Physiological and Neural Changes with Rehabilitation Training in a 53-Year Amputee: A Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
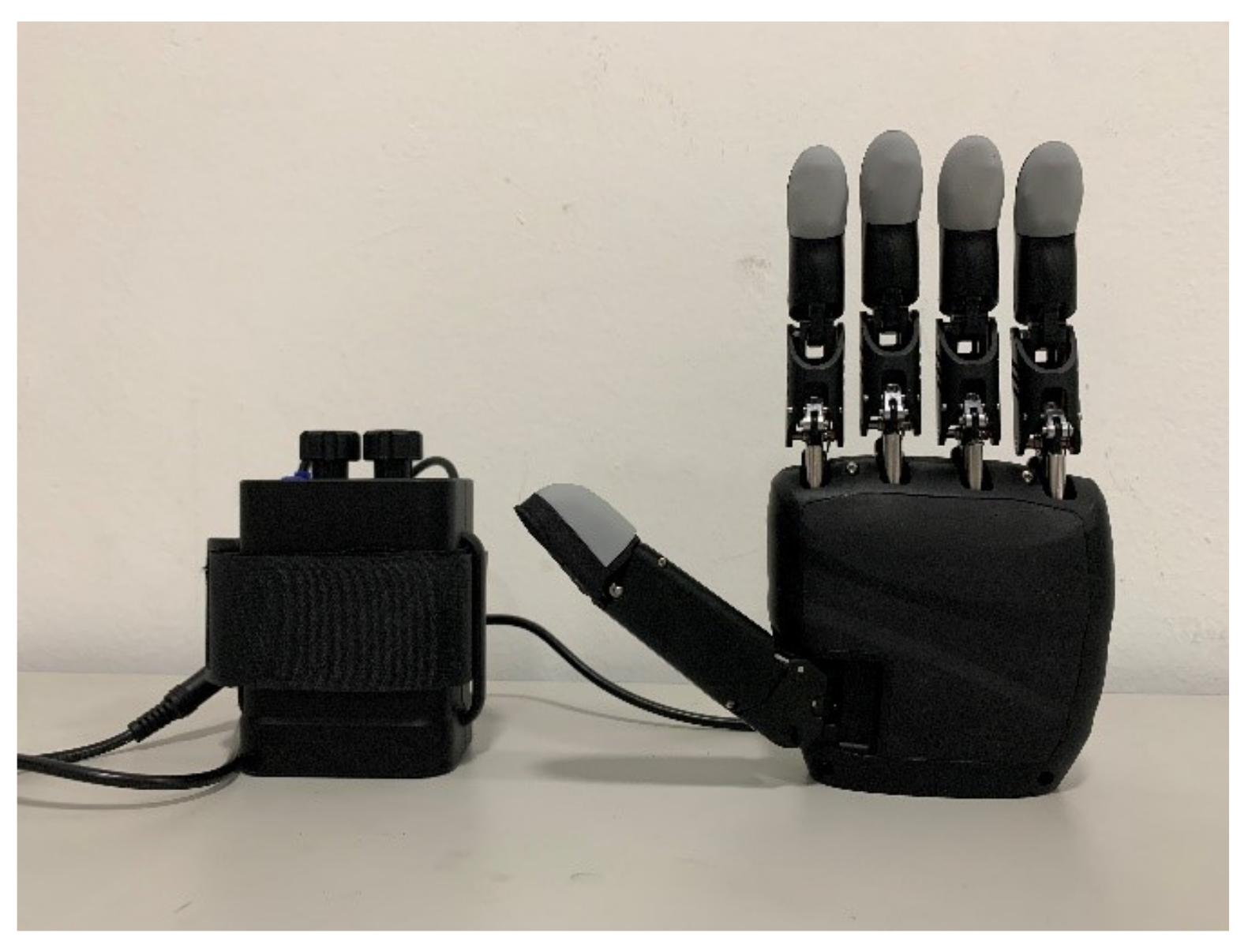
2.2. Semg Prosthesis
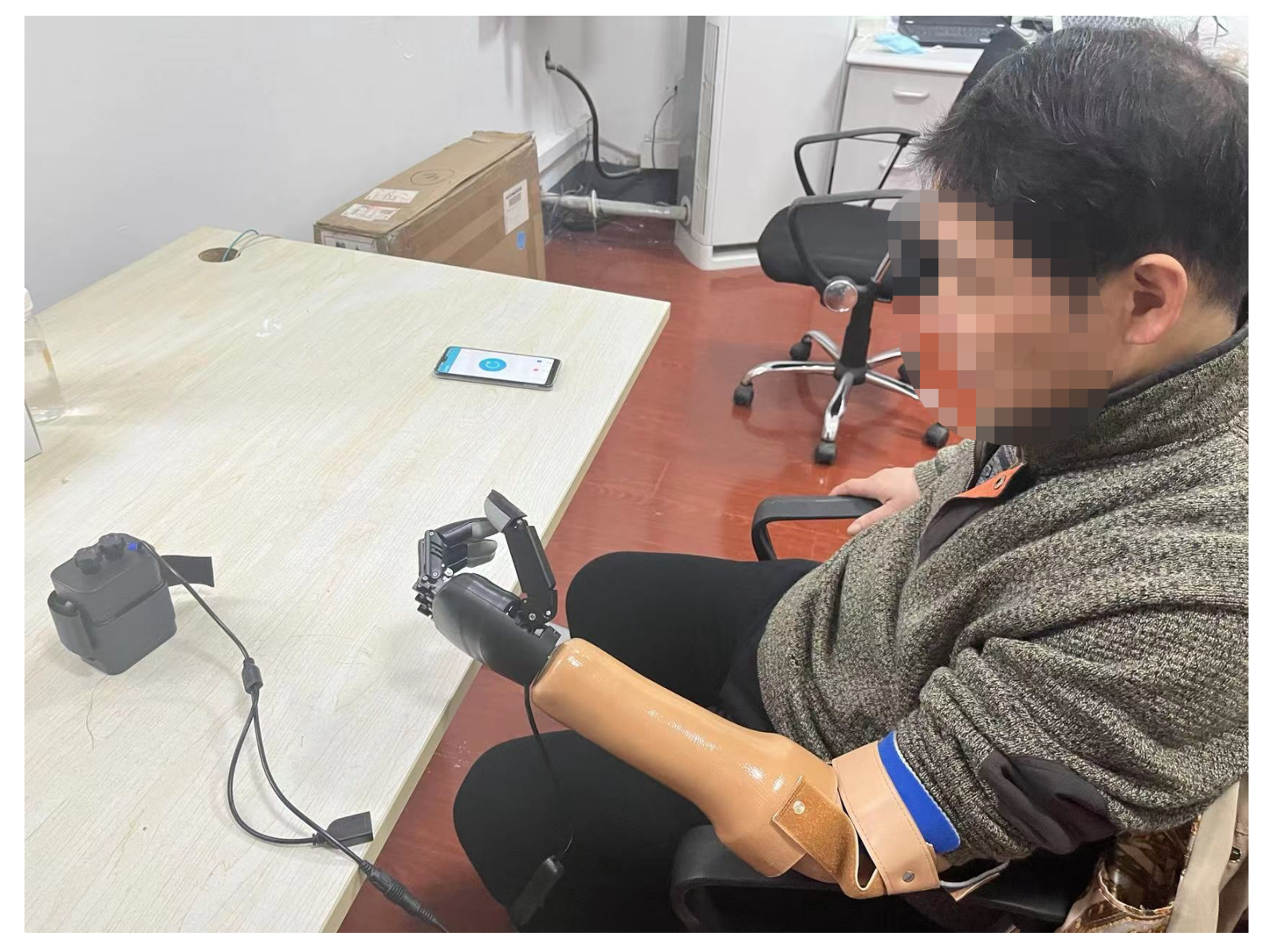
2.3. Prosthetic Training
2.4. Data Acquisition
2.4.1. sEMG
2.4.2. EEG
2.4.3. Resting Motor Threshold (rMT)
2.5. Data Processing
2.5.1. sEMG
2.5.2. EEG
3. Results
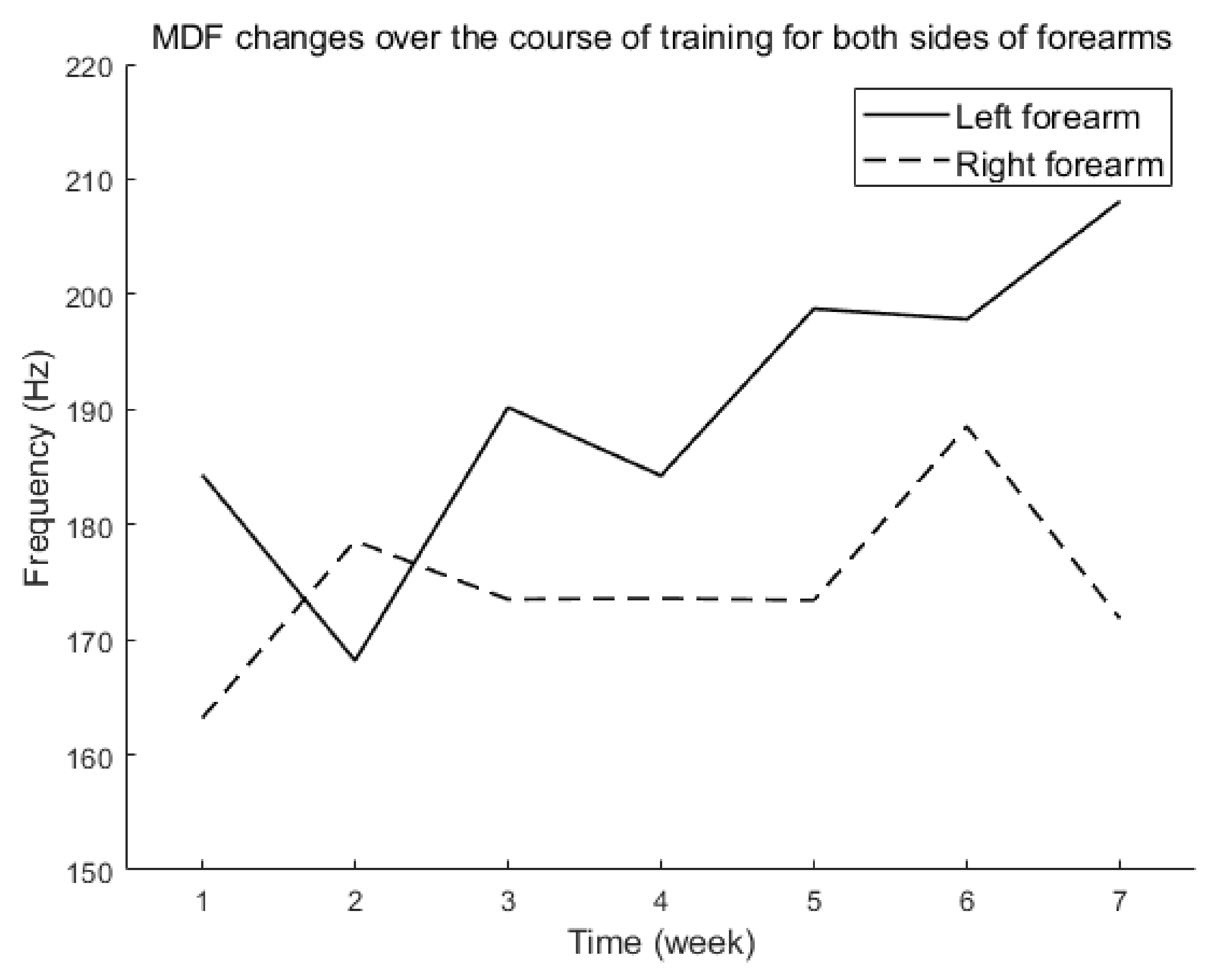
3.1. sEMG
3.2. EEG
3.3. rMT
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ephraim, P.L.; Dillingham, T.R.; Sector, M.; Pezzin, L.E.; MacKenzie, E.J. Epidemiology of limb loss and congenital limb deficiency: A review of the literature11No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the author(s) or upon any organization with which the author(s) is/are associated. Arch. Phys. Med. Rehabil. 2003, 84, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, N.; Hamada, M.; Diedrichsen, J. Hand use predicts the structure of representations in sensorimotor cortex. Nat. Neurosci. 2015, 18, 1034–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kew, J.J.; Ridding, M.C.; Rothwell, J.C.; Passingham, R.E.; Leigh, P.N.; Sooriakumaran, S.; Frackowiak, R.S.; Brooks, D.J. Reorganization of cortical blood flow and transcranial magnetic stimulation maps in human subjects after upper limb amputation. J. Neurophysiol. 1994, 72, 2517–2524. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, S.; Liu, H.; Chen, Y. The activation of the cortical hand area by toe tapping in two bilateral upper-extremities amputees with extraordinary foot movement skill. Magn. Reson. Imaging 2006, 24, 45–50. [Google Scholar] [CrossRef]
- Flor, H.; Elbert, T.; Knecht, S.; Wienbruch, C.; Pantev, C.; Birbaumer, N.; Larbig, W.; Taub, E. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature 1995, 375, 482–484. [Google Scholar] [CrossRef]
