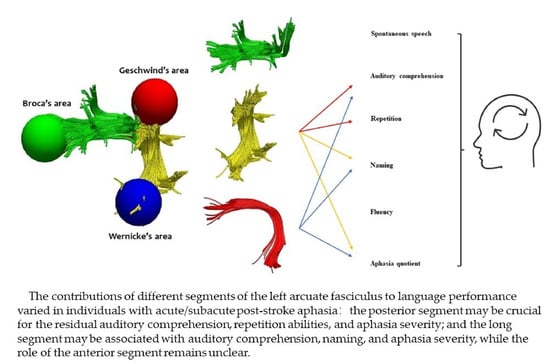
Integrity of the Left Arcuate Fasciculus Segments Significantly Affects Language Performance in Individuals with Acute/Subacute Post-Stroke Aphasia: A Cross-Sectional Diffusion Tensor Imaging Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Language Assessment
2.3. MRI Acquisition and Preprocessing
2.4. Lesion Overlay Map and Lesion Load
2.5. Reconstruction of the AF
2.6. Statistical Analyses
3. Results
3.1. Demographic and Clinical Characteristics
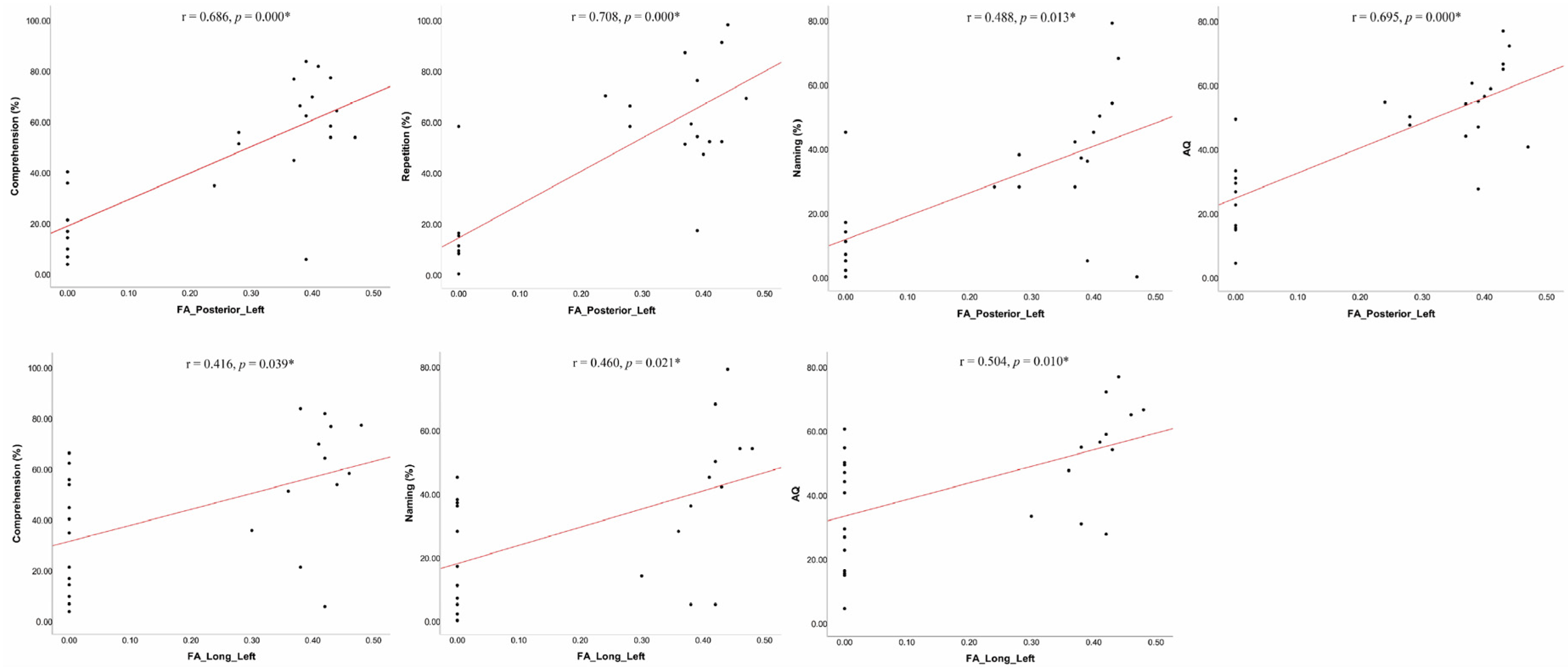
3.2. Correlation Analyses between Language Performance and MRI Measures
3.3. Intergroup Demographic and Stroke-Related Variables Analyses
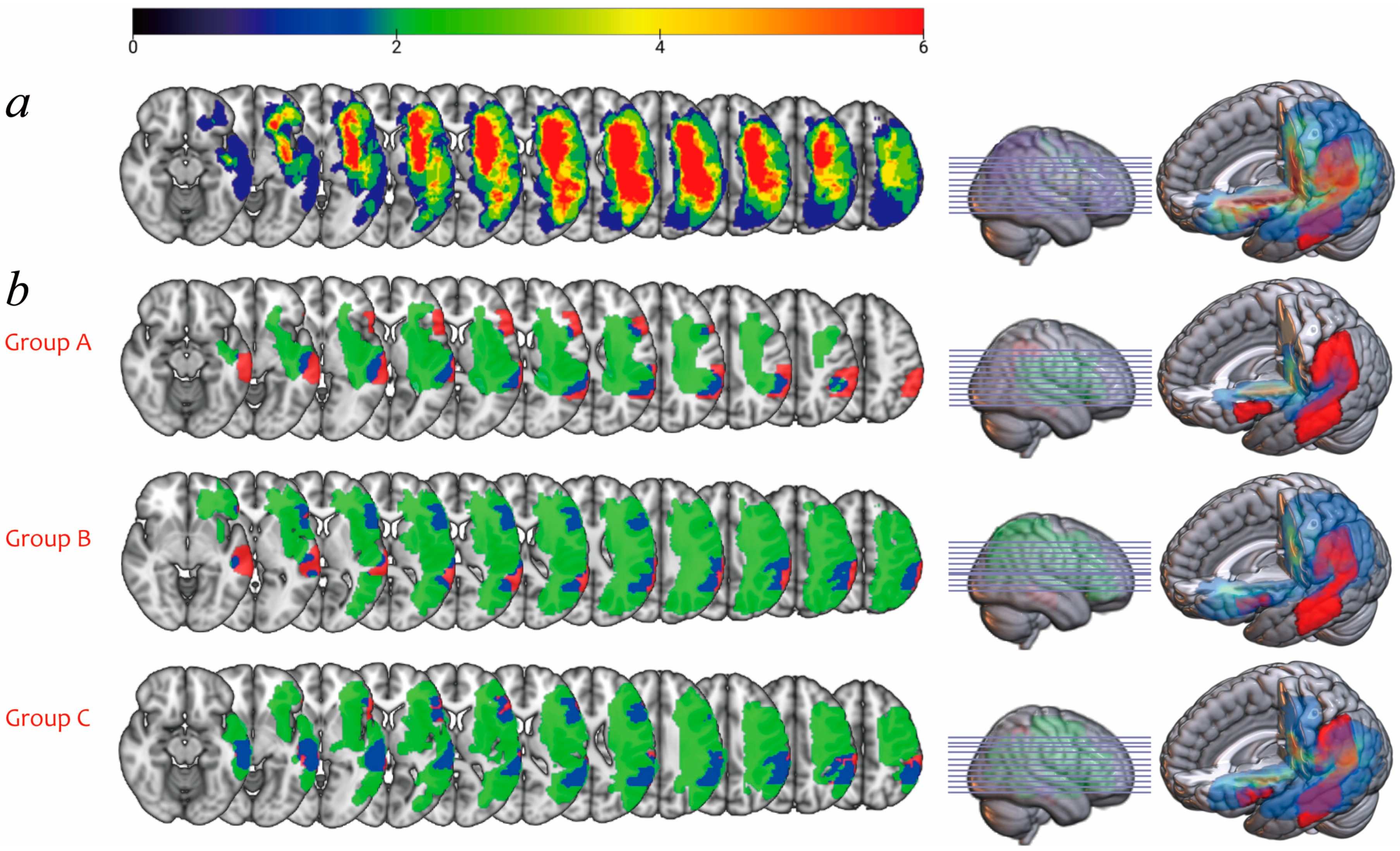
3.4. Lesion Overlay Map and Lesion Load Analyses
3.5. Intergroup Language Performance Analyses
3.6. Intergroup Diffusion Indices Analyses
4. Discussion
4.1. The Importance of Lesion Load of Cortical Language Areas in PSA
4.2. The Linguistic Roles of the Left ASAF in PSA
4.3. The Linguistic Contributions of the Left LSAF in PSA
4.4. The Linguistic Functions of the Left PSAF in PSA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flowers, H.L.; Skoretz, S.A.; Silver, F.L.; Rochon, E.; Fang, J.; Flamand-Roze, C.; Martino, R. Poststroke Aphasia Frequency, Recovery, and Outcomes: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2016, 97, 2188–2201.e8. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.J.; Jang, S.H. Relationships among language ability, the arcuate fasciculus and lesion volume in patients with putaminal hemorrhage: A diffusion tensor imaging study. J. Integr. Neurosci. 2021, 20, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Flinker, A.; Korzeniewska, A.; Shestyuk, A.Y.; Franaszczuk, P.J.; Dronkers, N.F.; Knight, R.T.; Crone, N.E. Redefining the role of Broca’s area in speech. Proc. Natl. Acad. Sci. USA 2015, 112, 2871–2875. [Google Scholar] [CrossRef] [PubMed]
- Matchin, W.; Basilakos, A.; Ouden, D.-B.D.; Stark, B.C.; Hickok, G.; Fridriksson, J. Functional differentiation in the language network revealed by lesion-symptom mapping. NeuroImage 2022, 247, 118778. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lambon Ralph, M.A.; Halai, A.D. Relating resting-state hemodynamic changes to the variable language profiles in post-stroke aphasia. NeuroImage Clin. 2018, 20, 611–619. [Google Scholar] [CrossRef]
- Hartwigsen, G.; Saur, D. Neuroimaging of stroke recovery from aphasia—Insights into plasticity of the human language network. NeuroImage 2019, 190, 14–31. [Google Scholar] [CrossRef]
- Kiran, S.; Thompson, C.K. Neuroplasticity of Language Networks in Aphasia: Advances, Updates, and Future Challenges. Front. Neurol. 2019, 10, 295. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, H.; Li, S.; Dai, Y. Predictive role of subcomponents of the left arcuate fasciculus in prognosis of aphasia after stroke: A retrospective observational study. Medicine 2019, 98, e15775. [Google Scholar] [CrossRef]
- Matsumoto, R.; Nair, D.R.; LaPresto, E.; Najm, I.; Bingaman, W.; Shibasaki, H.; Lüders, H.O. Functional connectivity in the human language system: A cortico-cortical evoked potential study. Brain 2004, 127 Pt 10, 2316–2330. [Google Scholar] [CrossRef]
