The Prophylactic and Multimodal Activity of Two Isatin Thiosemicarbazones against Alzheimer’s Disease In Vitro
Abstract
:1. Introduction
2. Materials and Methods
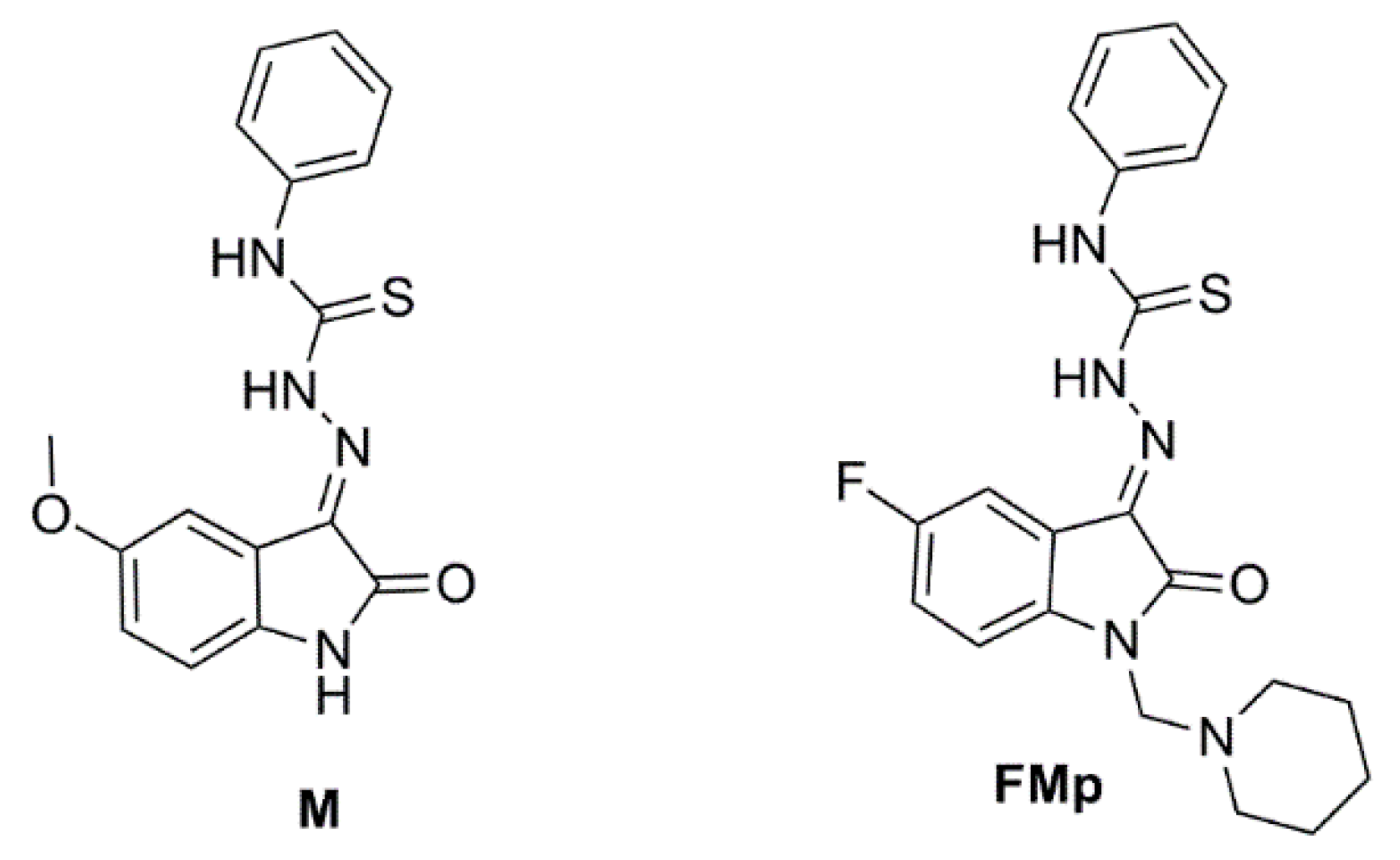
2.1. Synthesis and Characterization
2.2. Cell Viability Assay (MTT)
2.3. Cell Culture, Cell Lysis and Immunoblotting
2.4. Preparation of Aβ Stock and Solutions
2.5. Soybean LOX Inhibition Study In Vitro
2.6. Inhibition of Linoleic Acid Lipid Peroxidation
2.7. Inhibition of Acetyl-Cholinesterase
2.8. Statistical Analysis
3. Results and Discussion
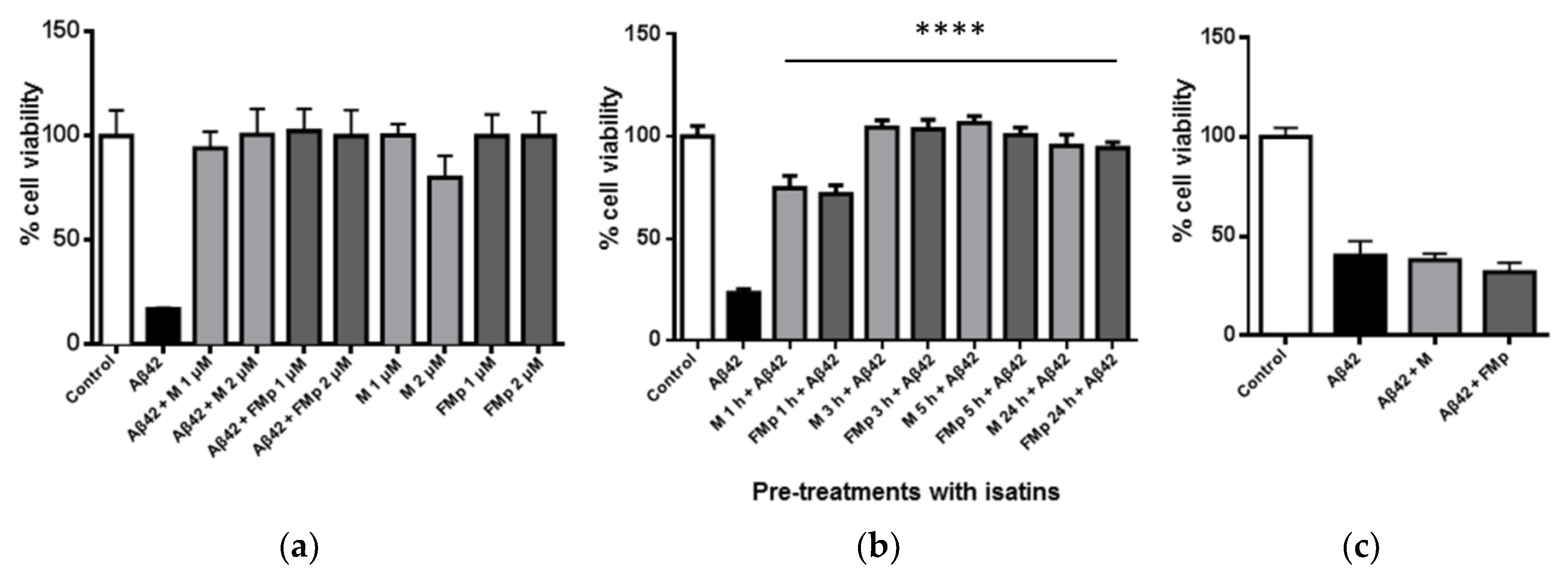
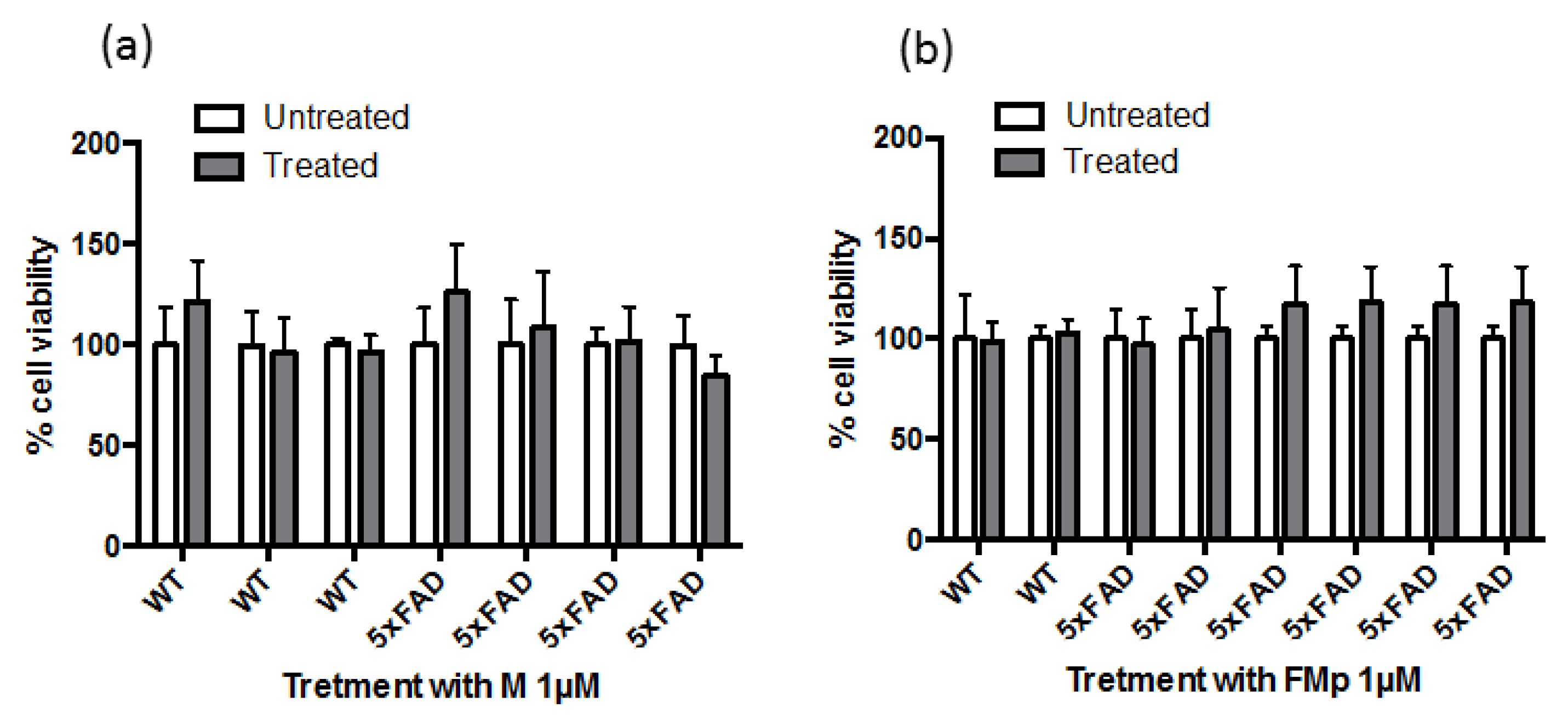
3.1. ITCSs Rescue SK-N-SH Neuroblastoma Cells from Aβ-Induced Toxicity in a Prophylactic Manner
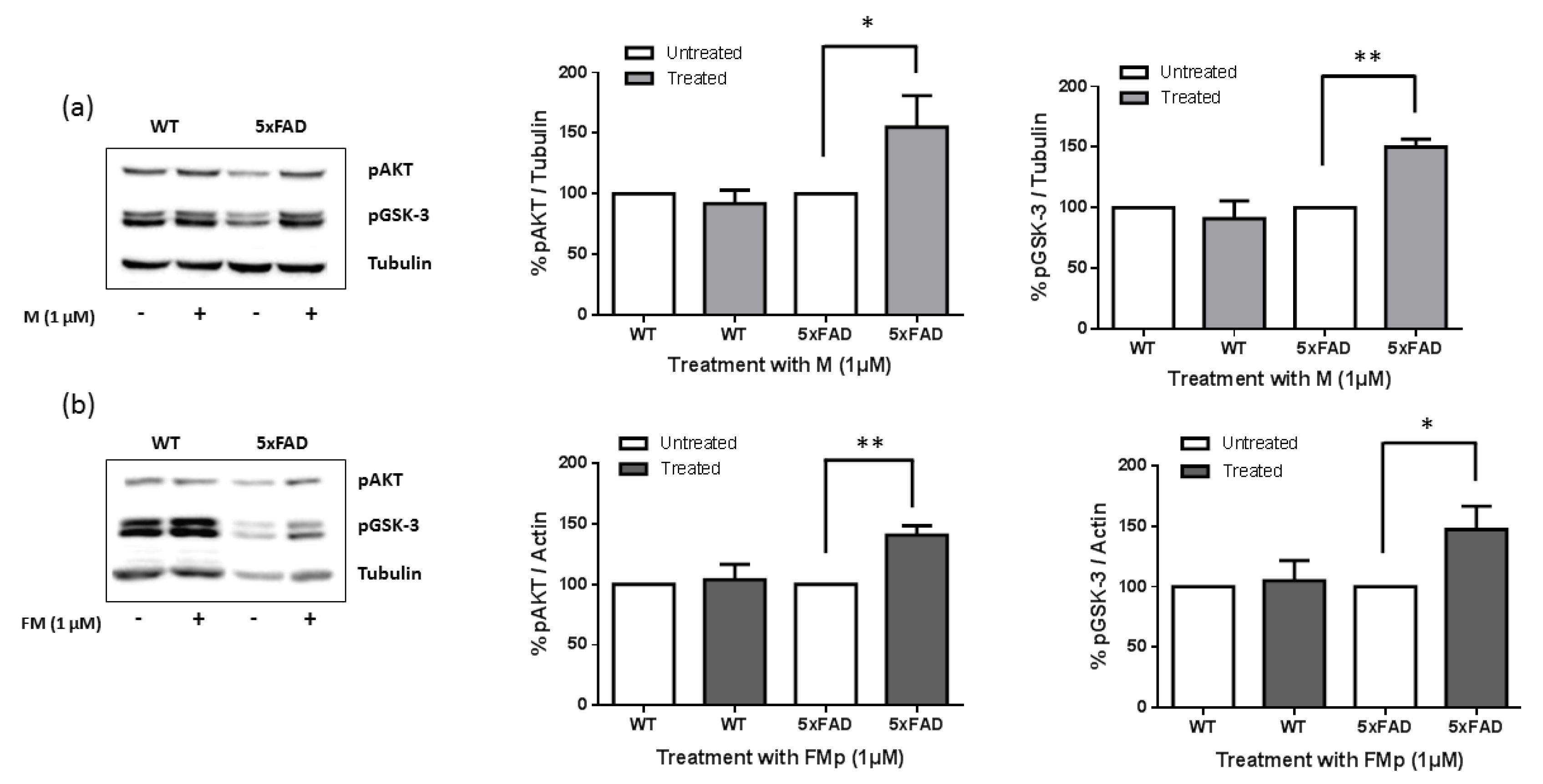
3.2. ITSCs Restore Aβ-Induced Reduction in Ser473 Phosphorylation of Akt and in Ser9 Phosphorylation of GSK-3b in Primary Hippocampal Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Paudel, P.; Seong, S.H.; Zhou, Y.; Park, H.J.; Jung, H.A.; Choi, J.S. Anti-Alzheimer’s Disease Activity of Bromophenols from a Red Alga, Symphyocladia latiuscula (Harvey) Yamada. ACS Omega 2019, 4, 12259–12270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayed, R.; Head, E.; Thompson, J.L.; Mcintire, T.M.; Milton, S.C.; Cotman, C.W.; Glabe, C.G. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 2003, 300, 486–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Li, S.; Xu, H.; Walsh, D.M.; Selkoe, D.J. Large soluble oligomers of amyloid beta-protein from Alzheimer brain are far less neuroactive than the smaller oligomers to which they dissociate. J. Neurosci. 2017, 37, 152–163. [Google Scholar] [CrossRef]
