Abstract
Functional abnormalities in brain areas within the fronto-limbic network have been widely reported in obsessive–compulsive disorder (OCD). However, region- and network-level brain activities of the fronto-limbic network at rest have not been simultaneously investigated in OCD. In this study, 40 medicine-free and non-comorbidity patients with OCD and 38 age-, education-, and gender-matched healthy controls (HCs) underwent a resting-state functional magnetic-resonance-imaging scan. Fractional amplitude of low-frequency fluctuations (fALFF), network homogeneity (NH), and support vector machine were used to analyze the data. Patients with OCD showed increased fALFF in the right orbital frontal cortex (OFC), increased NH in the left OFC, and decreased NH in the right putamen. Decreased NH of the right putamen was negatively correlated with the Y-BOCS total and compulsive behavior scores. Furthermore, a combination of NH in the left OFC and right putamen could be applied to differentiate OCD from HCs with optimum specificity and sensitivity. The current findings emphasize the crucial role of the fronto-limbic network in the etiology of OCD.
1. Introduction
In addition to intrusive thoughts and/or compulsive behavior, anxiety, dysregulated fear, and uncertainty are the primary clinical characteristics of obsessive–compulsive disorder (OCD), which may affect daily life and social function of the patients [1,2,3,4]. Increasing findings demonstrated that these apparent characteristics may be caused by the dysfunction of brain circuits rather than a single brain region [5].
The cortico-striato-thalamo-cortical circuit including cortical areas, striatum, and thalamus has been a considerable model involving the neural basis of OCD over the years [6,7,8]. According to recent reviews, five parallel and segregated neural networks are related to different clinical characteristics of OCD: the fronto-limbic, dorsal cognitive, ventral cognitive, ventral affective, and sensorimotor networks [6]. The fronto-limbic circuit connecting the ventromedial prefrontal cortex (vmPFC), amygdala, and hippocampus is crucial for dysregulated fear and uncertainty of OCD [6].
Altered structure and function of the fronto-limbic network have been discovered in patients with OCD. For example, increased and/or decreased gray-matter volume in the vmPFC, hippocampus, and thalamus has been found in OCD [9,10,11]. Dysfunctional activities during emotional processing and increased activities during uncertainty and decision-making within the fronto-limbic network have been noted in OCD [2,12,13]. At the same time, hyperconnectivities within the fronto-limbic network (i.e., between caudate and orbital frontal cortex [OFC] and anterior cingulate cortex [ACC], and between vmPFC and ACC) have been discovered in OCD at rest [14]. Moreover, decreased amygdala-vmPFC functional connectivity and right amygdala degree centrality may predict a better outcome of cognitive behavior therapy in OCD [15,16]. The above-mentioned research related alterations in the fronto-limbic network to the pathophysiology of OCD.
Previous studies demonstrated that the local and network properties of brain function at rest are closely related [17,18,19]. However, region- and network-level brain activities of the fronto-limbic network at rest have not been simultaneously investigated in OCD. In the current research, fractional amplitude of low-frequency fluctuations (fALFF) combined with network homogeneity (NH) were applied to comprehensively assess the local and network properties of the fronto-limbic network in OCD at rest. FALFF explores the intensity of regional brain spontaneous activities, and it can be used to detect the regional brain activities at rest [20]. The NH approach evaluates the synchronization of a voxel with all other brain voxels in a brain network and supplies an assessment of a given network with an unbiased hypothesis-driven manner [21]. The combination of these two methods may provide complementary information underlying the fronto-limbic network involved in OCD [22]. We hypothesized that OCD could display changed fALFF and/or NH values within the fronto-limbic network at rest, which could be related to clinical characteristics (i.e., symptom severity and illness duration) and can be used to identify patients with OCD from HCs.
2. Materials and Methods
2.1. Participants
The participants comprised 40 patients with OCD (13 females and 27 males) and 38 HCs (13 females and 25 males). They were all Han Chinese, right-handed, and 18–50 years old. All subjects were informed of the aims and procedures of our study and sighed an informed consent form. This research was confirmed by the Research Ethics Committee of Qiqihar Medical University.