- Bernal, B.; Ardila, A. The role of the arcuate fasciculus in conduction aphasia. Brain 2009, 132, 2309–2316. [Google Scholar] [CrossRef]
- Catani, M.; Jones, D.K.; Ffytche, D.H. Perisylvian language networks of the human brain. Ann. Neurol. 2005, 57, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Glasser, M.F.; Rilling, J.K. DTI Tractography of the Human Brain’s Language Pathways. Cereb. Cortex 2008, 18, 2471–2482. [Google Scholar] [CrossRef] [PubMed]
- Friederici, A.D.; Gierhan, S.M. The language network. Curr. Opin. Neurobiol. 2013, 23, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Forkel, S.J.; Rogalski, E.; Sancho, N.D.; D’Anna, L.; Laguna, P.L.; Sridhar, J.; Dell’Acqua, F.; Weintraub, S.; Thompson, C.; Mesulam, M.-M.; et al. Anatomical evidence of an indirect pathway for word repetition. Neurology 2020, 94, e594–e606. [Google Scholar] [CrossRef]
- Ivanova, M.V.; Zhong, A.; Turken, A.; Baldo, J.V.; Dronkers, N.F. Functional Contributions of the Arcuate Fasciculus to Language Processing. Front. Hum. Neurosci. 2021, 15, 672665. [Google Scholar] [CrossRef]
- Ryu, H.; Park, C.-H. Structural Characteristic of the Arcuate Fasciculus in Patients with Fluent Aphasia Following Intracranial Hemorrhage: A Diffusion Tensor Tractography Study. Brain Sci. 2020, 10, 280. [Google Scholar] [CrossRef]
- Jang, S.H.; Lee, A.Y.; Shin, S.M. Injury of the arcuate fasciculus in the dominant hemisphere in patients with mild traumatic brain injury: A retrospective cross-sectional study. Medicine 2016, 95, 1–5. [Google Scholar] [CrossRef]
- Lahiri, D.; Dubey, S.; Ardila, A.; Ray, B.K. Factors affecting vascular aphasia severity. Aphasiology 2021, 35, 633–641. [Google Scholar] [CrossRef]
- Clarke, W.T.; Stagg, C.J.; Jbabdi, S. FSL-MRS: An end-to-end spectroscopy analysis package. Magn. Reson. Med. 2021, 85, 2950–2964. [Google Scholar] [CrossRef]
- Wan, C.Y.; Zheng, X.; Marchina, S.; Norton, A.; Schlaug, G. Intensive therapy induces contralateral white matter changes in chronic stroke patients with Broca’s aphasia. Brain Lang. 2014, 136, 1–7. [Google Scholar] [CrossRef]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006, 31, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.; Woolrich, M.W.; Smith, S.M. FSL. NeuroImage 2012, 62, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Benner, T.; Sorensen, A.G.; Wedeen, V.J. Diffusion toolkit: A software package for diffusion imaging data processing and tractography. Proc. Intl. Soc. Mag. Reson. Med. 2007, 15, 3720. Available online: http://www.trackvis.org/faq/2007_ISMRM_diffusion_toolkit.pdf (accessed on 12 March 2022).
- Forkel, S.J.; de Schotten, M.T.; Dell’Acqua, F.; Kalra, L.; Murphy, D.G.M.; Williams, S.C.R.; Catani, M. Anatomical predictors of aphasia recovery: A tractography study of bilateral perisylvian language networks. Brain 2014, 137 Pt 7, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jang, S.H. Prediction of aphasia outcome using diffusion tensor tractography for arcuate fasciculus in stroke. AJNR Am. J. Neuroradiol. 2013, 34, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Kourtidou, E.; Kasselimis, D.; Angelopoulou, G.; Karavasilis, E.; Velonakis, G.; Kelekis, N.; Zalonis, I.; Evdokimidis, I.; Potagas, C.; Petrides, M. The Role of the Right Hemisphere White Matter Tracts in Chronic Aphasic Patients After Damage of the Language Tracts in the Left Hemisphere. Front. Hum. Neurosci. 2021, 15, 635750. [Google Scholar] [CrossRef] [PubMed]