- Sengupta, U.; Nilson, A.N.; Kayed, R. The Role of Amyloid-β Oligomers in Toxicity, Propagation, and Immunotherapy. EBioMedicine 2016, 6, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Viola, K.L.; Klein, W.L. Amyloid β oligomers in Alzheimer’s disease pathogenesis, treatment, and diagnosis. Acta Neuropathol. 2015, 129, 183–206. [Google Scholar] [CrossRef]
- Katzmarski, N.; Ziegler-Waldkirch, S.; Scheffler, N.; Witt, C.; Abou-Ajram, C.; Nuscher, B.; Prinz, M.; Haass, C.; Meyer-Luehmann, M. Aβ oligomers trigger and accelerate Aβ seeding. Brain Pathol. 2020, 30, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Hurtado, D.E.; Molina-Porcel, L.; Carroll, J.C.; MacDonald, C.; Aboagye, A.K.; Trojanowski, J.Q.; Lee, V.M. Selectively silencing GSK-3 isoforms reduces plaques and tangles in mouse models of Alzheimer’s disease. J. Neurosci. 2012, 32, 7392–7402. [Google Scholar] [CrossRef] [Green Version]
- Takashima, A.; Noguchi, K.; Michel, G.; Mercken, M.; Hoshi, M.; Ishiguro, K.; Imahori, K. Exposure of rat hippocampal neurons to amyloid B peptide (25–35) induces the inactivation of phosphatidyl inositol-3 kinase and the activation of tau protein kinase I/glycogen synthase kinase-3β. Neurosci. Lett. 1996, 203, 33–36. [Google Scholar] [CrossRef]
- Beurel, E.; Jope, R.S. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog. Neurobiol. 2006, 79, 173–189. [Google Scholar] [CrossRef] [Green Version]
- DaRocha-Souto, B.; Coma, M.; Perez-Nievas, B.G.; Scotton, T.C.; Siao, M.; Sánchez-Ferrer, P.; Hashimoto, T.; Fan, Z.; Hudry, E.; Barroeta, I.; et al. Activation of Glycogen synthase kinase-3 beta mediates β-amyloid induced neuritic damage in Alzheimer’s disease. Neurobiol. Dis. 2012, 45, 425–437. [Google Scholar] [CrossRef] [Green Version]
