Effects of Sound Interventions on the Permeability of the Blood–Brain Barrier and Meningeal Lymphatic Clearance
Abstract
:1. Introduction
1.1. Music and Body and Brain Function Health
1.2. Music and Autonomic Function
1.3. Music and Cognitive Function
1.4. Music and Neuronal Function/Health
1.5. Relationships between Music, Body/Brain, and Glymphatic Clearance
2. Purpose
Scientific Rationale
3. Methods
3.1. Design
3.2. Search Strategies
3.3. Inclusion/Exclusion Criteria
4. Results
4.1. Music/Sound and the Blood–Brain Barrier
4.2. Music and Neurodegenerative/Neurological Injuries
4.3. Supplemental Practices Involving Glymphatic Manipulation
4.4. Music and Mood
4.5. Variabilities in Intensity and Exposure Time
4.6. Effects of Music and Sound on BDNF Production
5. Discussion
5.1. Limitations
5.2. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Mesquita, S.; Fu, Z.; Kipnis, J. The Meningeal Lymphatic System: A New Player in Neurophysiology. Neuron 2018, 100, 375–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Földi, M.; Gellért, A.; Kozma, M.; Poberai, M.; Zoltán, Ö.T.; Csanda, E. New contributions to the anatomical connections of the brain and the lymphatic system. Cells Tissues Organs 1966, 64, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Jessen, N.A.; Munk, A.S.F.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyrtsos, C.R.; Baras, J.S. Modeling the Role of the Glymphatic Pathway and Cerebral Blood Vessel Properties in Alzheimer’s Disease Pathogenesis. PLoS ONE 2015, 10, e0139574. [Google Scholar] [CrossRef] [Green Version]
- Golden, T.L.; Springs, S.; Kimmel, H.J.; Gupta, S.; Tiedemann, A.; Sandu, C.C.; Magsamen, S. The Use of Music in the Treatment and Management of Serious Mental Illness: A Global Scoping Review of the Literature. Front. Psychol. 2021, 12, 880. [Google Scholar] [CrossRef]
- Bradt, J.; Dileo, C.; Potvin, N. Music for Stress and Anxiety Reduction in Coronary Heart Disease Patients. Cochrane Database Syst. Rev. 2013, 2013, CD006577. [Google Scholar] [CrossRef]
- Bradt, J.; Dileo, C. Music Interventions for Mechanically Ventilated Patients. Cochrane Database Syst. Rev. 2014, 2014, CD006902. [Google Scholar] [CrossRef]
- Bradt, J.; Dileo, C.; Magill, L.; Teague, A. Music Interventions for Improving Psychological and Physical Outcomes in Cancer Patients. Cochrane Database Syst. Rev. 2016, 8, CD006911. [Google Scholar] [CrossRef]
- Mojtabavi, H.; Saghazadeh, A.; Valenti, V.E.; Rezaei, N. Can Music Influence Cardiac Autonomic System? A Systematic Review and Narrative Synthesis to Evaluate Its Impact on Heart Rate Variability. Complement. Ther. Clin. Pract. 2020, 39, 101162. [Google Scholar] [CrossRef]