- Lachenbruch, R. Review: Statistical power analysis for the behavioral sciences (2nd ed.). by Jacob Cohen. J. Am. Stat. Assoc. 1989, 84, 1096. [Google Scholar] [CrossRef]
- Hillis, A.E.; Gold, L.; Kannan, V.; Cloutman, L.; Kleinman, J.T.; Newhart, M.; Heidler-Gary, J.; Davis, C.; Aldrich, E.; Llinas, R.; et al. Site of the ischemic penumbra as a predictor of potential for recovery of functions. Neurology 2008, 71, 184–189. [Google Scholar] [CrossRef]
- Schlaug, G. Even when right is all that’s left: There are still more options for recovery from aphasia. Ann. Neurol. 2018, 83, 661–663. [Google Scholar] [CrossRef]
- Zhu, L.L.; Lindenberg, R.; Alexander, M.P.; Schlaug, G. Lesion Load of the Corticospinal Tract Predicts Motor Impairment in Chronic Stroke. Stroke 2010, 41, 910–915. [Google Scholar] [CrossRef]
- Breier, J.I.; Hasan, K.M.; Zhang, W.; Men, D.; Papanicolaou, A.C. Language Dysfunction After Stroke and Damage to White Matter Tracts Evaluated Using Diffusion Tensor Imaging. Am. J. Neuroradiol. 2008, 29, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Stockert, A.; Wawrzyniak, M.; Klingbeil, J.; Wrede, K.; Kümmerer, D.; Hartwigsen, G.; Kaller, C.; Weiller, C.; Saur, D. Dynamics of language reorganization after left temporo-parietal and frontal stroke. Brain 2020, 143, 844–861. [Google Scholar] [CrossRef] [PubMed]
- Marchina, S.; Zhu, L.L.; Norton, A.; Zipse, L.; Wan, C.Y.; Schlaug, G. Impairment of Speech Production Predicted by Lesion Load of the Left Arcuate Fasciculus. Stroke 2011, 42, 2251–2256. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Marchina, S.; Norton, A.C.; Wan, C.Y.; Schlaug, G. Predicting speech fluency and naming abilities in aphasic patients. Front. Hum. Neurosci. 2013, 7, 831. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.M.H.; Seghier, M.L.; Prejawa, S.; Leff, A.P.; Price, C.J. Distinguishing the effect of lesion load from tract disconnection in the arcuate and uncinate fasciculi. NeuroImage 2016, 125, 1169–1173. [Google Scholar] [CrossRef]
- Griffis, J.C.; Metcalf, N.V.; Corbetta, M.; Shulman, G.L. Structural Disconnections Explain Brain Network Dysfunction after Stroke. Cell Rep. 2019, 28, 2527–2540.e9. [Google Scholar] [CrossRef]
- Szaflarski, J.P.; Allendorfer, J.B.; Banks, C.; Vannest, J.; Holland, S.K. Recovered vs. not-recovered from post-stroke aphasia: The contributions from the dominant and non-dominant hemispheres. Restor. Neurol. Neurosci. 2013, 31, 347–360. [Google Scholar] [CrossRef]
- Lazar, R.M.; Antoniello, D. Variability in recovery from aphasia. Curr. Neurol. Neurosci. Rep. 2008, 8, 497–502. [Google Scholar] [CrossRef]
- Geva, S.; Correia, M.M.; Warburton, E.A. Contributions of bilateral white matter to chronic aphasia symptoms as assessed by diffusion tensor MRI. Brain Lang. 2015, 150, 117–128. [Google Scholar] [CrossRef]
