol. Genet. 2018, 27, R250–R258. [Google Scholar] [CrossRef]
- Talwar, D.; Tseng, T.-S.; Foster, M.; Xu, L.; Chen, L.-S. Genetics/genomics education for nongenetic health professionals: A systematic literature review. Genet. Med. 2017, 19, 725–732. [Google Scholar] [CrossRef]
- Browning, M.; Bilderbeck, A.C.; Dias, R.; Dourish, C.T.; Kingslake, J.; Deckert, J.; Goodwin, G.M.; Gorwood, P.; Guo, B.; Harmer, C.J.; et al. The clinical effectiveness of using a predictive algorithm to guide antidepressant treatment in primary care (PReDicT): An open-label, randomised controlled trial. Neuropsychopharmacology 2021, 46, 1307–1314. [Google Scholar] [CrossRef]
- Murray, E.; Treweek, S.; Pope, C.; Macfarlane, A.; Ballini, L.; Dowrick, C.; Finch, T.; Kennedy, A.; Mair, F.; O’Donnell, K.; et al. Normalisation process theory: A framework for developing, evaluating and implementing complex interventions. BMC Med. 2010, 8, 63. [Google Scholar] [CrossRef]
- Jacobsen, A.D.M.; Azevedo, R.D.M.; Juty, N.; Batista, D.; Coles, S.; Cornet, R.; Courtot, M.; Crosas, M.; Dumontier, M.; Evelo, C.T.; et al. FAIR Principles: Interpretations and Implementation Considerations. Data Intell. 2020, 2, 10–29. [Google Scholar] [CrossRef]
- Glasgow, R.E.; Vogt, T.M.; Boles, S.M. Evaluating the public health impact of health promotion interventions: The RE-AIM framework. Am. J. Public Health 1999, 89, 1322–1327. [Google Scholar] [CrossRef]
- Powell, B.J.; Waltz, T.J.; Chinman, M.J.; Damschroder, L.J.; Smith, J.L.; Matthieu, M.M.; Proctor, E.K.; Kirchner, J.E. A refined compilation of implementation strategies: Results from the Expert Recommendations for Implementing Change (ERIC) project. Implement. Sci. 2015, 10, 21. [Google Scholar] [CrossRef]
- Vis, C.; Mol, M.; Kleiboer, A.; Bührmann, L.; Finch, T.; Smit, J.; Riper, H. Improving Implementation of eMental Health for Mood Disorders in Routine Practice: Systematic Review of Barriers and Facilitating Factors. JMIR Ment. Health 2018, 5, e20. [Google Scholar] [CrossRef] [PubMed]
- Vis, C.; Bührmann, L.; Riper, H.; Ossebaard, H.C. Health technology assessment frameworks for eHealth: A systematic review. Int. J. Technol. Assess. Health Care 2020, 36, 204–216. [Google Scholar] [CrossRef] [PubMed]




| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
|
| NA |
|
|
|
|
| NA |
|
|
|
|
|
|
|
|
|
|
| NA |
|
| NA |
|
| NA |
| First Author, Date | Location | Research Method | Field of Psychiatry | Type of Precision Model | Sample | Level of Evidence | Summary of Barriers | Summary of Facilitators |
|---|---|---|---|---|---|---|---|---|
| Bellón, 2014 [31] | Spain | Focus groups | Depression | Individualised risk prediction algorithm | 52 service-users | 1 | Resistance to knowledge of risk scores; Negative attitudes towards psychiatry | Adequate skills and competence training; Availability of effective interventions and counselling |
| Brown, 2020 [32] | United States | Survey | Suicidal behaviours | Individualised risk prediction algorithm | 139 health care professionals (psychologists, social workers, psychiatrists, nurses and other allied health professionals) | 1 | Poor accuracy and utility of the model; Poor transparency and complexity of the model | N/A |
| Chan, 2017 [33] | Singapore | Survey | General psychiatry | Pharmaco-genomics | 167 doctors and 27 pharmacists (n = 194) | 1 | Poor perceived competence in precision medicine; Negative staff perceptions of precision medicine; Cost and time investments; Lack of clear guidelines; Potential psychological harm; Potential economic and occupational harm | Availability of associated infrastructure |
| Doraiswamy, 2020 [34] | North America, South America, Europe and Asia-Pacific | Survey | General psychiatry | General AI/ML applications | 791 psychiatrists | 1 | Poor perceived relative advantage of the model; Negative staff perceptions of precision medicine | N/A |
| Dunbar, 2012 [27] † | New Zealand | Surveys and interviews | General psychiatry | Pharmaco-genomics | 33 senior medical officers and registrars | 1 | Lack of clinical resources; Poor perceived competence in precision medicine; Poor perceived relative advantage of the model; Cost and time investments; Potential psychological harm | N/A |