- MacIver, K.; Lloyd, D.; Kelly, S.; Roberts, N.; Nurmikko, T. Phantom limb pain, cortical reorganization and the therapeutic effect of mental imagery. Brain J. Neurol. 2008, 131, 2181–2191. [Google Scholar] [CrossRef]
- Flor, H.; Denke, C.; Schaefer, M.; Grüsser, S. Effect of sensory discrimination training on cortical reorganisation and phantom limb pain. Lancet 2001, 357, 1763–1764. [Google Scholar] [CrossRef]
- Preißler, S.; Feiler, J.; Dietrich, C.; Hofmann, G.O.; Miltner, W.H.R.; Weiss, T. Gray Matter Changes Following Limb Amputation with High and Low Intensities of Phantom Limb Pain. Cereb. Cortex 2012, 23, 1038–1048. [Google Scholar] [CrossRef] [Green Version]
- Al-Timemy, A.H.; Khushaba, R.N.; Bugmann, G.; Escudero, J. Improving the Performance Against Force Variation of EMG Controlled Multifunctional Upper-Limb Prostheses for Transradial Amputees. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 650–661. [Google Scholar] [CrossRef] [Green Version]
- Farina, D.; Jiang, N.; Rehbaum, H.; Holobar, A.; Graimann, B.; Dietl, H.; Aszmann, O.C. The Extraction of Neural Information from the Surface EMG for the Control of Upper-Limb Prostheses: Emerging Avenues and Challenges. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 797–809. [Google Scholar] [CrossRef]
- Mioton, L.; Dumanian, G. Targeted muscle reinnervation and prosthetic rehabilitation after limb loss. J. Surg. Oncol. 2018, 118, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Marasco, P.D.; Kim, K.; Colgate, J.E.; Peshkin, M.A.; Kuiken, T.A. Robotic touch shifts perception of embodiment to a prosthesis in targeted reinnervation amputees. Brain 2011, 134, 747–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Colgate, J.E.; Santos-MunnÉ, J.J.; Makhlin, A.; Peshkin, M.A. On the Design of Miniature Haptic Devices for Upper Extremity Prosthetics. IEEE/ASME Trans. Mechatro. 2010, 15, 27–39. [Google Scholar] [CrossRef]