- Fridriksson, J.; Guo, D.; Fillmore, P.; Holland, A.; Rorden, C. Damage to the anterior arcuate fasciculus predicts non-fluent speech production in aphasia. Brain 2013, 136 Pt 11, 3451–3460. [Google Scholar] [CrossRef]
- Basilakos, A.; Fillmore, P.T.; Rorden, C.; Guo, D.; Bonilha, L.; Fridriksson, J. Regional white matter damage predicts speech fluency in chronic post-stroke aphasia. Front. Hum. Neurosci. 2014, 8, 845. [Google Scholar] [CrossRef] [PubMed]
- van Geemen, K.; Herbet, G.; Moritz-Gasser, S.; Duffau, H. Limited plastic potential of the left ventral premotor cortex in speech articulation: Evidence From intraoperative awake mapping in glioma patients. Hum. Brain Mapp. 2014, 35, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Gajardo-Vidal, A.; Lorca-Puls, D.L.; Team, P.; Warner, H.; Pshdary, B.; Crinion, J.T.; Leff, A.P.; Hope, T.M.H.; Geva, S.; Seghier, M.L.; et al. Damage to Broca’s area does not contribute to long-term speech production outcome after stroke. Brain 2021, 144, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, M.V.; Isaev, D.Y.; Dragoy, O.V.; Akinina, Y.S.; Petrushevskiy, A.G.; Fedina, O.N.; Shklovsky, V.M.; Dronkers, N.F. Diffusion-tensor imaging of major white matter tracts and their role in language processing in aphasia. Cortex 2016, 85, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Hillis, A.E. The ‘Standard’ for Poststroke Aphasia Recovery. Stroke 2010, 41, 1316–1317. [Google Scholar] [CrossRef][Green Version]
- Wilson, S.M.; Henry, M.L.; Besbris, M.; Ogar, J.M.; Dronkers, N.F.; Jarrold, W.; Miller, B.L.; Gorno-Tempini, M.L. Connected speech production in three variants of primary progressive aphasia. Brain 2010, 133 Pt 7, 2069–2088. [Google Scholar] [CrossRef]
- Sierpowska, J.; Gabarrós, A.; Fernandez-Coello, A.; Camins, A.; Castañer, S.; Juncadella, M.; Morís, J.; Rodríguez-Fornells, A. Words are not enough: Nonword repetition as an indicator of arcuate fasciculus integrity during brain tumor resection. J. Neurosurg. 2017, 126, 435–445. [Google Scholar] [CrossRef]
- Catani, M.; Bambini, V. A model for Social Communication And Language Evolution and Development (SCALED). Curr. Opin. Neurobiol. 2014, 28, 165–171. [Google Scholar] [CrossRef]
- Tak, H.J.; Kim, J.H.; Son, S.M. Developmental process of the arcuate fasciculus from infancy to adolescence: A diffusion tensor imaging study. Neural Regen. Res. 2016, 11, 937–943. [Google Scholar] [CrossRef]
- López-Barroso, D.; Catani, M.; Ripollés, P.; Dell’Acqua, F.; Rodríguez-Fornells, A.; de Diego-Balaguer, R. Word learning is mediated by the left arcuate fasciculus. Proc. Natl. Acad. Sci. USA 2013, 110, 13168–13173. [Google Scholar] [CrossRef]
- Gullick, M.M.; Booth, J.R. The direct segment of the arcuate fasciculus is predictive of longitudinal reading change. Dev. Cogn. Neurosci. 2015, 13, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Friederici, A.D. The Brain Basis of Language Processing: From Structure to Function. Physiol. Rev. 2011, 91, 1357–1392. [Google Scholar] [CrossRef] [PubMed]
