Integrating Distribution-Based and Anchor-Based Techniques to Identify Minimal Important Change for the Tinnitus Functional Index (TFI) Questionnaire
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection Schedule
2.2. Inclusion Criteria
2.3. Participants
2.4. Measures
2.4.1. Tinnitus Functional Index
2.4.2. Global Rating of Perceived Problem with Tinnitus (Perceived Problem Rating)
2.4.3. Clinical Global Impression of Perceived Change in Tinnitus (CGI)
2.5. Analysis Methods
2.5.1. Visual Anchor-Based Minimal Important Change (MIC) Distribution
2.5.2. Distribution-Based Techniques
2.5.3. Anchor-Based Techniques
3. Results
3.1. Descriptive Statistics
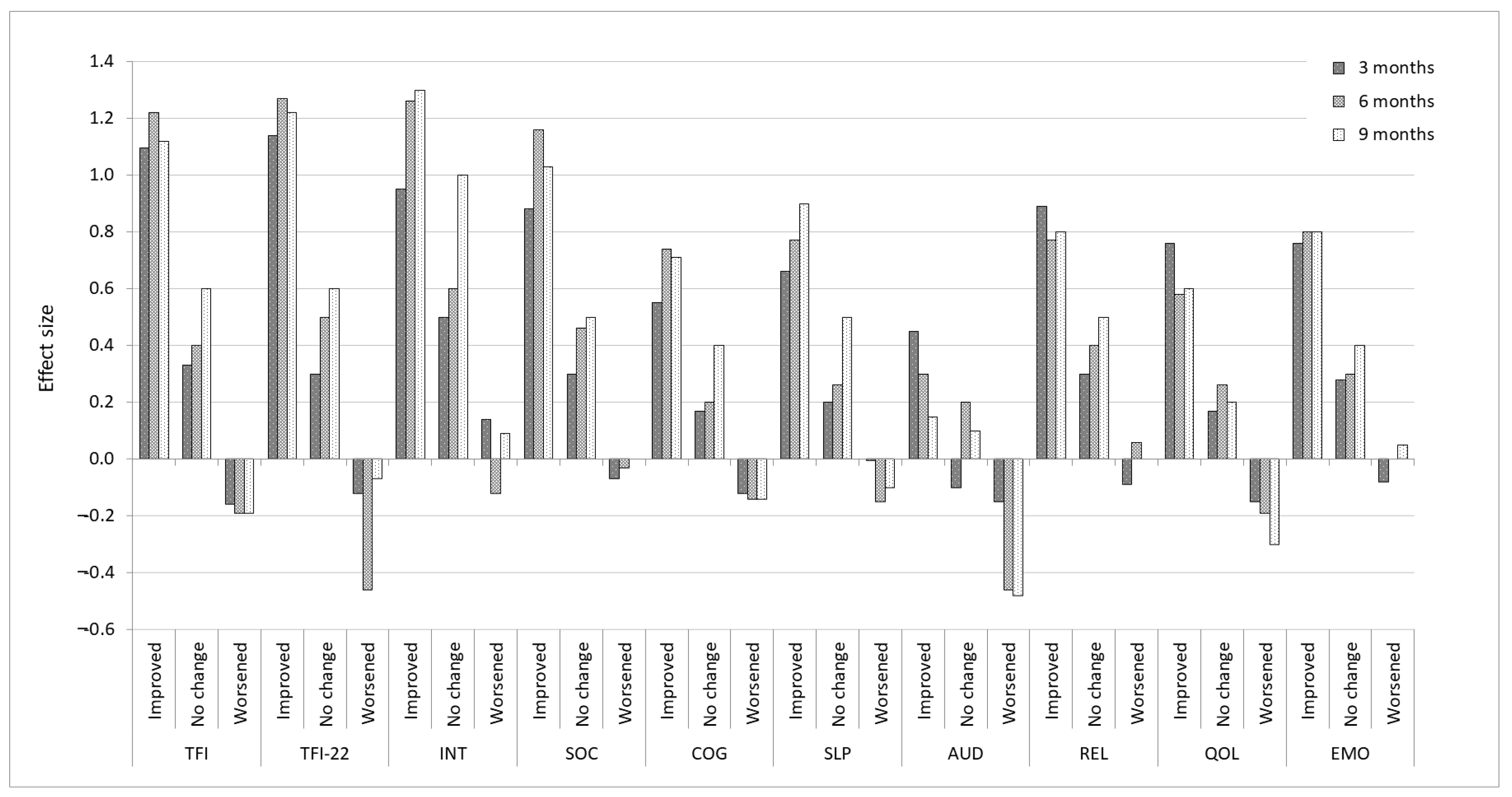
3.2. Distribution-Based Estimates
3.3. Anchor-Based Estimates
3.3.1. Identifying Meaningful Change for Improvement
3.3.2. Identifying Meaningful Change for Worsening
3.4. Visual Anchor-Based MIC Distribution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watts, E.J.; Fackrell, K.; Smith, S.; Sheldrake, J.; Haider, H.; Hoare, D.J. Why Is Tinnitus a Problem? A Qualitative Analysis of Problems Reported by Tinnitus Patients. Trends Hear. 2018, 22. [Google Scholar] [CrossRef]
- Baguley, D.; McFerran, D.; Hall, D. Tinnitus. Lancet 2013, 382, 1600–1607. [Google Scholar] [CrossRef]
- Hall, D.A.; Haider, H.; Szczepek, A.J.; Lau, P.; Rabau, S.; Jones-Diette, J.; Londero, A.; Edvall, N.K.; Cederroth, C.R.; Mielczarek, M.; et al. Systematic review of outcome domains and instruments used in clinical trials of tinnitus treatments in adults. Trials 2016, 17, 270. [Google Scholar] [CrossRef] [PubMed]
- Fackrell, K.; Hall, D.A.; Barry, J.G.; Hoare, D.J. Tools for tinnitus measurement: Development and validity of questionnaires to assess handicap and treatment effects. In Tinnitus: Causes, Treatment and Short & Long-Term Health Effects; Signorelli, F., Turjman, F., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2014; pp. 13–60. [Google Scholar]
- Meikle, M.; Henry, J.; Griest, S.; Stewart, B.; Abrams, H.; McArdle, R.; Myers, P.; Newman, C.; Sandridge, S.; Turk, D.; et al. The Tinnitus Functional Index: Development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear. 2012, 33, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.A.; Griest, S.; Thielman, E.; McMillan, G.; Kaelin, C.; Carlson, K.F. Tinnitus Functional Index: Development, validation, outcomes research, and clinical application. Hear. Res. 2016, 334, 58–64. [Google Scholar] [CrossRef]
- Skarżyński, H.; Gos, E.; Dziendziel, B.; Raj-Koziak, D.; Włodarczyk, E.A.; Skarżyński, P.H. Clinically important change in tinnitus sensation after stapedotomy. Health Qual. Life Outcomes 2018, 16, 208. [Google Scholar] [CrossRef] [PubMed]
- Fackrell, K.; Hall, D.A.; Barry, J.G.; Hoare, D.J. Psychometric properties of the Tinnitus Functional Index (TFI): Assessment in a UK research volunteer population. Hear. Res. 2016, 335, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Folmer, R.L. Reply to: Psychometric properties of the Tinnitus Functional Index (TFI): Assessment in a UK research volunteer population. Hear. Res. 2016, 335, 236. [Google Scholar] [CrossRef] [PubMed]