The diagnoses for each patient with OCD were conducted by two psychiatrists according to the Structured Clinical Interview for DMS-IV (SCID) patient version. The clinical symptoms of OCD were evaluated with the Yale–Brown Obsessive–Compulsive Scale (Y-BOCS), Hamilton Anxiety Rating Scale (HAMA), and 17-item Hamilton Depression Rating Scale (HAMD). All patients had Y-BOCS total score ≥16 and 17-HAMD score <18 and were psychotropic medication-free for at least 4 weeks. Eighteen patients with OCD were drug-naive; fourteen patients had a history of antiobsessive/antidepressant/anxiolytic medication (i.e., selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors, benzodiazepines, and buspirone); and eight patients had a history of antipsychotics (i.e., aripiprazole 5–20 mg/day). None of the patients were undergoing any systematic behavioral therapy or cognitive behavioral therapy. HCs were screened using the SCID non-patient version. Exclusion criteria of all participants included (1) any other psychiatric disorder; (2) serious physical or neurological disease; (3) drug or alcohol dependence; and (4) contraindication for an MRI scan.
2.2. MRI Data Acquisition
MRI images were obtained with a 3.0-Tesla GE 750 Signa-HDX scanner. All subjects were instructed to lie quietly, close their eyes, and stay awake. Resting-state functional magnetic resonance imaging (rs-fMRI) data were acquired with an echo-planar imaging sequence: 33 axial slices, 2000 ms repetition time, 30 ms echo time, 3.5 mm slice thickness, 0.6 mm inter-slice gap, 90° flip angle, 200 × 200 mm2 field of view, 64 × 64 data matrix, and 240 volumes in total. All subjects manifested no significantly structural abnormalities in the brain.
2.3. fMRI Data Preprocessing
The Data Processing Assistant for Brain Imaging (DPABI) software was used to conduct the fMRI data preprocessing [23], which included the following steps: discarding the first 10 functional volumes, slice timing and head motion correction, normalization and spatial resampling to 3 × 3 × 3 mm3, smoothing with an isotropic Gaussian kernel of 8 mm, band-pass filtering (0.01–0.08 Hz), regression of the nuisance covariates (i.e., cerebrospinal fluid, white matter, and 24 motion parameters), and scrubbing of time points with a threshold of 0.2 mm of framewise displacement (FD) [24].
2.4. Fronto-Limbic Network Mask Identification
We established the fronto-limbic network mask based on the anatomical automatic labeling templates, including the superior medial frontal gyrus, superior frontal gyrus, middle frontal gyrus, inferior frontal gyrus, ACC, medial OFC, amygdala, thalamus, and hippocampus [25] (Figure S1 in Supplementary Materials).
2.5. FALFF Analysis
FALFF analysis was conducted with REST software with the following steps [26]. First, the time series were transformed into the frequency domain to obtain the power spectrum using fast Fourier transform. Second, the square root of the spectral power spectrum was calculated at each frequency and averaged across 0.01–0.08 Hz in each voxel. Finally, for standardization purposes, the sum of amplitude was further divided by the whole frequency range within the fronto-limbic network mask for each subject, and the fALFF value was obtained.
2.6. NH Analysis
NH analysis was conducted based on MATLAB. For each participant, correlation coefficients were obtained for each voxel in relation to all other voxels within the fronto-limbic network mask. The mean correlation coefficient refers to the NH of a given voxel. Then average NH of each voxel of the fronto-limbic network was generated. A Gaussian kernel of 4 mm was used to smooth the averaged NH maps twice [21]. Finally, the NH maps of the fronto-limbic network were used for group comparison.
2.7. Statistical Analysis
Two-sample t-tests and chi-square test were used to analyze the continuous variables and categorical data of demographic characteristics between OCD and HCs, respectively. Two-sample t-tests were utilized to compare the fALFF and NH values between the two groups in voxel-wise within the fronto-limbic network, with age, sex, and mean FD values as covariates for minimizing the potential effects. p < 0.05 corrected by Gaussian random field (voxel significance: p < 0.001, cluster significance: p < 0.05) was the significant level.