- Turken, A.U.; Dronkers, N.F. The Neural Architecture of the Language Comprehension Network: Converging Evidence from Lesion and Connectivity Analyses. Front. Syst. Neurosci. 2011, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Matchin, W.; Hickok, G. The Cortical Organization of Syntax. Cereb. Cortex 2020, 30, 1481–1498. [Google Scholar] [CrossRef] [PubMed]
- Friederici, A.D. The cortical language circuit: From auditory perception to sentence comprehension. Trends Cogn. Sci. 2012, 16, 262–268. [Google Scholar] [CrossRef]
- Wilson, S.M.; Galantucci, S.; Tartaglia, M.C.; Rising, K.; Patterson, D.K.; Henry, M.; Ogar, J.M.; Deleon, J.; Miller, B.L.; Gorno-Tempini, M.L. Syntactic Processing Depends on Dorsal Language Tracts. Neuron 2011, 72, 397–403. [Google Scholar] [CrossRef]
- Magnusdottir, S.; Fillmore, P.; den Ouden, D.B.; Hjaltason, H.; Rorden, C.; Kjartansson, O.; Bonilha, L.; Fridriksson, J. Damage to left anterior temporal cortex predicts impairment of complex syntactic processing: A lesion-symptom mapping study. Hum. Brain Mapp. 2013, 34, 2715–2723. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, D.G.; You, H.; Son, S.M.; Cho, Y.W.; Chang, M.C.; Lee, J.; Jang, S.H. The clinical application of the arcuate fasciculus for stroke patients with aphasia: A diffusion tensor tractography study. NeuroRehabilitation 2011, 29, 305–310. [Google Scholar] [CrossRef]
- Berthier, M.L.; Lambon Ralph, M.A.; Pujol, J.; Green, C. Arcuate fasciculus variability and repetition: The left sometimes can be right. Cortex 2012, 48, 133–143. [Google Scholar] [CrossRef]
- Dronkers, N.F.; Wilkins, D.P.; Van Valin, R.D., Jr.; Redfern, B.B.; Jaeger, J.J. Lesion analysis of the brain areas involved in language comprehension. Cognition 2004, 92, 145–177. [Google Scholar] [CrossRef]
- Song, X.; Dornbos, D., 3rd; Lai, Z.; Zhang, Y.; Li, T.; Chen, H.; Yang, Z. Diffusion tensor imaging and diffusion tensor imaging-fibre tractograph depict the mechanisms of Broca-like and Wernicke-like conduction aphasia. Neurol. Res. 2011, 33, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Woolnough, O.; Rollo, P.S.; Roccaforte, Z.J.; Segaert, K.; Hagoort, P.; Tandon, N. Minimal Phrase Composition Revealed by Intracranial Recordings. J. Neurosci. 2022, 42, 3216–3227. [Google Scholar] [CrossRef] [PubMed]
- Mesulam, M.-M.; Rader, B.M.; Sridhar, J.; Nelson, M.J.; Hyun, J.; Rademaker, A.; Geula, C.; Bigio, E.H.; Thompson, C.K.; Gefen, T.D.; et al. Word comprehension in temporal cortex and Wernicke area: A PPA perspective. Neurology 2019, 92, e224–e233. [Google Scholar] [CrossRef] [PubMed]
- Fridriksson, J.; Kjartansson, O.; Morgan, P.S.; Hjaltason, H.; Magnusdottir, S.; Bonilha, L.; Rorden, C. Impaired Speech Repetition and Left Parietal Lobe Damage. J. Neurosci. 2010, 30, 11057–11061. [Google Scholar] [CrossRef]
- Baldo, J.V.; Katseff, S.; Dronkers, N.F. Brain regions underlying repetition and auditory-verbal short-term memory deficits in aphasia: Evidence from voxel-based lesion symptom mapping. Aphasiology 2012, 26, 338–354. [Google Scholar] [CrossRef]
- Levelt, W.J.M.; Roelofs, A.; Meyer, A.S. A theory of lexical access in speech production. Behav. Brain Sci. 1999, 22, 1–38. [Google Scholar] [CrossRef]