- Diaz Abrahan, V.; Shifres, F.; Justel, N. Cognitive Benefits from a Musical Activity in Older Adults. Front. Psychol. 2019, 10, 652. [Google Scholar] [CrossRef] [Green Version]
- Ferreri, L.; Aucouturier, J.-J.; Muthalib, M.; Bigand, E.; Bugaiska, A. Music Improves Verbal Memory Encoding While Decreasing Prefrontal Cortex Activity: An FNIRS Study. Front. Microbiol. 2013, 7, 779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mammarella, N.; Fairfield, B.; Cornoldi, C. Does Music Enhance Cognitive Performance in Healthy Older Adults? The Vivaldi Effect. Aging Clin. Exp. Res. 2007, 19, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.G.; Moulin, C.J.A.; Hayre, S.; Jones, R.W. Music Enhances Category Fluency in Healthy Older Adults and Alzheimer’s Disease Patients. Exp. Aging Res. 2005, 31, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Degé, F.; Kerkovius, K. The Effects of Drumming on Working Memory in Older Adults. Ann. N. Y. Acad. Sci. 2018, 1423, 242–250. [Google Scholar] [CrossRef]
- Bugos, J.A. The Effects of Bimanual Coordination in Music Interventions on Executive Functions in Aging Adults. Front. Integr. Neurosci. 2019, 13, 68. [Google Scholar] [CrossRef] [Green Version]
- Gaser, C.; Schlaug, G. Brain Structures Differ between Musicians and Non-Musicians. J. Neurosci. 2003, 23, 9240–9245. [Google Scholar] [CrossRef] [Green Version]
- Sutoo, D.; Akiyama, K. Music Improves Dopaminergic Neurotransmission: Demonstration Based on the Effect of Music on Blood Pressure Regulation. Brain Res. 2004, 1016, 255–262. [Google Scholar] [CrossRef]
- Böhmer, M.R.; Chlon, C.H.T.; Raju, B.I.; Chin, C.T.; Shevchenko, T.; Klibanov, A.L. Focused Ultrasound and Microbubbles for Enhanced Extravasation. J. Control. Release 2010, 148, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Semyachkina-Glushkovskaya, O.; Abdurashitov, A.; Dubrovsky, A.; Bragin, D.; Bragina, O.; Shushunova, N.; Maslyakova, G.; Navolokin, N.; Bucharskaya, A.; Tuchind, V.; et al. Application of Optical Coherence Tomography for in Vivo Monitoring of the Meningeal Lymphatic Vessels during Opening of Blood–Brain Barrier: Mechanisms of Brain Clearing. J. Biomed. Opt. 2017, 22, 121719. [Google Scholar] [CrossRef] [Green Version]
- Semyachkina-Glushkovskaya, O.; Chekhonin, V.; Bragin, D.; Bragina, O.; Vodovozova, E.; Alekseeva, A.; Salmin, V.; Morgun, A.; Malinovskaya, N.; Osipova, E.; et al. Loud Music and the Specific Sound Stress Open the Blood-Brain Barrier: New Fundamental, Biomedical, and Social Aspects. BioRxiv 2018, 509042. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, Y.; Park, E.