Pearson analysis was conducted to evaluate the correlation between the altered fALFF/NH values (mean z values of fALFF/NH) and clinical characteristics in OCD. p < 0.05 (Bonferroni corrected) was the significant level.
Support vector machine (SVM) analysis was conducted using LIBSVM (https://github.com/cjlin1/libsvm (accessed on 16 August 2020)) to explore whether changed fALFF and/or NH values within the fronto-limbic network can be utilized to identify OCD from HCs. The SVM model consisted of a training dataset for selecting discriminating clusters and testing dataset for checking the classification performance. SVM analysis was executed via a “leave-one-out” approach.
3. Results
3.1. Demographic and Clinical Data
Age, gender, education, and FD values were not significantly different between the patients with OCD and HCs. However, patients with OCD had higher Y-BOCS total and subscale scores, HAMD, and HAMA scores relative to HCs (Table 1).

Table 1.
Sociodemographic and clinical characteristics of participants.
3.2. Group Differences of fALFF within the Fronto-Limbic Network

Table 2.
Regions with altered fALFF/NH in fronto-limbic network at rest in OCD.
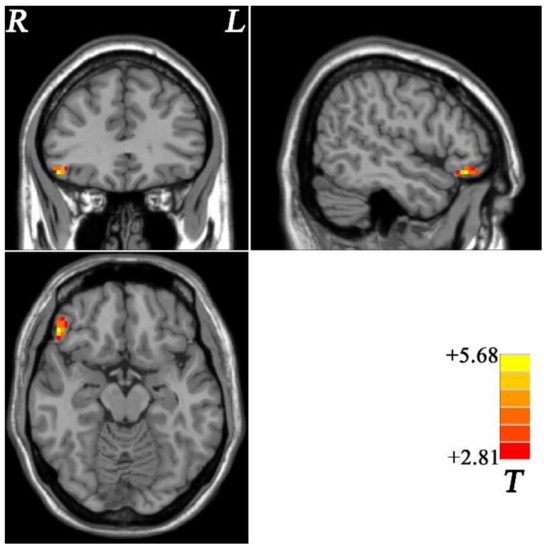
Figure 1.
Brain regions with altered fALFF within fronto-limbic network at rest in OCD. The color bar indicates the T values from two-sample t-tests. fALFF = fractional amplitude of low-frequency fluctuations, OCD = obsessive–compulsive disorder, L = left, R = right.
3.3. Group Deviations of NH within the Fronto-Limbic Network
OCD displayed increased NH in the left OFC and decreased NH in the right putamen compared with HCs (Table 2 and Figure 2).
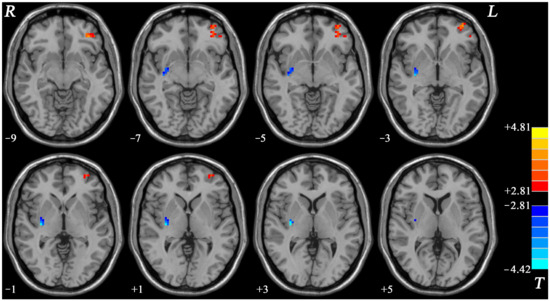
Figure 2.
Brain regions with changed NH of fronto-limbic network at rest in OCD. The color bar indicates the T values from two-sample t-tests. NH = network homogeneity, OCD = obsessive–compulsive disorder, L = left, R = right.
3.4. Relationship between fALFF/NH Values and Clinical Characteristics in OCD
The decreased NH value of the right putamen was negatively related to the Y-BOCS total scores (r = −0.332, p = 0.036) and compulsive behavior scores (r = −0.336, p = 0.034) (Figure 3).