- Czapski, G.A.; Czubowicz, K.; Strosznajder, J.B.; Strosznajder, R.P. The lipoxygenases: Their regulation and implication in Alzheimer’s disease. Neurochem. Res. 2016, 41, 243–257. [Google Scholar] [CrossRef] [Green Version]
- Pihlaja, R.; Haaparanta-Solin, M.; Rinne, O.M. The anti-inflammatory effects of lipoxygenase and cyclo-oxygenase inhibitors in inflammation-induced human fetal glia cells and the Aβ degradation capacity of human fetal astrocytes in an ex vivo assay. Front. Neurosci. 2017, 11, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peña-Bautista, C.; Baquero, M.; Vento, M.; Cháfer-Pericás, C. Free radicals in Alzheimer’s disease: Lipid peroxidation biomarkers. Clin. Chim. Acta 2019, 491, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Dean, R.L.; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Hamaue, N.; Minami, M.; Hirafuji, M.; Terado, M.; Machida, M.; Yamazaki, N.; Yoshioka, M.; Ogata, A.; Tashiro, K. Isatin, an endogenous MAO inhibitor, as a new biological modulator. CNS Drug Rev. 1999, 5, 331–346. [Google Scholar] [CrossRef]
- Medvedev, A.; Buneeva, O.; Gnedenko, O.; Ershov, P.; Ivanov, A. Isatin, an endogenous nonpeptide biofactor: A review of its molecular targets, mechanisms of actions, and their biomedical implications. Biofactors 2018, 44, 95–108. [Google Scholar] [CrossRef]
- Medvedev, A.; Buneeva, O.; Kopylov, A.; Gnedenko, O.V.; Medvedeva, M.V.; Kozin, S.A.; Ivanov, A.S.; Zgoda, V.G.; Makarov, A.A. The effects of endogenous non-peptide molecule isatin and hydrogen peroxide on proteomic profiling of rat brain amyloid-β binding proteins: Relevance to Alzheimer’s disease? Int. J. Mol. Sci. 2015, 16, 476–495. [Google Scholar] [CrossRef] [Green Version]