- Folmer, R.L.; Theodoroff, S.M.; Casiana, L.; Shi, Y.; Griest, S.; Vachhani, J. Repetitive transcranial magnetic stimulation treatment for chronic tinnitus: A randomized clinical trial. JAMA Otolaryngol.-Head Neck Surg. 2015, 141, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Chandra, N.; Chang, K.; Lee, A.; Shekhawat, G.S.; Searchfield, G.D. Psychometric validity, reliability, and responsiveness of the tinnitus functional index. J. Am. Acad. Audiol. 2018, 29, 609–625. [Google Scholar] [CrossRef]
- Fackrell, K.; Hall, D.A.; Barry, J.G.; Hoare, D.J. Performance of the Tinnitus Functional Index as a diagnostic instrument in an UK clinical population. Hear. Res. 2018, 358, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Crosby, R.D.; Kolotkin, R.L.; Williams, G.R. An integrated method to determine meaningful changes in health-related quality of life. J. Clin. Epidemiol. 2004, 57, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Yost, K.J.; Eton, D.T. Combining distribution- and anchor-based approaches to determine minimally important differences: The FACIT experience. Eval. Health Prof. 2005, 28, 172–191. [Google Scholar] [CrossRef] [PubMed]
- De Vet, H.C.W.; Ostelo, R.W.J.G.; Terwee, C.B.; Van Der Roer, N.; Knol, D.L.; Beckerman, H.; Boers, M.; Bouter, L.M. Minimally important change determined by a visual method integrating an anchor-based and a distribution-based approach. Qual. Life Res. 2007, 16, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Vernon, J.; Griest, S.; Press, L. Plight of unreturned tinnitus questionnaires. Br. J. Audiol. 1992, 26, 137–138. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Window, Version 21.0; IBM Corp.: Armonk, NY, USA, 2012. [Google Scholar]
- Lipsey, M.W. A scheme for assessing measurement sensitivity in program evaluation and other applied research. Psychol. Bull. 1983, 94, 152–165. [Google Scholar] [CrossRef]
- Crosby, R.D.; Kolotkin, R.L.; Williams, G.R. Defining clinically meaningful change in health-related quality of life. J. Clin. Epidemiol. 2003, 56, 395–407. [Google Scholar] [CrossRef]
- Revicki, D.; Hays, R.D.; Cella, D.; Sloan, J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J. Clin. Epidemiol. 2008, 61, 102–109. [Google Scholar] [CrossRef]
- Terwee, C.B.; Roorda, L.D.; Knol, D.L.; De Boer, M.R.; De Vet, H.C.W.W.; De Boer, M.R.; Vet, H.C.W. De Linking measurement error to minimal important change of patient-reported outcomes. J. Clin. Epidemiol. 2009, 62, 1062–1067. [Google Scholar] [CrossRef]
- De Vet, H.C.W.; Terwee, C.B.; Mokkink, L.B.; Knol, D.L. Measurement in Medicine: A Practical Guide; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Copay, A.G.; Subach, B.R.; Glassman, S.D.; Polly, D.W.; Schuler, T.C. Understanding the minimum clinically important difference: A review of concepts and methods. Spine J. 2007, 7, 541–546. [Google Scholar] [CrossRef]