-J.; Kwon, S.; Kim, H.; Lee, J.Y.; Lee, D.S. Improvement of Glymphatic–Lymphatic Drainage of Beta-Amyloid by Focused Ultrasound in Alzheimer’s Disease Model. Sci. Rep. 2020, 10, 16144. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.I.; Carpenter, J.S.; Mehta, R.I.; Haut, M.W.; Ranjan, M.; Najib, U.; Lockman, P.; Wang, P.; D’haese, P.-F.; Rezai, A.R. Blood-Brain Barrier Opening with MRI-Guided Focused Ultrasound Elicits Meningeal Venous Permeability in Humans with Early Alzheimer Disease. Radiology 2021, 298, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Abrahao, A.; Heyn, C.C.; Bethune, A.J.; Huang, Y.; Pople, C.B.; Aubert, I.; Hamani, C.; Zinman, L.; Hynynen, K.; et al. Glymphatics Visualization after Focused Ultrasound-Induced Blood–Brain Barrier Opening in Humans. Ann. Neurol. 2019, 86, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Greenhalgh, T.; Westhorp, G.; Buckingham, J.; Pawson, R. RAMESES Publication Standards: Meta-Narrative Reviews. BMC Med. 2013, 11, 20. [Google Scholar] [CrossRef] [Green Version]
- Angelucci, F.; Ricci, E.; Padua, L.; Sabino, A.; Tonali, P.A. Music Exposure Differentially Alters the Levels of Brain-Derived Neurotrophic Factor and Nerve Growth Factor in the Mouse Hypothalamus. Neurosci. Lett. 2007, 429, 152–155. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, J.; Shen, G.; Ji, X.; Sun, L.; Li, X.; Xu, F.; Gu, J. Music Therapy Alleviates Motor Dysfunction in Rats with Focal Cerebral Ischemia–Reperfusion Injury by Regulating BDNF Expression. Front. Neurol. 2021, 12, 1027. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.S.-G.; Abdurashitov, A.; Pavlov, A.; Shirokov, A.; Navolokin, N.; Pavlova, O.; Gekalyuk, A.; Ulanova, M.; Shushunova, N.; Bodrova, A.; et al. Laser Speckle Imaging and Wavelet Analysis of Cerebral Blood Flow Associated with the Opening of the Blood–Brain Barrier by Sound. Chin. Opt. Lett. 2017, 15, 090002. [Google Scholar] [CrossRef] [Green Version]
- Semyachkina-Glushkovskaya, O.; Esmat, A.; Bragin, D.; Bragina, O.; Shirokov, A.A.; Navolokin, N.; Yang, Y.; Abdurashitov, A.; Khorovodov, A.; Terskov, A.; et al. Phenomenon of Music-Induced Opening of the Blood-Brain Barrier in Healthy Mice. Proc. R. Soc. B Biol. Sci. 2020, 287, 20202337. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Khorovodov, A.; Fedosov, I.; Pavlov, A.; Shirokov, A.; Sharif, A.E.; Dubrovsky, A.; Blokhina, I.; Terskov, A.; Navolokin, N.; et al. A Novel Method to Stimulate Lymphatic Clearance of Beta-Amyloid from Mouse Brain Using Noninvasive Music-Induced Opening of the Blood–Brain Barrier with EEG Markers. Appl. Sci. 2021, 11, 10287. [Google Scholar] [CrossRef]