- Rakesh, K.; Manukumar, H.; Gowda, D.C. Schiff’s bases of quinazolinone derivatives: Synthesis and SAR studies of a novel series of potential anti-inflammatory and antioxidants. Bioorg. Med. Chem. Lett. 2015, 25, 1072–1077. [Google Scholar] [CrossRef] [Green Version]
- Campagna, F.; Catto, M.; Purgatorio, R.; Altomare, C.D.; Carotti, A.; de Stradis, A.; Palazzo, G. Synthesis and biophysical evaluation of arylhydrazono-1H-2-indolinones as β-amyloid aggregation inhibitors. Eur. J. Med. Chem. 2011, 46, 275–284. [Google Scholar] [CrossRef]
- Purgatorio, R.; de Candia, M.; de Palma, A.; de Santis, F.; Pisani, L.; Campagna, F.; Cellamare, S.; Altomare, C.D.; Catto, M. Insights into structure-activity relationships of 3-arylhydrazonoindolin-2-one derivatives for their multitarget activity on β-amyloid aggregation and neurotoxicity. Molecules 2018, 23, 1544. [Google Scholar] [CrossRef] [Green Version]
- Akrami, H.; Mirjalili, B.F.; Khoobi, M.; Nadri, H.; Moradi, A.; Sakhteman, A.; Emami, S.; Foroumadi, A.; Shafiee, A. Indolinone-based acetylcholinesterase inhibitors: Synthesis, biological activity and molecular modeling. Eur. J. Med. Chem. 2014, 84, 375–381. [Google Scholar] [CrossRef]
- Catto, M.; Aliano, R.; Carotti, A.; Cellamare, S.; Palluotto, F.; Purgatorio, R.; de Stradis, A.; Campagna, F. Design, synthesis and biological evaluation of indane-2-arylhydrazinylmethylene-1,3-diones and indol-2-aryldiazenylmethylene-3-ones as β-amyloid aggregation inhibitors. Eur. J. Med. Chem. 2010, 45, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Catto, M.; Arnesano, F.; Palazzo, G.; de Stradis, A.; Calò, V.; Losacco, M.; Purgatorio, R.; Campagna, F. Investigation on the influence of (Z)-3-(2-(3-chlorophenyl)hydrazono)-5,6-dihydroxyindolin-2-one (PT2) on β-amyloid(1-40) aggregation and toxicity. Arch. Biochem. Biophys. 2014, 560, 73–82. [Google Scholar] [CrossRef]