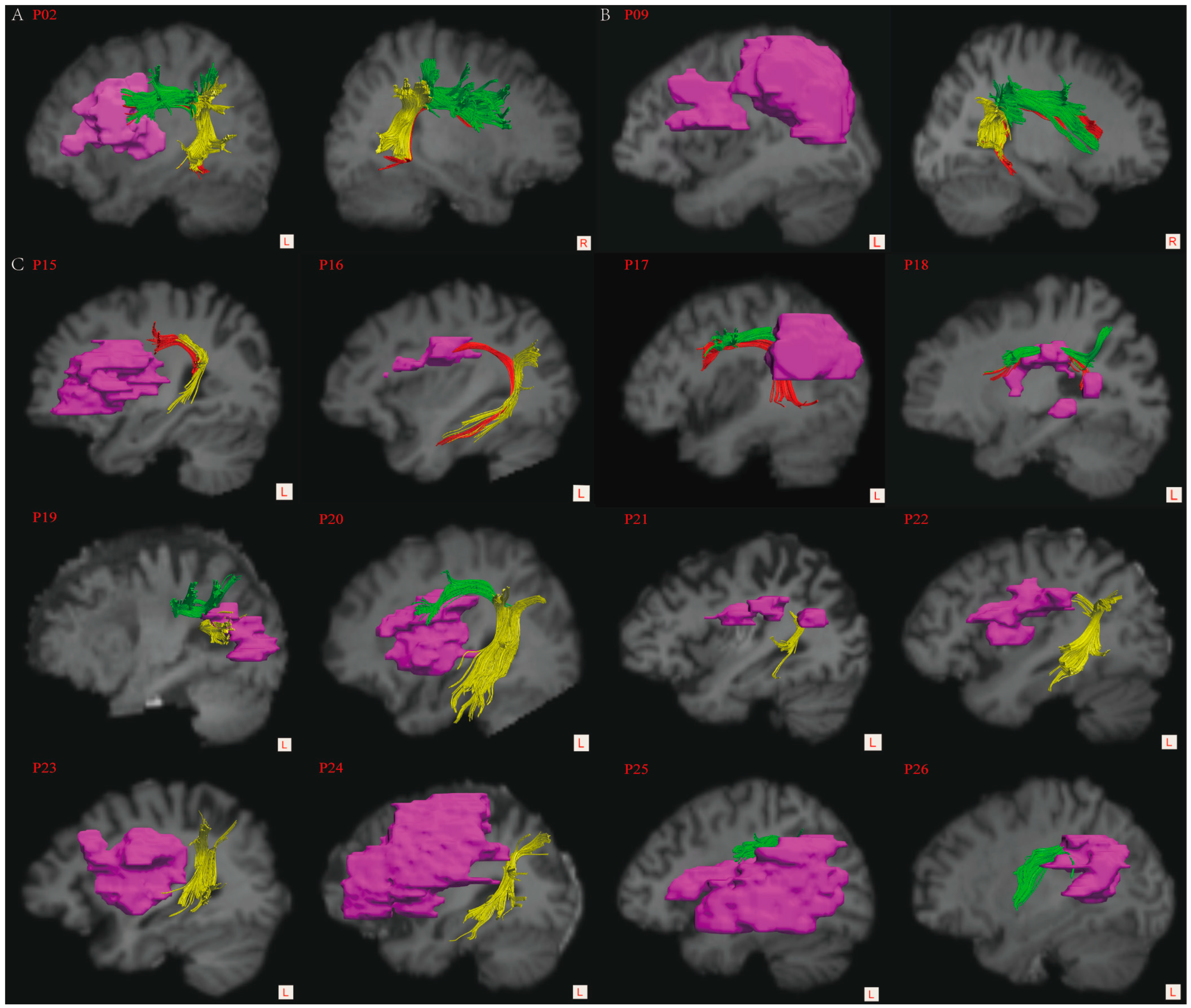

| Patient ID | Age/Sex | Education (Years) | Time Post Onset (Days) | Stroke Type | Aphasia Type | Aphasia Severity | Lesion Site | Lesion Volume (cm3) | Lesion Load (%) |
|---|---|---|---|---|---|---|---|---|---|
| 01 | M/52 | 9 | 8 | Infarction | TSA | mild | Basal ganglia, corona radiate, and centrum semiovale | 7.94 | 0 |
| 02 | M/49 | 15 | 10 | Infarction | Broca | moderate | Basal ganglia, frontotemporal parietal lobe, and corona radiata | 43.43 | 3.02 |
| 03 | F/59 | 6 | 12 | Infarction | TSA | moderate | Basal ganglia, corona radiate | 8.19 | 0 |
| 04 | M/36 | 9 | 16 | Infarction | Broca | moderate | Basal ganglia, temporal lobe, corona radiata, and centrum semiovale | 27.79 | 0 |
| 05 | M/59 | 12 | 17 | ICH | Wernicke | severe | Temporal lobe, insula | 75.34 | 0.7 |
| 06 | F/56 | 12 | 18 | ICH | Wernicke | severe | Temporal parietal lobe | 68.07 | 26.88 |
| 07 | F/56 | 9 | 81 | ICH | Conduction | moderate | Basal ganglia, corona radiata | 17.15 | 0 |
| 08 | M/38 | 15 | 83 | ICH | Broca | moderate | Basal ganglia, frontal lobe | 27.34 | 0.02 |
| 09 | M/55 | 12 | 16 | Infarction | Global | very severe | Basal ganglia, frontotemporal parietal lobe, and corona radiata | 200.50 | 26.29 |
| 10 | M/61 | 12 | 45 | Infarction | Conduction | severe | Frontotemporal parietal lobe | 21.02 | 1.97 |
| 11 | M/64 | 12 | 75 | Infarction | Global | very severe | Frontotemporal parietal and occipital lobe | 50.87 | 9.36 |
| 12 | F/71 | 6 | 89 | Infarction | Global | very severe | Frontotemporal parietal and occipital lobe | 150.50 | 35.14 |
| 13 | M/56 | 15 | 85 | Infarction | Global | severe | Basal ganglia, frontoparietal lobe, and insula | 76.59 | 2.24 |
| 14 | M/34 | 12 | 77 | Infarction | Global | very severe | Frontotemporal parietal lobe | 54.30 | 15.19 |
| 15 | M/61 | 9 | 21 | Infarction | Anomic | moderate | Basal ganglia, frontal lobe | 38.50 | 2.9 |
| 16 | M/72 | 16 | 23 | Infarction | Broca | moderate | Basal ganglia, corona radiate | 12.50 | 0.03 |
| 17 | F/33 | 6 | 5 | Infarction | Global | severe | Temporal and parietal lobe, insula | 40.26 | 32.01 |
| 18 | M/64 | 9 | 8 | Infarction | Global | severe | Temporal and parietal lobe, insula | 11.51 | 0.89 |
| 19 | M/69 | 12 | 5 | Infarction | TSA | moderate | Basal ganglia, temporal and parietal lobe, and corona radiata | 14.42 | 0.28 |
| 20 | M/48 | 9 | 88 | ICH | MTA | severe | Basal ganglia, frontotemporal lobe | 29.30 | 0.17 |
| 21 | F/50 | 12 | 15 | Infarction | Broca | severe | Temporal and parietal lobe, corona radiata, and insula | 10.84 | 6.33 |
| 22 | M/43 | 16 | 53 | Infarction | Anomic | moderate | Frontoparietal lobe, insula | 26.95 | 2.07 |
| 23 | M/69 | 6 | 68 | ICH | TMA | severe | Basal ganglia, frontal lobe | 46.70 | 0.19 |
| 24 | M/64 | 15 | 73 | Infarction | MTA | severe | Frontoparietal lobe | 151.30 | 21.02 |
| 25 | M/48 | 9 | 24 | Infarction | Global | very severe | Frontotemporal parietal lobe, basal ganglia, and insula | 1.44 | 48.30 |
| 26 | M/50 | 9 | 11 | Infarction | Wernicke | severe | Temporo-occipital junction, insula | 35.26 | 15.73 |