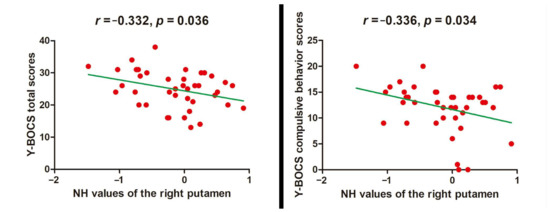
Figure 3.
Relationship between NH values and clinical characteristics in OCD. NH = network homogeneity, OCD = obsessive–compulsive disorder, Y-BOCS = Yale–Brown Obsessive–compulsive Scale.
3.5. SVM Results
SVM analysis proceeded with three abnormal fALFF/NH values (1 = right OFC, 2 = left OFC, 3 = right putamen) discovered in OCD and pairwise combinations. The results were as follows: 1 accuracy = 76.92% (60/78; classification); 2 accuracy = 78.21% (61/78; classification); 3 accuracy = 70.51% (55/78; classification); 12 accuracy = 80.77% (63/78; classification); 13 accuracy = 79.49% (62/78; classification); and 23 accuracy = 89.74% (70/78; classification). The accuracy of the combination of 2 and 3 was the highest (Figure 4), and thus it could be utilized to distinguish OCD from HCs with a sensitivity of 95.00% (38/40), a specificity of 84.21% (32/38), and an accuracy of 89.74% (70/78) (Figure 5).
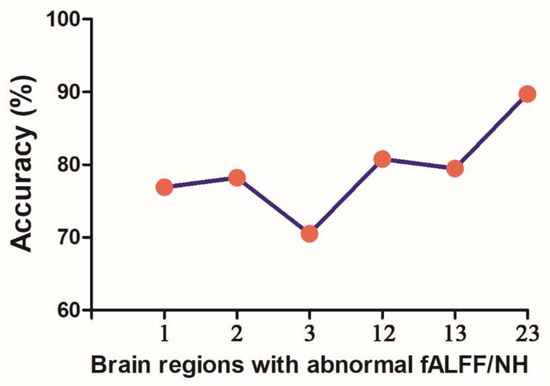
Figure 4.
Accuracy (%) of SVM using three brain regions with altered fALFF/NH values of fronto-limbic network to discriminate OCD from HCs. fALFF = fractional amplitude of low-frequency fluctuations, NH = network homogeneity, 1 = right orbitofrontal cortex, 2 = left orbitofrontal cortex, 3 = right putamen, SVM = support vector machine, OCD = obsessive–compulsive disorder, HCs = healthy controls.
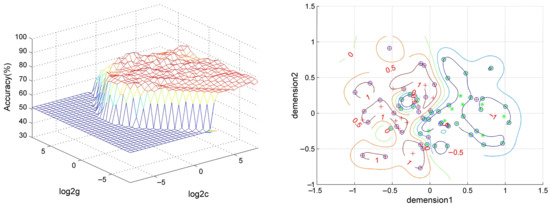
Figure 5.
Visualization of SVM results using NH values of left orbitofrontal cortex and right putamen. Left: 3D visualization of SVM with the best parameters; right: classification map of the NH values of left orbitofrontal cortex and right putamen. SVM = support vector machine, NH = network homogeneity, log 2c and log 2g = the range and step size of c and g (c and g are the parameters of the kernel functions).
4. Discussion
In the current research, fALFF and NH approaches were used to investigate the regional- and network-level brain activities within the fronto-limbic network at rest in medicine-free OCD. The results manifested that increased fALFF in the right OFC, increased NH in the left OFC, and decreased NH in the right putamen were discovered at rest in OCD. In addition, decreased NH of the right putamen was negatively related to the Y-BOCS total and compulsive behavior scores. A combination of NH in the left OFC and right putamen could be utilized to identify OCD from HCs with optimum specificity and sensitivity.