- Rai, S.K.; Yazdany, J.; Fortin, P.R.; Antonio Aviña-Zubieta, J. Approaches for estimating minimal clinically important differences in systemic lupus erythematosus. Arthritis Res. Ther. 2015, 17, 143. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Agreement between methods of measurement with multple observations per individual. J. Biopharm. Stat. 2007, 17, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.P. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J. Strength Cond. Res. 2005, 19, 231–240. [Google Scholar] [CrossRef]
- Streiner, D.L.; Norman, G.R. Health Measurement Scales: A practical Guide to Their Development and Use, 4th ed.; Oxford University Press: Oxford, UK, 2008; ISBN 9780199231881. [Google Scholar]
- Wyrwich, K.W.; Norquist, J.M.; Lenderking, W.R.; Acaster, S. Methods for interpreting change over time in patient-reported outcome measures. Qual. Life Res. 2013, 22, 475–483. [Google Scholar] [CrossRef]
- Terwee, C.B.; Bot, S.D.M.; de Boer, M.R.; van der Windt, D.; Knol, D.L.; Dekker, J.; Bouter, L.M.; de Vet, H.C.W. Quality criteria were proposed for measurement properties of health status questionnaires. J. Clin. Epidemiol. 2007, 60, 34–42. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Norman, G.R.; Sloan, J.A.; Wyrwich, K.W. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med. Care 2003, 41, 582–592. [Google Scholar] [CrossRef]
- Angst, F.; Aeschlimann, A.E.; Angst, J. The minimal clinically important difference raised the significance of outcome effects above the statistical level, with methodological implications for future studies. J. Clin. Epidemiol. 2017, 82, 128–136. [Google Scholar] [CrossRef]
- De Vet, H.C.W.; Terluin, B.; Knol, D.L.; Roorda, L.D.; Mokkink, L.B.; Ostelo, R.W.J.G.; Hendriks, E.J.M.; Bouter, L.M.; Terwee, C.B. Three ways to quantify uncertainty in individually applied “minimally important change” values. J. Clin. Epidemiol. 2010, 63, 37–45. [Google Scholar] [CrossRef]
- Terwee, C.B.; Roorda, L.D.; Dekker, J.; Bierma-Zeinstra, S.M.; Peat, G.; Jordan, K.P.; Croft, P.; de Vet, H.C.W. Mind the MIC: Large variation among populations and methods. J. Clin. Epidemiol. 2010, 63, 524–534. [Google Scholar] [CrossRef]
- Ward, M.M.; Guthrie, L.C.; Alba, M. Dependence of the minimal clinically important improvement on the baseline value is a consequence of floor and ceiling effects and not different expectations by patients. J. Clin. Epidemiol. 2014, 67, 689–696. [Google Scholar] [CrossRef]
- De Vet, H.C.W.; Foumani, M.; Scholten, M.A.; Jacobs, W.C.H.; Stiggelbout, A.M.; Knol, D.L.; Peul, W.C. Minimally important change values of a measurement instrument depend more on baseline values than on the type of intervention. J. Clin. Epidemiol. 2015, 68, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Eng, J. Receiver operating characteristic analysis: A primer. Acad. Radiol. 