| Lesion Volume | Lesion Load | FA Value in Left AF Segments | Fiber Number in Left AF Segments | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anterior | Posterior | Long | Anterior | Posterior | Long | |||||||||||
| ρ | P | ρ | P | ρ | P | ρ | P | ρ | P | ρ | P | ρ | P | ρ | P | |
| Spontaneous speech | −0.447 | 0.022 * | −0.547 | 0.004 * | 0.348 | 0.081 | 0.467 | 0.016 * | 0.525 | 0.006 * | 0.346 | 0.083 | 0.281 | 0.165 | 0.405 | 0.040 * |
| Comprehension | −0.393 | 0.047 * | −0.688 | 0.000 * | 0.237 | 0.244 | 0.717 | 0.000 * | 0.521 | 0.006 * | 0.193 | 0.346 | 0.687 | 0.000 * | 0.395 | 0.046 * |
| Repetition | −0.316 | 0.116 | −0.548 | 0.004 * | 0.333 | 0.097 | 0.725 | 0.000 * | 0.366 | 0.066 | 0.094 | 0.646 | 0.629 | 0.001 * | 0.237 | 0.244 |
| Naming | −0.436 | 0.026 * | −0.642 | 0.000 * | 0.150 | 0.464 | 0.587 | 0.002 * | 0.591 | 0.001 * | 0.126 | 0.541 | 0.366 | 0.066 | 0.381 | 0.055 |
| Fluency | −0.169 | 0.410 | −0.299 | 0.138 | 0.363 | 0.068 | 0.195 | 0.340 | 0.438 | 0.025 * | 0.422 | 0.032 * | 0.055 | 0.791 | 0.375 | 0.059 |
| AQ | −0.497 | 0.010 * | −0.741 | 0.000 * | 0.351 | 0.079 | 0.766 | 0.000 * | 0.635 | 0.000 * | 0.240 | 0.237 | 0.592 | 0.001 * | 0.445 | 0.023 * |
| FA Value in Left AF Segments | Fiber Number in Left AF Segments | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anterior | Posterior | Long | Anterior | Posterior | Long | |||||||
| r | P | r | P | r | P | r | P | r | P | r | P | |
| Spontaneous speech | 0.161 | 0.442 | 0.168 | 0.421 | 0.325 | 0.113 | 0.307 | 0.135 | 0.020 | 0.926 | 0.300 | 0.146 |
| Comprehension | 0.160 | 0.445 | 0.686 | 0.000 * | 0.416 | 0.039 * | 0.243 | 0.242 | 0.644 | 0.001 * | 0.333 | 0.104 |
| Repetition | 0.198 | 0.342 | 0.708 | 0.000 * | 0.270 | 0.191 | 0.104 | 0.620 | 0.576 | 0.003 * | 0.159 | 0.447 |
| Naming | 0.006 | 0.979 | 0.488 | 0.013 * | 0.460 | 0.021 * | 0.115 | 0.583 | 0.254 | 0.220 | 0.295 | 0.152 |
| Fluency | 0.231 | 0.267 | 0.002 | 0.993 | 0.290 | 0.159 | 0.362 | 0.075 | −0.107 | 0.610 | 0.313 | 0.127 |
| AQ | 0.199 | 0.341 | 0.695 | 0.000 * | 0.504 | 0.010 * | 0.249 | 0.231 | 0.500 | 0.011 * | 0.378 | 0.062 |
| Group A | Group B | Group C | p | |
|---|---|---|---|---|
| Spontaneous speech | 59.38 ± 12.94 | 41.67 ± 17.22 | 45.83 ± 20.43 | 0.149 |
| Comprehension | 60.81 ± 25.82 | 16.17 ± 13.34 | 44.71 ± 20.05 | 0.002 * |
| Repetition | 58.25 ± 23.89 | 6.50 ± 5.17 | 55.08 ± 30.29 | 0.004 * |
| Naming | 43.50 ± 21.73 | 14.33 ± 16.29 | 25.92 ± 20.36 | 0.036 * |
| Fluency | 67.50 ± 21.21 | 50.00 ± 16.73 | 44.17 ± 23.53 | 0.076 |
| AQ | 56.26 ± 14.70 | 24.07 ± 13.18 | 43.48 ± 17.72 | 0.004 * |
| Mean Diff. | 95% CI | Adjusted-p | |
|---|---|---|---|
| Comprehension | |||
| Group A–Group B | 44.65 | 15.60, 73.69 | 0.002 * |
| Group A–Group C | 16.10 | −8.45, 40.65 | 0.311 |
| Group B–Group C | −28.54 | −55.43, −1.65 | 0.035 * |
| Repetition | Test Statistic | Std. Error | Adjusted-p |
| Group A–Group B | 11.85 | 4.13 | 0.012 * |
| Group A–Group C | −0.10 | 3.49 | 1.000 |
| Group B–Group C | −11.96 | 3.82 | 0.005 * |
| Naming | |||
| Group A–Group B | 29.17 | 1.29, 57.04 | 0.038 * |
| Group A–Group C | 17.58 | −5.98, 41.14 | 0.199 |
| Group B–Group C | −11.58 | −37.39, 14.23 | 0.775 |
| AQ | |||