- Heiligenberg, F.; Orlov, T.; Macdonald, S.; Duff, E.; Henderson Slater, D.; Beckmann, C.; Johansen-Berg, H.; Culham, J.; Makin, T. Artificial limb representation in amputees. Brain J. Neurol. 2018, 141, 1422–1433. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zheng, X.; Li, P.; Xu, L.; He, L.; Mei, Z.; Zhu, Y.; Huang, G.; Zhong, C.; Song, S. Cerebral blood perfusion changes in amputees with myoelectric hands after rehabilitation: A SPECT computer-aided analysis. BMC Neurosci. 2016, 17, 59. [Google Scholar] [CrossRef] [Green Version]
- Serino, A.; Akselrod, M.; Salomon, R.; Martuzzi, R.; Blefari, M.L.; Canzoneri, E.; Rognini, G.; van der Zwaag, W.; Iakova, M.; Luthi, F.; et al. Upper limb cortical maps in amputees with targeted muscle and sensory reinnervation. Brain 2017, 140, 2993–3011. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A.; Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef] [Green Version]
- Hétu, S.; Gagné, M.; Reilly, K.; Mercier, C. Short-term reliability of transcranial magnetic stimulation motor maps in upper limb amputees. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2011, 18, 728–730. [Google Scholar] [CrossRef]
- Ciciliot, S.; Rossi, A.C.; Dyar, K.A.; Blaauw, B.; Schiaffino, S. Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2191–2199. [Google Scholar] [CrossRef]
- Loughna, P.; Izumo, S.; Goldspink, G.; Nadal-Ginard, B. Disuse and passive stretch cause rapid alterations in expression of developmental and adult contractile protein genes in skeletal muscle. Development 1990, 109, 217–223. [Google Scholar] [CrossRef]