Consistent with previous neuroimaging studies, our findings manifested increased fALFF value in the right OFC at rest in OCD [27,28,29]. Moreover, we discovered the increased NH value in the left OFC in OCD. As a component of the fronto-limbic network, the OFC has a crucial role in OCD etiology by emotional regulation and reward processing [30]. Increased fALFF and NH in the OFC may underpin aberrant coordination within the fronto-limbic network at rest, and it may be related to more effort to modulate negative emotion (i.e., anxiety and fear) and tolerance of uncertainty in patients with OCD. By contrast, the changed brain regions were not the same using fALFF and NH approaches in the current study. We infer that the increased spontaneous neuronal activity of the right OFC may contribute to enhanced connectivity of the left OFC within the fronto-limbic network at rest due to the dynamic brain networks [25].
Our research revealed decreased NH of the right putamen at rest in patients with OCD. Previous studies found increased gray-matter volume, lowered functional connectivity at rest, and increased activation during emotional processing in the right putamen in OCD [2,31,32]. As a part of the striatum, putamen receives and integrates information from the cortex [33]. Furthermore, putamen, a key region of the fronto-limbic network, participates in the sensorimotor network in OCD [6]. OCD pathology (i.e., maladaptive habituation behavior mediated by the sensorimotor network) may cause the decreased NH of the right putamen at rest in OCD [6], which may interpret the relationship between decreased NH values of the right putamen and clinical symptoms of OCD discovered in the current study. The SVM results showed that a combination of NH in the left OFC and right putamen may be used to differentiate individuals with OCD from HCs, suggesting the crucial role of the network-level brain activities of the fronto-limbic network at rest in the neurocircuit-based classification in patients with OCD.
In the fronto-limbic model, we found that patients with OCD showed increased regional- and network-level brain activities in the frontal cortex and decreased network-level in the limbic system at rest. Notably, these brain regions also participate in other networks (i.e., ventral affective and sensorimotor network) involving other functions [6]. Therefore, the fronto-limbic network may work with other networks involved in OCD [6].
As important brain regions of the fronto-limbic network, vmPFC, ACC, and amygdala showed no altered regional- and network-level brain activities at rest in patients with OCD in our research, which was inconsistent with previous findings [14,15]. Heterogeneity of clinical samples (i.e., sample sizes, comorbidity, and medication status) may explain these inconsistencies [34]. In addition, the low statistical power of a relatively small sample size may lead to the low reproducibility of neuroimaging results [35]. The activities of these brain regions are highly sensitive to emotional stimuli and symptomatic provocation but not particularly changed at rest [1].
Compared with medicine-free patients, OCD-medicated patients showed decreased activation of the OFC during symptom provocation tasks [36] and increased functional connectivity between the right putamen and the left frontal cortex at rest [37]. Drug treatment may normalize OCD-related impaired segregation of the functional network in the whole brain [38]. For this reason, some patients with OCD had a history of psychotropic medication in the current study, which may affect the local and network properties of the fronto-limbic network at rest. Some possible unmeasured variables, such as social effects and physical effects, may also affect the current results [39,40]. For these reasons, the current findings should be prudently interpreted.
This study has several limitations. First, we explored the regional- and network-level brain activities in OCD at rest, not the task state, which is closely related to the function of the fronto-limbic network. Second, we did not investigate the causality between the cortical and subcortical brain areas of the fronto-limbic network in OCD. Finally, whether the current altered regional- and network-level brain activities change with treatment needs to be determined in longitudinal studies.
5. Conclusions
We found altered regional- and network-level brain activities within the fronto-limbic network in medicine-free and non-comorbidity patients with OCD at rest. Our findings emphasize the crucial role of the fronto-limbic network in the etiology of OCD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/brainsci12070857/s1, Figure S1: Fronto-limbic network mask.
Author Contributions
Methodology, W.G.; software, Z.D. and X.Y.; validation, Z.Y. and G.Z.; formal analysis, R.Y.; resources, J.M., C.Z. and Z.S.; data curation, D.L., T.S. and J.X.; writing—original draft preparation, Y.C. and Y.O.; writing—review and editing, X.W., W.G. and P.L.; funding acquisition, P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from Heilongjiang Natural Science Foundation of China (LH2019H064).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the the Research Ethics Committee of Qiqihar Medical University (protocol code 003, 2013).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Our data may be available upon reasonable request. Please contact lipingchxyy@163.com for details.