| Group A–Group B | 32.20 | 9.98, 54.41 | 0.003 * |
| Group A–Group C | 12.79 | −5.99, 31.56 | 0.276 |
| Group B–Group C | −19.41 | −39.97, 1.16 | 0.069 |
| Segments | Group A | Group B | Group C | p | |
|---|---|---|---|---|---|
| FA value | Anterior | 0.40 ± 0.03 | 0.00 | 0.17 ± 0.18 | 0.001 * |
| Posterior | 0.39 ± 0.05 | 0.00 | 0.25 ± 0.19 | 0.003 * | |
| Long | 0.42 ± 0.04 | 0.00 | 0.13 ± 0.19 | 0.000 * | |
| Fiber number | Anterior | 275.25 ± 58.92 | 0.00 | 97.58 ± 108.09 | 0.000 * |
| Posterior | 320.50 ± 60.88 | 0.00 | 181.42 ± 154.14 | 0.002 * | |
| Long | 309.00 ± 82.90 | 0.00 | 64.75 ± 98.12 | 0.000 * |
| Segment | Test Statistic | Std. Error | p | Adjusted-p | |
|---|---|---|---|---|---|
| FA value | Anterior | ||||
| Group A–Group B | 14.63 | 3.92 | 0.000 | 0.001 * | |
| Group A–Group C | 9.21 | 3.31 | 0.005 | 0.016 * | |
| Group B–Group C | −5.42 | 3.63 | 0.136 | 0.407 | |
| Posterior | |||||
| Group A–Group B | 13.63 | 4.01 | 0.001 | 0.002 * | |
| Group A–Group C | 5.38 | 3.39 | 0.112 | 0.337 | |
| Group B–Group C | −8.25 | 3.71 | 0.006 | 0.019 * | |
| Long | |||||
| Group A–Group B | 14.19 | 3.79 | 0.000 | 0.001 * | |
| Group A–Group C | 10.35 | 3.21 | 0.001 | 0.003 * | |
| Group B–Group C | −3.54 | 3.51 | 0.313 | 0.939 | |
| Fiber number | Anterior | ||||
| Group A–Group B | 14.88 | 3.92 | 0.000 | 0.000 * | |
| Group A–Group C | 9.63 | 3.32 | 0.004 | 0.011 * | |
| Group B–Group C | −5.25 | 3.63 | 0.148 | 0.445 | |
| Posterior | |||||
| Group A–Group B | 14.38 | 4.01 | 0.000 | 0.001 * | |
| Group A–Group C | 6.63 | 3.39 | 0.051 | 0.152 | |
| Group B–Group C | −7.75 | 3.72 | 0.007 | 0.021 * | |
| Long | |||||
| Group A–Group B | 14.75 | 3.80 | 0.000 | 0.000 * | |
| Group A–Group C | 11.58 | 3.21 | 0.000 | 0.001 * | |
| Group B–Group C | −3.17 | 3.51 | 0.368 | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Sun, Y.; Liao, X.; Qian, W.; Ye, T. Integrity of the Left Arcuate Fasciculus Segments Significantly Affects Language Performance in Individuals with Acute/Subacute Post-Stroke Aphasia: A Cross-Sectional Diffusion Tensor Imaging Study. Brain Sci. 2022, 12, 907. https://doi.org/10.3390/brainsci12070907
Yu Q, Sun Y, Liao X, Qian W, Ye T. Integrity of the Left Arcuate Fasciculus Segments Significantly Affects Language Performance in Individuals with Acute/Subacute Post-Stroke Aphasia: A Cross-Sectional Diffusion Tensor Imaging Study. Brain Sciences. 2022; 12(7):907. https://doi.org/10.3390/brainsci12070907
Chicago/Turabian StyleYu, Qiwei, Yan Sun, Xiaoyu Liao, Wenjun Qian, and Tianfen Ye. 2022. "Integrity of the Left Arcuate Fasciculus Segments Significantly Affects Language Performance in Individuals with Acute/Subacute Post-Stroke Aphasia: A Cross-Sectional Diffusion Tensor Imaging Study" Brain Sciences 12, no. 7: 907. https://doi.org/10.3390/brainsci12070907
APA StyleYu, Q., Sun, Y., Liao, X., Qian, W., & Ye, T. (2022). Integrity of the Left Arcuate Fasciculus Segments Significantly Affects Language Performance in Individuals with Acute/Subacute Post-Stroke Aphasia: A Cross-Sectional Diffusion Tensor Imaging Study. Brain Sciences, 12(7), 907. https://doi.org/10.3390/brainsci12070907