- Gerdle, B.; Henriksson-Larsen, K.; Lorentzon, R.; Wretling, M.L. Dependence of the mean power frequency of the electromyogram on muscle force and fibre type. Acta Physiol. Scand. 1991, 142, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Gerdle, B.; Karlsson, S.; Crenshaw, A.G.; Elert, J.; Fridén, J. The influences of muscle fibre proportions and areas upon EMG during maximal dynamic knee extensions. Eur. J. Appl. Physiol. 2000, 81, 2–10. [Google Scholar] [CrossRef]
- Gerdle, B.; Karlsson, S.; Crenshaw, A.G.; Friden, J. The relationships between EMG and muscle morphology throughout sustained static knee extension at two submaximal force levels. Acta Physiol. Scand. 1997, 160, 341–351. [Google Scholar] [CrossRef]
- Gerdle, B.; Wretling, M.L.; Henriksson-Larsén, K. Do the fibre-type proportion and the angular velocity influence the mean power frequency of the electromyogram? Acta Physiol. Scand. 1988, 134, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Moritani, T.; Gaffney, F.; Carmichael, T.; Hargis, J. Interrelationships among muscle fiber types, electromyogram and blood pressure during fatiguing isometric contraction. In Biomechanics IX-A International Series on Biomechanics; Human Kinetics: Champaign, IL, USA, 1985; pp. 287–292. [Google Scholar]
- Wretling, M.; Gerdle, B.; Henriksson-Larsén, K. EMG: A non-invasive method for determination of fibre type proportion. Acta Physiol. Scand. 1987, 131, 627–628. [Google Scholar] [CrossRef]
- Bassett, D.; Bullmore, E. Human Brain Networks in Health and Disease. Curr. Opin. Neurol. 2009, 22, 340–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leistedt, S.J.; Coumans, N.; Dumont, M.; Lanquart, J.P.; Stam, C.J.; Linkowski, P. Altered sleep brain functional connectivity in acutely depressed patients. Hum. Brain Mapp. 2009, 30, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Latora, V.; Marchiori, M. Efficient Behavior of Small-World Networks. Phys. Rev. Lett. 2001, 87, 198701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ‘small-world’ networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef]