- Pisani, L.; de Palma, A.; Giangregorio, N.; Miniero, D.V.; Pesce, P.; Nicolotti, O.; Campagna, F.; Altomare, C.D.; Catto, M. Mannich base approach to 5-methoxyisatin 3-(4-isopropylphenyl)hydrazone: A water-soluble prodrug for a multitarget inhibition of cholinesterases, beta-amyloid fibrillization and oligomer-induced cytotoxicity. Eur. J Pharm. Sci. 2017, 109, 381–388. [Google Scholar] [CrossRef]
- Sagnou, M.; Mavroidi, B.; Kaminari, A.; Boukos, N.; Pelecanou, M. Novel isatin thiosemicarbazone derivatives as potent inhibitors of β-amyloid peptide aggregation and toxicity. ACS Chem. Neurosci. 2020, 11, 2266–2276. [Google Scholar] [CrossRef]
- Kaminari, A.; Giannakas, N.; Tzinia, A.; Tsilibary, E.C. Overexpression of matrix metalloproteinase-9 (MMP-9) rescues insulin-mediated impairment in the 5XFAD model of Alzheimer’s disease. Sci. Rep. 2017, 7, 683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadjipavlou-Litina, D.; Garnelis, T.; Athanassopoulos, C.M.; Papaioannou, D. Kukoamine A analogs with lipoxygenase inhibitory activity. J. Enzym. Inhib. Med. Chem. 2009, 24, 1188–1193. [Google Scholar] [CrossRef]
- Liargkova, T.; Eleftheriadis, N.; Dekker, F.; Voulgari, E.; Avgoustakis, C.; Sagnou, M.; Mavroidi, B.; Pelecanou, M.; Hadjipavlou-Litina, D. Small multitarget molecules incorporating the enone moiety. Molecules 2019, 24, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malafaia, D.; Albuquerque, H.M.T.; Silva, A.M.S. Amyloid-β and tau aggregation dual-inhibitors: A synthetic and structure-activity relationship focused review. Eur. J. Med. Chem. 2021, 214, 113209. [Google Scholar] [CrossRef]