Conflicts of Interest
The authors declare no conflict of interest.
References
- van den Heuvel, O.A.; van Wingen, G.; Soriano-Mas, C.; Alonso, P.; Chamberlain, S.R.; Nakamae, T.; Denys, D.; Goudriaan, A.E.; Veltman, D.J. Brain circuitry of compulsivity. Eur. Neuropsychopharmacol. 2016, 26, 810–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorsen, A.L.; Hagland, P.; Radua, J.; Mataix-Cols, D.; Kvale, G.; Hansen, B.; Heuvel, O.A.V.D. Emotional processing in obsessive-compulsive disorder: A systematic review and meta-analysis of 25 functional neuroimaging studies. Biol. Psychiatry 2018, 3, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Pinciotti, C.M.; Riemann, B.C.; Abramowitz, J.S. Intolerance of uncertainty and obsessive-compulsive disorder dimensions. J. Anxiety Disord. 2021, 81, 102417. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tang, Z.; Wang, X.; Ma, X.; Cheng, Y.; Wang, B.; Sun, P.; Tang, W.; Luo, J.; Wang, C.; et al. The cost of obsessive-compulsive disorder (OCD) in China: A multi-center cross-sectional survey based on hospitals. Gen. Psychiatr. 2021, 34, e100632. [Google Scholar] [CrossRef]
- Gürsel, D.A.; Avram, M.; Sorg, C.; Brandl, F.; Koch, K. Frontoparietal areas link impairments of large-scale intrinsic brain networks with aberrant fronto-striatal interactions in OCD: A meta-analysis of resting-state functional connectivity. Neurosci. Biobehav. Rev. 2018, 87, 151–160. [Google Scholar] [CrossRef]
- Shephard, E.; Stern, E.R.; van den Heuvel, O.A.; Costa, D.L.C.; Batistuzzo, M.C.; Godoy, P.B.G.; Lopes, A.C.; Brunoni, A.R.; Hoexter, M.Q.; Shavitt, R.G.; et al. Toward a neurocircuit-based taxonomy to guide treatment of obsessive-compulsive disorder. Mol. Psychiatry 2021, 26, 4583–4604. [Google Scholar] [CrossRef]
- Soriano-Mas, C. Functional Brain Imaging and OCD. Curr. Top. Behav. Neurosci. 2021, 49, 269–300. [Google Scholar]
- Stein, D.J.; Costa, D.L.C.; Lochner, C.; Miguel, E.C.; Reddy, Y.C.J.; Shavitt, R.G.; van den Heuvel, O.A.; Simpson, H.B. Obsessive-compulsive disorder. Nat. Rev. Dis. Primers 2019, 5, 52. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Du, M.; Chen, L.; Li, L.; Zhou, M.; Zhang, L.; Liu, Q.; Lu, L.; Mreedha, K.; Huang, X.; et al. Meta-analytic investigations of common and distinct grey matter alterations in youths and adults with obsessive-compulsive disorder. Neurosci. Biobehav. Rev. 2017, 78, 91–103. [Google Scholar] [CrossRef]
- Peng, Z.; Lui, S.S.; Cheung, E.F.; Jin, Z.; Miao, G.; Jing, J.; Chan, R.C. Brain structural abnormalities in obsessive-compulsive disorder: Converging evidence from white matter and grey matter. Asian J. Psychiatr. 2012, 5, 290–296. [Google Scholar] [CrossRef]
- Rao, S.; Raveendranathan, D.; Shivakumar, V.; Narayanaswamy, J.C.; Venkatasubramanian, G.; Reddy, Y.C.J. Hippocampus volume alterations and the clinical correlates in medication naive obsessive compulsive disorder. J. Affect. Disord. 2018, 236, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Weidt, S.; Lutz, J.; Rufer, M.; Delsignore, A.; Jakob, N.J.; Herwig, U.; Bruehl, A.B. Common and differential alterations of general emotion processing in obsessive-compulsive and social anxiety disorder. Psychol. Med. 2016, 46, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Stern, E.R.; Welsh, R.C.; Gonzalez, R.; Fitzgerald, K.D.; Abelson, J.L.; Taylor, S.F. Subjective uncertainty and limbic hyperactivation in obsessive-compulsive disorder. Hum. Brain Mapp. 2013, 34, 1956–1970. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Cao, L.; Li, H.; Gao, Y.; Bu, X.; Liang, K.; Bao, W.; Zhang, S.; Qiu, H.; Li, X.; et al. Abnormal resting-state functional connectivity in patients with obsessive-compulsive disorder: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2022, 135, 104574. [Google Scholar] [CrossRef] [PubMed]