- Nayak, S.; Wheeler, B.L.; Shiflett, S.C.; Agostinelli, S. Effect of Music Therapy on Mood and Social Interaction among Individuals with Acute Traumatic Brain Injury and Stroke. Rehabil. Psychol. 2000, 45, 274–283. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Kurths, J.; Borisova, E.; Sokolovski, S.; Mantareva, V.; Angelov, I.; Shirokov, A.; Navolokin, N.; Shushunova, N.; Khorovodov, A.; et al. Photodynamic Opening of Blood-Brain Barrier. Biomed. Opt. Express 2017, 8, 5040. [Google Scholar] [CrossRef] [PubMed]
- Jung, O.; Thomas, A.; Burks, S.R.; Dustin, M.L.; Frank, J.A.; Ferrer, M.; Stride, E. Neuroinflammation associated with ultrasound-mediated permeabilization of the blood–brain barrier. Trends Neurosci. 2022, 3, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Suzukamo, Y.; Sato, M.; Izumi, S.-I. Effects of Music Therapy on Behavioral and Psychological Symptoms of Dementia: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2013, 12, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Cheung, D.S.K.; Lai, C.K.Y.; Wong, F.K.Y.; Leung, M.C.P. Is Music-With-Movement Intervention Better than Music Listening and Social Activities in Alleviating Agitation of People with Moderate Dementia? A Randomized Controlled Trial. Dementia 2018, 22, 1413–1425. [Google Scholar] [CrossRef]
- Bakerjian, D.; Bettega, K.; Cachu, A.M.; Azzis, L.; Taylor, S. The Impact of Music and Memory on Resident Level Outcomes in California Nursing Homes. J. Am. Med. Dir. Assoc. 2020, 21, 1045–1050. [Google Scholar] [CrossRef]
- Hidayati, A.; Joewono, H.T.; Widjiati, W. Increased Brain Derived Neurothropic Factor in the cerebrum and cerebellum of Rattus norvegicus newborn with exposure to Mozart’s music in default sequence compared with the reversed sequence and without exposure during gestation. Maj. Obstet. Ginekol. 2018, 26, 67. [Google Scholar] [CrossRef] [Green Version]
- Marzban, M.; Shahbazi, A.; Tondar, M.; Soleimani, M.; Bakhshayesh, M.; Moshkforoush, A.; Sadati, M.; Siamak, A.Z.; Mohammad, T.J. Effect of Mozart Music on Hippocampal Content of BDNF in Postnatal Rats. Basic Clin. Neurosci. 2011, 2, 21–26. [Google Scholar]
- Chikahisa, S.; Sei, H.; Morishima, M.; Sano, A.; Kitaoka, K.; Nakaya, Y.; Morita, Y. Exposure to music in the perinatal period enhances learning performance and alters BDNF/TrkB signaling in mice as adults. Behav. Brain Res. 2006, 169, 312–319. [Google Scholar] [CrossRef]
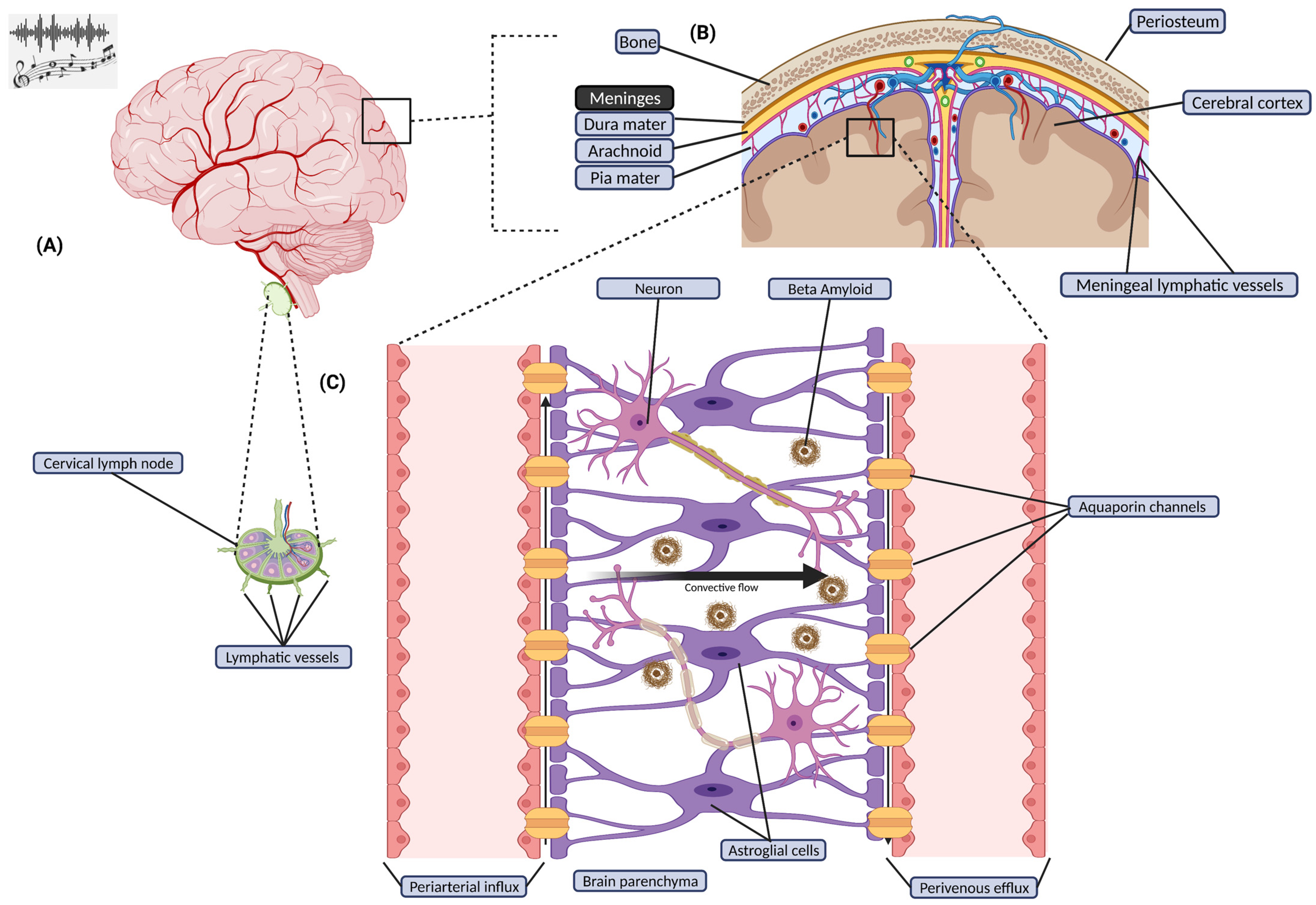
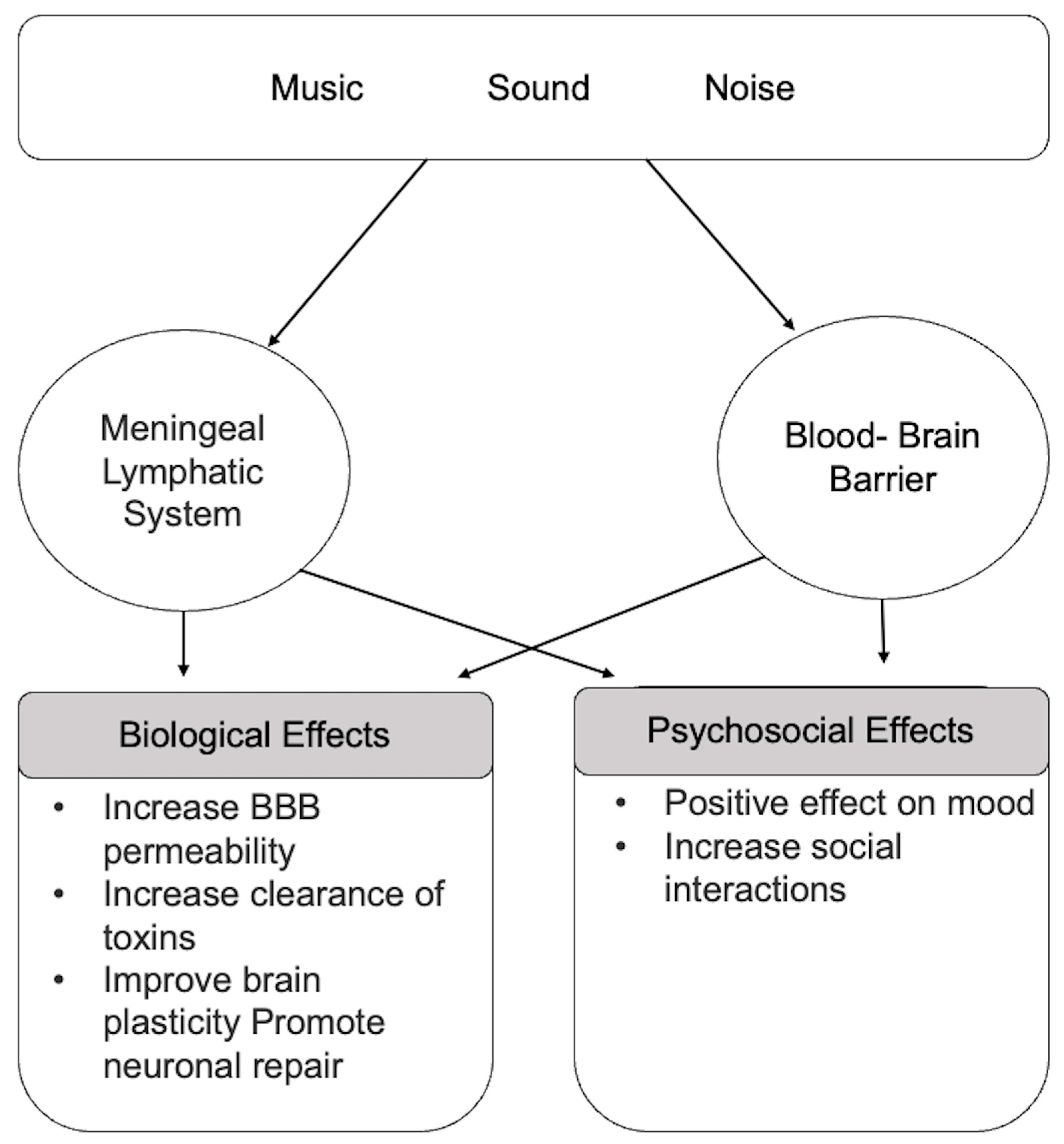
| Reference | Test Subjects | Research Methodology | Anatomical/Functional Outcomes |
|---|---|---|---|