- Humphries, M.D.; Gurney, K. Network ‘small-world-ness’: A quantitative method for determining canonical network equivalence. PLoS ONE 2008, 3, e0002051. [Google Scholar] [CrossRef]
- Gilbertson, T.; Lalo, E.; Doyle, L.; Di Lazzaro, V.; Cioni, B.; Brown, P. Existing Motor State Is Favored at the Expense of New Movement during 13–35 Hz Oscillatory Synchrony in the Human Corticospinal System. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 7771–7779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maltz, M. New Psycho-Cybernetics; Penguin: London, UK, 2002. [Google Scholar]


| Measure Time | Stimulate Site | Collection Site | Intensity of RMT (%) |
|---|---|---|---|
| Enrollment experiment | Motor cortex M1 area | Abductor pollicis brevis | 70 |
| The third week | Motor cortex M1 area | Abductor pollicis brevis | 42 |
| The sixth week | Motor cortex M1 area | Abductor pollicis brevis | 41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, L.; Lu, X.; Yu, C.; Yin, K. Physiological and Neural Changes with Rehabilitation Training in a 53-Year Amputee: A Case Study. Brain Sci. 2022, 12, 832. https://doi.org/10.3390/brainsci12070832
Mao L, Lu X, Yu C, Yin K. Physiological and Neural Changes with Rehabilitation Training in a 53-Year Amputee: A Case Study. Brain Sciences. 2022; 12(7):832. https://doi.org/10.3390/brainsci12070832
Chicago/Turabian StyleMao, Lin, Xiao Lu, Chao Yu, and Kuiying Yin. 2022. "Physiological and Neural Changes with Rehabilitation Training in a 53-Year Amputee: A Case Study" Brain Sciences 12, no. 7: 832. https://doi.org/10.3390/brainsci12070832
APA StyleMao, L., Lu, X., Yu, C., & Yin, K. (2022). Physiological and Neural Changes with Rehabilitation Training in a 53-Year Amputee: A Case Study. Brain Sciences, 12(7), 832. https://doi.org/10.3390/brainsci12070832