2005, 12, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Uslu, R.I.; Kapci, E.G.; Oncu, B.; Ugurlu, M.; Turkcapar, H. Psychometric properties and cut-off scores of the beck depression inventory-II in Turkish adolescents. J. Clin. Psychol. Med. Settings 2008, 15, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.H.; Malley, A.J.O.; Mauri, L.; O’Malley, A.J.; Mauri, L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation 2007, 115, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.A.; Thielman, E.; Zaugg, T. Reply to: Psychometric properties of the Tinnitus Functional Index (TFI): Assessment in a UK research volunteer population. Hear. Res. 2017, 350, 222–223. [Google Scholar] [CrossRef] [PubMed]
- Fackrell, K.; Hall, D.A.; Barry, J.G.; Hoare, D.J. Response to Letter: Psychometric properties of the Tinnitus Functional Index (TFI): Assessment in a UK research volunteer population. Hear. Res. 2016, 335, 237–238. [Google Scholar] [CrossRef]
- Fackrell, K.; Hall, D.A.; Barry, J.G.; Hoare, D.J. Response to letter: Psychometric properties of the Tinnitus Functional Index (TFI): Assessment in a UK research volunteer population. Hear. Res. 2017, 350, 224–225. [Google Scholar] [CrossRef]
- Sirois, F.M.; Davis, C.G.; Morgan, M.S. “Learning to live with what you can’t rise above”: Control beliefs, symptom control, and adjustment to tinnitus. Health Psychol. 2006, 25, 119–123. [Google Scholar] [CrossRef]
- Vollmann, M.; Kalkouskaya, N.; Langguth, B.; Scharloo, M. When the ringing in the ears gets unbearable: Illness representations, self-instructions and adjustment to tinnitus. J. Psychosom. Res. 2012, 73, 108–111. [Google Scholar] [CrossRef][Green Version]
- Dawes, P.; Newall, J.; Stockdale, D.; Baguley, D.M. Natural history of tinnitus in adults: A cross-sectional and longitudinal analysis. BMJ Open 2020, 10, e041290. [Google Scholar] [CrossRef] [PubMed]
- Searchfield, G.D. Tinnitus what and where: An ecological framework. Front. Neurol. 2014, 5, 271. [Google Scholar] [CrossRef] [PubMed]
- Mokkink, L.B.; Terwee, C.B.; Patrick, D.L.; Alonso, J.; Stratford, P.W.; Knol, D.L.; Bouter, L.M.; de Vet, H.C.W. The COSMIN Checklist Manual; VU University Medical Centre: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Lipsey, M.W.; Cordray, D. Evaluation methods for social intervention. Annu. Rev. Psychol. 2000, 51, 345–375. [Google Scholar] [CrossRef] [PubMed]
- Adamchic, I.; Tass, P.A.; Langguth, B.; Hauptmann, C.; Koller, M.; Schecklmann, M.; Zeman, F.; Landgrebe, M. Linking the Tinnitus Questionnaire and the subjective Clinical Global Impression: Which differences are clinically important? Health Qual. Life Outcomes 2012, 10, 79. [Google Scholar] [CrossRef]
- Phillips, J.S.; McFerran, D.J.; Hall, D.A.; Hoare, D.J. The natural history of subjective tinnitus in adults: A systematic review and meta-analysis of no-intervention periods in controlled trials. Laryngoscope 2018, 128, 217–227. [Google Scholar] [CrossRef]

| T1 | T2 | T3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CGI | n | Mean (SD) | MCID | n | Mean (SD) | MCID | n | Mean (SD) | MCID | |