| [25] Music exposure differentially alters the levels of brain-derived neurotrophic factor and nerve growth factor in the mouse hypothalamus. | Disease Condition: None Species: Mouse Strain: BALB/c (Charles River, Italy) Weight: 30 g Avg. Age: 40 day N: 20 | Mice were exposed to slow rhythmic music for 6 h for 21 d. Music had mild sound pressure levels, around 50–60 dB. Control mice were placed in a similar environment but without music. Animals had free access to food and water and were placed on a 12 h light/dark schedule. The music was played during nighttime hours due to the mice being nocturnal. On day 22, mice were sacrificed, and the hypothalamus was extracted to measure BDNF and NGF production levels. Sound: Slow rhythm music (~50 and 60 dB) |
|
| [26] Music therapy alleviates motor dysfunction in rats with focal cerebral ischemia-reperfusion injury by regulating BDNF expression. | Disease Condition: Focal cerebral ischemia-reperfusion injury Species: Rat Strain: Sprague-Dawley (Nantong, China) Weight: 220–250 g N: 90 Sex: M | Rats underwent middle cerebral artery occlusion (MCAO) for 1h followed by reperfusion. Rats that survived were separated into 4 groups:
Sound: Mozart K.448 (65–75 dB) |
|
| [19] Application of optical coherence tomography for in vivo monitoring of the meningeal lymphatic vessel during the opening of blood–brain barrier: mechanisms of brain clearing. | Disease condition: None Species: Mouse Strain: N/D Weight: 25 g N: 89 Sex: M | Evans Blue and FITC-Dextran were used to monitor large and small molecule intravasation through the BBB, respectively. Analyses of Evans Blue were performed in 4 groups:
Sound: Audible sound (100 dB, 370 Hz) |
|
| [27] Laser speckle imaging and wavelet analysis of cerebral blood flow associated with the opening of the blood-brain barrier by sound. | Disease Condition: None Species: Mouse Strain: N/D Weight: 20–25 g Avg. Age: 2 mo N: 40 Sex: M | Analyses of BBB permeability were conducted in 4 groups: no music 90 min after sound exposure 4 h after sound exposure 24 h after sound exposure Each group included 10 mice. To trigger the BBB opening, the audible sound was applied in 60 s on/60 s off intervals for 2 h. Then, EB was administered and circulated in the blood for 30 min; FITCD was administered and circulated for 2 min. Afterward, mice were sacrificed, and tissues were analyzed. Sound: Audible sound (110 dB, 370 Hz) |
|
| [20] Loud music and specific sound stress open BBB. | Disease Condition: None Species: Mouse and Rat Strain: Mongrel and Wistar Weight: 20–25 g Avg. Age: “Wistar male rats of corresponding age been used.” N: 60 Sex: M | 4 groups:
Sound: The music played was “Still Loving You” by Scorpions. |
|
| [28] Phenomenon of music-induced opening of the blood–brain barrier in healthy mice. | Disease Condition: None Species: Mouse Strain: Mongrel Weight: 20–25 g N: 50 Sex: M | 4 groups:
Sound: The music played was “Still Loving You” by Scorpions. The control group of mice (n = 15) was not exposed to the song or music. |
|
| [29] A novel method to stimulate lymphatic clearance of beta-amyloid from mouse brain using non-invasive music-induced opening of the blood-brain barrier with EEG markers. | Disease Condition: None Species: Mouse Strain: Mongrel Weight: 20–25 g N: 74 Sex: M | 8 groups:
Sound: The music played was “Still Loving You” by Scorpions. |
|
| Reference | Test Subjects | Research Methodology | Anatomical/Functional Outcomes |
|---|---|---|---|
| [30] Effect of music therapy on mood and social interaction among individuals with acute traumatic brain injury and stroke. | Disease Condition: TBI; Stroke Avg. Age: 59.89 yr N: 18 Sex: 33% M Duration: 10 wks | 18 individuals with TBI or stroke were assigned to one of the following conditions:
Sound: Simple pitched instruments, including percussion and melodic. Singing and listening were also incorporated. Different instruments were used based on each participant’s preference. | As a result of the study, they concluded that music therapy had a beneficial effect on the behavioral and social outcomes of the participants with stroke or TBI and showed trends in respect to mood. Furthermore, in the standard rehabilitation process, the effect allegedly facilitated participation. The difference amongst the two groups on the Faces Scale was F = 3.46 (p < 0.01). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sachdeva, S.; Persaud, S.; Patel, M.; Popard, P.; Colverson, A.; Doré, S. Effects of Sound Interventions on the Permeability of the Blood–Brain Barrier and Meningeal Lymphatic Clearance. Brain Sci. 2022, 12, 742. https://doi.org/10.3390/brainsci12060742
Sachdeva S, Persaud S, Patel M, Popard P, Colverson A, Doré S. Effects of Sound Interventions on the Permeability of the Blood–Brain Barrier and Meningeal Lymphatic Clearance. Brain Sciences. 2022; 12(6):742. https://doi.org/10.3390/brainsci12060742
Chicago/Turabian StyleSachdeva, Sean, Sushmita Persaud, Milani Patel, Peyton Popard, Aaron Colverson, and Sylvain Doré. 2022. "Effects of Sound Interventions on the Permeability of the Blood–Brain Barrier and Meningeal Lymphatic Clearance" Brain Sciences 12, no. 6: 742. https://doi.org/10.3390/brainsci12060742
APA StyleSachdeva, S., Persaud, S., Patel, M., Popard, P., Colverson, A., & Doré, S. (2022). Effects of Sound Interventions on the Permeability of the Blood–Brain Barrier and Meningeal Lymphatic Clearance. Brain Sciences, 12(6), 742. https://doi.org/10.3390/brainsci12060742