| Erickson, 2013 [35] | United States | Survey | Mood disorders | Genetic testing | 147 service users, caregivers and community members, and mental health professionals | 1 | Negative staff perceptions of precision medicine; Scepticism in genetics | Availability of associated infrastructure |
| Evanoff, 2016 [36] * | United States | Stakeholder meetings | General psychiatry | Genomics | Health care professionals, research scientists, and community members (n = unspecified) | 1 | Poor accuracy and utility of the model | Engagement and community outreach |
| Finn, 2005 [37] | United States | Survey | General psychiatry | Genetic testing | 844 psychiatrists or psychiatrists-in-training | 1 | Potential stigmatisation; Potential economic and occupational harm; Lack of clinical resources; Poor perceived competence in precision medicine | N/A |
| Goodspeed, 2019 [38] | United States | Focus groups and prototype development | General psychiatry | Pharmaco-genomics integrated into a clinical decision support system | 16 mental health clinicians | 1 | Poor perceived relative advantage of the model; Poor previous experience; Cost and time investments | Integration into current workflow |
| Henshall, 2017 [39] | United Kingdom | Focus groups and prototype feedback | General psychiatry | Clinical decision support system | 12 consultant psychiatrists, 11 primary care practitioners and 8 patients/carers (n = 31) | 1 | Poor perceived relative advantage of the model; Potential psychological harm; Poor transparency and complexity of the model; Cost and time investments; Poor accuracy and utility of the model | Collaborative usage; Trusting service user/clinician relationship; Multi-modal models; Simplicity and usability of the model |
| Hoop, 2008 [40] | United States | Survey | General psychiatry | Genetic testing | 45 psychiatrists | 1 | Lack of clinical resources; Poor perceived competence in precision medicine | Availability of associated infrastructure |
| Hoop, 2010 [28] † | United States | Survey | General psychiatry | Pharmaco-genomics | 75 psychiatry attending physicians and residents | 1 | Potential psychological harm; Potential economic and occupational harm; Poor accuracy and utility of the model; Cost and time investments; Lack of clinical resources; Negative staff perceptions of precision medicine | Confidentiality of personal data; Adequate skills and competence training; Availability of effective interventions and counselling |
| Illes, 2008 [41] | United States | Survey | Major depression | Functional brain imaging | 52 psychiatrists or psychologists, and 72 inpatient and outpatient service users (n = 124) | 1 | Cost and time investments; Potential psychological harm; Potential economic and occupational harm | N/A |
| Jenkins, 2016 [42] | United Kingdom | Face-to-face and telephone interviews | General psychiatry | Genetic testing | 9 psychiatric staff nurses and consultant psychiatrists | 1 | Poor transparency and complexity of the model; Poor accuracy and utility of the model; Poor perceived competence in precision medicine; Weak demand and engagement; Potential psychological harm; Potential stigmatisation | Availability of associated infrastructure; Adequate skills and competence training |
| Laegsgaard, 2008 [43] | Denmark | Questionnaire | General psychiatry | Genetic testing | 681 patients and relatives | 1 | Potential misuse of personal data; Scepticism in genetics | Confidentiality of personal data |
| Lucero, 2020 [44] * | United States | Survey | General psychiatry | Pharmaco-genomics | 830 psychiatrists | 1 | N/A | Adequate skills and competence training |
| Mathews, 2018 [24] *,† | United States | Feasibility study | Child psychiatry | Pharmaco-genomics | Parents and associated clinicians of 73 young service users | 2 | Cost and time investments; Fear of invasive procedures | Adequate skills and competence training |
| Moreno-Peral, 2018 [29] † | Spain | Face-to-face semi-structured interviews | Major depression | Individualised risk prediction algorithm | 67 family physicians | 2 | Cost and time investments; Poor transparency and complexity of the model; Lack of motivation to address mental health in primary care; Potential psychological harm | Simplicity and usability of the model; Stratification over precision; Integration into current workflow; Adequate skills and competence training; Effective time management and organisation |