- Pagano, K.; Tomaselli, S.; Molinari, H.; Ragona, L. Natural compounds as inhibitors of Aβ peptide aggregation: Chemical requirements and molecular mechanisms. Front. Neurosci. 2020, 14, 619667. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yang, C.; Zheng, J.; Chen, Y.; Xiao, Y.; Huang, K. Non-polyphenolic natural inhibitors of amyloid aggregation. Eur. J. Med. Chem. 2020, 192, 112197. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.; Patel, B.; Makwana, V.; Jadhav, H.R.; Kiefel, M.; Davey, A.; Reekie, T.A.; Rudrawar, S.; Kassiou, M. Peptides peptidomimetics, and carbohydrate-peptide conjugates as amyloidogenic aggregation inhibitors for Alzheimer’s disease. ACS Chem. Neurosci. 2018, 9, 1530–1551. [Google Scholar] [CrossRef]
- Purgatorio, R.; Gambacorta, N.; Catto, M.; de Candia, M.; Pisani, L.; Espargaró, A.; Sabaté, R.; Cellamare, S.; Nicolotti, O.; Altomare, C.D. Pharmacophore modeling and 3D-QSAR study of indole and isatin derivatives as antiamyloidogenic agents targeting Alzheimer’s disease. Molecules 2020, 25, 5773. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Li, F.; Maiese, K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog. Neurobiol. 2005, 75, 207–246. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, S.; Torres, M.; Vizuete, M.; Sanchez-Varo, R.; Sanchez-Mejias, E.; Trujilo-Estrada, L.; Carmona-Cuenca, I.; Caballero, C.; Ruano, D.; Gutierrez, A.; et al. Age-dependent accumulation of soluble amyloid β (Aβ) oligomer reverses the neuroprotective effect of soluble amyloid precursor protein-α (sAPPα) by modulating phosphatidylinositol 3-kinase (PI3K)/Akt-GSK-3β pathway in Alzheimer mouse model. J. Biol. Chem. 2011, 286, 18414–18425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito, M.A. Pharmacokinetic study with computational tools in the medicinal chemistry course. Braz. J. Pharm. Sci. 2011, 47, 797–805. [Google Scholar] [CrossRef]