- Fullana, M.A.; Zhu, X.; Alonso, P.; Cardoner, N.; Real, E.; Lopez-Sola, C.; Segalas, C.; Subira, M.; Galfalvy, H.; Menchon, J.M.; et al. Basolateral amygdala-ventromedial prefrontal cortex connectivity predicts cognitive behavioural therapy outcome in adults with obsessive-compulsive disorder. J. Psychiatry Neurosci. 2017, 42, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Gottlich, M.; Kramer, U.M.; Kordon, A.; Hohagen, F.; Zurowski, B. Resting-state connectivity of the amygdala predicts response to cognitive behavioral therapy in obsessive compulsive disorder. Biol. Psychol. 2015, 111, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Zalesky, A.; Fornito, A.; Egan, G.F.; Pantelis, C.; Bullmore, E.T. The relationship between regional and inter-regional functional connectivity deficits in schizophrenia. Hum. Brain Mapp. 2012, 33, 2535–2549. [Google Scholar] [CrossRef]
- Di, X.; Kim, E.H.; Huang, C.C.; Tsai, S.J.; Lin, C.P.; Biswal, B.B. The influence of the amplitude of low-frequency fluctuations on resting-state functional connectivity. Front. Hum. Neurosci. 2013, 7, 118. [Google Scholar] [CrossRef] [Green Version]
- Riedl, V.; Bienkowska, K.; Strobel, C.; Tahmasian, M.; Grimmer, T.; Förster, S.; Friston, K.J.; Sorg, C.; Drzezga, A. Local activity determines functional connectivity in the resting human brain: A simultaneous FDG-PET/fMRI study. J. Neurosci. 2014, 34, 6260–6266. [Google Scholar] [CrossRef]
- Zou, Q.H.; Zhu, C.Z.; Yang, Y.; Zuo, X.N.; Long, X.Y.; Cao, Q.J.; Wang, Y.F.; Zang, Y.F. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J. Neurosci. Methods 2008, 172, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Uddin, L.Q.; Kelly, A.M.C.; Biswal, B.B.; Margulies, D.S.; Shehzad, Z.; Shaw, D.; Ghaffari, M.; Rotrosen, J.; Adler, L.A.; Castellanos, F.X. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J. Neurosci. Methods 2008, 169, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Fan, J.; Liu, W.; Du, H.; Zhu, J.; Yi, J.; Tan, C.; Zhu, X. Functional connectivity within the salience network differentiates autogenous- from reactive-type obsessive-compulsive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 98, 109813. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.G.; Wang, X.D.; Zuo, X.N.; Zang, Y.F. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 2016, 14, 339–351. [Google Scholar] [PubMed]
- Power, J.D.; Barnes, K.A.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 2012, 59, 2142–2154. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Liu, F.; Yu, M.; Zhang, J.; Zhang, Z.; Liu, J.; Xiao, C.; Zhao, J. Decreased regional activity and network homogeneity of the fronto-limbic network at rest in drug-naive major depressive disorder. Aust. N. Z. J. Psychiatry 2015, 49, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Song, X.W.; Dong, Z.Y.; Long, X.Y.; Li, S.F.; Zuo, X.N.; Zhu, C.Z.; He, Y.; Yan, C.G.; Zang, Y.F. REST: A toolkit for resting-state functional magnetic resonance imaging data processing. PLoS ONE 2011, 6, e25031. [Google Scholar]