| Oliver, 2020 [25] † | United Kingdom | Feasibility study | Clinical high-risk for psychosis | Transdiagnostic risk calculator | Clinicians of 3722 patients screened and independent consultation with an unspecified number of service users and clinicians | 2 | N/A | Routinely collected predictors; Outreach to local clinicians and clinical prompts |
| Reger, 2019 [26] † | United States | Case example | Suicidal behaviours | Clinical prediction model | A clinical implementation team of professionals | 2 | Lack of effective interventions; Lack of clinical resources | Outreach to local clinicians and clinical prompts; Compliance with law and regulatory pathways |
| Salm, 2014 [45] | United States | Survey | General psychiatry | Genetic testing | 372 psychiatrists and 163 neurologists (n = 535) | 1 | Potential misuse of personal data; Poor perceived competence in precision medicine; Potential psychological harm; Potential economic and occupational harm | Adequate skills and competence training |
| Smith, 1996 [46] | United States | Survey | Bipolar disorder | Genetic testing | 48 members of a bipolar disorder support group, 35 medical students and 30 psychiatry residents (n = 113) | 1 | Lack of effective interventions | N/A |
| Trippitelli, 1998 [47] | United States | Questionnaire | Bipolar disorder | Genetic testing | 90 service users and their spouses | 1 | Potential misuse of personal data; Potential stigmatisation; Potential economic and occupational harm | N/A |
| Wachtler, 2018 [48] | Australia | Focus group, prototype development and semi-structured interviews | Depression | Clinical prediction model | 17 members and of the community and 7 service users (n = 24) | Poor transparency and complexity of the model;Ethics of risk communication; Potential psychological harm; Poor accuracy and utility of the model | Adaptability of the model | |
| Walden, 2015 [30] † | Canada | Survey | General psychiatry | Pharmaco-genomics | 168 physicians who had ordered pharmaco-genomic tests for psychotropic medication | 1 | Negative staff perceptions of precision medicine | N/A |
| Wilde, 2010 [49] | Australia | Focus groups | Major depression | Genetic testing | 36 members of the public (14 with disclosure of family history of mental illness) | 1 | Poor perceived relative advantage of the model; Poor accuracy and utility of the model; Potential misuse of personal data; Lack of effective interventions; Potential psychological harm; Potential stigmatisation; Potential economic and occupational harm | Integration into workflow |
| Williams, 2016 [50] | United States | Semi-structured interviews | Alcohol use disorders | Genetic testing | 24 primary care providers | 1 | Cost and time investments; Poor accuracy and utility of the model; Lack of clinical resources; Negative staff perceptions of precision medicine; Potential psychological harm | Patient engagement |
| Zhou, 2014 [51] | Australia | Survey | Schizophrenia, bipolar disorder and major depression. | Genetic testing | 104 psychiatrists, 36 genetic counsellors, and 17 medical geneticists (n = 157) | 1 | Poor perceived competence in precision medicine; Potential economic and occupational harm | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldwin, H.; Loebel-Davidsohn, L.; Oliver, D.; Salazar de Pablo, G.; Stahl, D.; Riper, H.; Fusar-Poli, P. Real-World Implementation of Precision Psychiatry: A Systematic Review of Barriers and Facilitators. Brain Sci. 2022, 12, 934. https://doi.org/10.3390/brainsci12070934
Baldwin H, Loebel-Davidsohn L, Oliver D, Salazar de Pablo G, Stahl D, Riper H, Fusar-Poli P. Real-World Implementation of Precision Psychiatry: A Systematic Review of Barriers and Facilitators. Brain Sciences. 2022; 12(7):934. https://doi.org/10.3390/brainsci12070934
Chicago/Turabian StyleBaldwin, Helen, Lion Loebel-Davidsohn, Dominic Oliver, Gonzalo Salazar de Pablo, Daniel Stahl, Heleen Riper, and Paolo Fusar-Poli. 2022. "Real-World Implementation of Precision Psychiatry: A Systematic Review of Barriers and Facilitators" Brain Sciences 12, no. 7: 934. https://doi.org/10.3390/brainsci12070934
APA StyleBaldwin, H., Loebel-Davidsohn, L., Oliver, D., Salazar de Pablo, G., Stahl, D., Riper, H., & Fusar-Poli, P. (2022). Real-World Implementation of Precision Psychiatry: A Systematic Review of Barriers and Facilitators. Brain Sciences, 12(7), 934. https://doi.org/10.3390/brainsci12070934