- Version 2016.10. Available online: https://www.molinspiration.com (accessed on 25 October 2020).
| Compound | LOX Inh. IC50 μΜ | % AChE inh @ 100 μΜ | LPI% @ 100 μΜ |
|---|---|---|---|
| M | 60.5 | 54 | 77 |
| FMp | 54 | 44 | 51 |
| trolox | 92 | ||
| Tacrine | - | 98 | |
| NDGA | 0.5 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavroidi, B.; Kaminari, A.; Matiadis, D.; Hadjipavlou-Litina, D.; Pelecanou, M.; Tzinia, A.; Sagnou, M. The Prophylactic and Multimodal Activity of Two Isatin Thiosemicarbazones against Alzheimer’s Disease In Vitro. Brain Sci. 2022, 12, 806. https://doi.org/10.3390/brainsci12060806
Mavroidi B, Kaminari A, Matiadis D, Hadjipavlou-Litina D, Pelecanou M, Tzinia A, Sagnou M. The Prophylactic and Multimodal Activity of Two Isatin Thiosemicarbazones against Alzheimer’s Disease In Vitro. Brain Sciences. 2022; 12(6):806. https://doi.org/10.3390/brainsci12060806
Chicago/Turabian StyleMavroidi, Barbara, Archontia Kaminari, Dimitris Matiadis, Dimitra Hadjipavlou-Litina, Maria Pelecanou, Athina Tzinia, and Marina Sagnou. 2022. "The Prophylactic and Multimodal Activity of Two Isatin Thiosemicarbazones against Alzheimer’s Disease In Vitro" Brain Sciences 12, no. 6: 806. https://doi.org/10.3390/brainsci12060806
APA StyleMavroidi, B., Kaminari, A., Matiadis, D., Hadjipavlou-Litina, D., Pelecanou, M., Tzinia, A., & Sagnou, M. (2022). The Prophylactic and Multimodal Activity of Two Isatin Thiosemicarbazones against Alzheimer’s Disease In Vitro. Brain Sciences, 12(6), 806. https://doi.org/10.3390/brainsci12060806