- Meng, Z.; Zhang, Z.; Fan, Q.; Li, Y. Altered Fractional Amplitude of Low Frequency Fluctuations in Unmedicated Female Patients with Obsessive-Compulsive Disorder. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2018, 2018, 1144–1147. [Google Scholar]
- Hou, J.M.; Wu, W.J.; Lin, Y.; Wang, J.; Zhou, D.Q.; Guo, J.W.; Gu, S.S.; He, M.; Ahmed, S.; Hu, J.N.; et al. Localization of cerebral functional deficits in patients with obsessive-compulsive disorder: A resting-state fMRI study. J. Affect. Disord. 2012, 138, 313–321. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, Q.; Zhang, H.; Qiu, J.; Tan, L.; Xiao, Z.; Tong, S.; Chen, J.; Li, Y. Altered intrinsic insular activity predicts symptom severity in unmedicated obsessive-compulsive disorder patients: A resting state functional magnetic resonance imaging study. BMC Psychiatry 2016, 16, 104. [Google Scholar] [CrossRef] [Green Version]
- Milad, M.R.; Rauch, S.L. Obsessive-compulsive disorder: Beyond segregated cortico-striatal pathways. Trends Cogn. Sci. 2012, 16, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Eng, G.K.; Sim, K.; Chen, S.H. Meta-analytic investigations of structural grey matter, executive domain-related functional activations, and white matter diffusivity in obsessive compulsive disorder: An integrative review. Neurosci. Biobehav. Rev. 2015, 52, 233–257. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hu, X.; Tang, W.; Li, B.; Yang, Y.; Gong, Q.; Huang, X. Intrinsic brain abnormalities in drug-naive patients with obsessive-compulsive disorder: A resting-state functional MRI study. J. Affect. Disord. 2019, 245, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Dagher, A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb. Cortex 2006, 16, 1508–1521. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Ou, Y.; Chen, Y.; Yang, R.; Zhong, Z.; Jia, C.; Sun, L.; Wang, Y.; Zhang, G.; Sun, Z.; et al. Increased cerebellar-default-mode network connectivity at rest in obsessive-compulsive disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Button, K.S.; Ioannidis, J.P.; Mokrysz, C.; Nosek, B.A.; Flint, J.; Robinson, E.S.; Munafo, M.R. Power failure: Why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013, 14, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Nakao, T.; Nakagawa, A.; Yoshiura, T.; Nakatani, E.; Nabeyama, M.; Yoshizato, C.; Kudoh, A.; Tada, K.; Yoshioka, K.; Kawamoto, M.; et al. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: A functional magnetic resonance imaging study. Biol. Psychiatry 2005, 57, 901–910. [Google Scholar] [CrossRef]
- Bernstein, G.A.; Cullen, K.R.; Harris, E.C.; Conelea, C.A.; Zagoloff, A.D.; Carstedt, P.A.; Lee, S.S.; Mueller, B.A. Sertraline Effects on Striatal Resting-State Functional Connectivity in Youth with Obsessive-Compulsive Disorder: A Pilot Study. J. Am. Acad Child. Adolesc. Psychiatry 2019, 58, 486–495. [Google Scholar] [CrossRef]
- Shin, D.J.; Jung, W.H.; He, Y.; Wang, J.; Shim, G.; Byun, M.S.; Jang, J.H.; Kim, S.N.; Lee, T.Y.; Park, H.Y.; et al. The effects of pharmacological treatment on functional brain connectome in obsessive-compulsive disorder. Biol. Psychiatry 2014, 75, 606–614. [Google Scholar] [CrossRef]
- Kempermann, G. Physical activity and brain function. Internist 2012, 53, 698–704. [Google Scholar] [CrossRef]
- Fuchs, E.; Flügge, G. Social stress in tree shrews: Effects on physiology, brain function, and behavior of subordinate individuals. Pharmacol. Biochem. Behav. 2002, 73, 247–258. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).