| TFI | Much improved | 8 | −37.2 (22.9) | −33.1 | 19 | −29.4 (16.5) | −22.3 | 14 | −29.1 (19.9) | −20.0 |
| Moderately improved | 22 | −16.6 (16.5) | −12.6 | 15 | −21.7 (17.7) | −14.6 | 21 | −22.8 (18.5) | −13.7 | |
| Slightly improved | 39 | −12.8 (10.2) | −8.8 | 33 | −12.7 (11.8) | −5.6 | 24 | −12.0 (16.7) | −2.9 | |
| No change | 101 | −4.1 (12.0) | 67 | −7.1 (13.5) | 48 | −9.1 (12.7) | ||||
| Slightly worse | 23 | 2.5 (12.1) | 6.5 | 30 | 1.4 (14.9) | 8.5 | 33 | −0.7 (16.1) | 8.4 | |
| Moderately worse | 3 | −2.5 (11.4) | 1.5 | 9 | 6.0 (13.4) | 13.2 | 14 | 7.2 (20.5) | 16.3 | |
| Much worse | 2 | 12.2 (22.3) | 19.3 | 11 | 8.1 (10.3) | 17.2 | ||||
| TFI-22 | Much improved | 8 | −37.7 (23.3) | −32.7 | 19 | −31.4 (17.4) | −23.8 | 14 | −31.9 (19.2) | −21.9 |
| Moderately improved | 22 | −18.4 (18.1) | −13.5 | 15 | −21.4 (17.0) | −13.8 | 21 | −24.7 (18.3) | −14.6 | |
| Slightly improved | 39 | −13.5 (10.9) | −8.5 | 33 | −14.7 (12.4) | −7.1 | 24 | −14.7 (17.0) | −4.7 | |
| No change | 101 | −4.9 (12.0) | 67 | −7.6 (14.5) | 48 | −10.0 (13.6) | ||||
| Slightly worse | 23 | 2.4 (14.0) | 7.4 | 30 | 0.4 (15.4) | 8.0 | 33 | −2.1 (17.0) | 7.9 | |
| Moderately worse | 3 | −3.6 (11.8) | 1.3 | 9 | 4.2 (13.5) | 11.9 | 14 | 5.8 (21.6) | 15.8 | |
| Much worse | 2 | 11.8 (22.5) | 19.4 | 11 | 6.3 (10.7) | 16.3 | ||||
| INT | Much improved | 8 | −54.6 (24.2) | −47.6 | 19 | −36.5 (24.2) | −24.6 | 14 | −35.7 (19.8) | −17.5 |
| Moderately improved | 22 | −16.4 (18.0) | −9.4 | 15 | −25.3 (25.0) | −13.4 | 21 | −30.3 (14.8) | −12.1 | |
| Slightly improved | 39 | −15.1 (16.5) | −8.1 | 33 | 19.7 (18.1) | −7.8 | 24 | −21.1 (23.3) | −2.9 | |
| No change | 101 | −7.0 (22.4) | 67 | −11.9 (18.1) | 48 | −18.2 (22.6) | ||||
| Slightly worse | 23 | −4.2 (23.8) | 2.8 | 30 | 3.9 (23.5) | 15.8 | 33 | −8.4 (22.5) | 9.8 | |
| Moderately worse | 3 | −11.1 (15.4) | −4.1 | 9 | 2.6 (16.1) | 14.5 | 14 | 3.1 (19.5) | 21.3 | |
| Much worse | 2 | 5.0 (7.1) | 16.9 | 11 | 7.9 (26.0) | 26.1 | ||||
| SOC | Much improved | 8 | −50.8 (27.2) | −46.7 | 19 | −40.5 (21.8) | −30.8 | 14 | −34.5 (30.0) | −24.4 |
| Moderately improved | 22 | −19.4 (28.6) | −15.2 | 15 | −21.6 (26.9) | −11.8 | 21 | −24.6 (23.6) | −14.5 | |
| Slightly improved | 39 | −15.3 (17.7) | −11.1 | 33 | −19.4 (16.0) | −9.6 | 24 | −15.1 (22.4) | −5.0 | |
| No change | 101 | −4.2 (18.3) | 67 | −9.8 (23.7) | 48 | −10.1 (20.2) | ||||
| Slightly worse | 23 | −0.6 (14.1) | 3.6 | 30 | −0.9 (26.0) | 8.9 | 33 | −4.6 (18.7) | 5.5 | |
| Moderately worse | 3 | 36.7 (49.8) | 40.8 | 9 | 3.7 (12.6) | 13.5 | 14 | 4.3 (33.0) | 14.4 | |
| Much worse | 2 | 11.7 (16.5) | 21.4 | 11 | 5.5 (10.1) | 15.6 | ||||
| COG | Much improved | 8 | −32.5 (25.0) | −27.7 | 19 | −24.9 (22.6) | −20.1 | 14 | −23.8 (28.4) | −17.1 |
| Moderately improved | 22 | −13.9 (21.9) | −9.1 | 15 | −13.9 (20.4) | −8.7 | 21 | −20.6 (26.7) | −13.9 | |
| Slightly improved | 39 | −7.1 (18.8) | −2.3 | 33 | −11.0 (17.8) | −6.2 | 24 | −16.9 (25.8) | −10.2 | |
| No change | 101 | −4.8 (21.6) | 67 | −4.8 (19.5) | 48 | −6.7 (15.4) | ||||
| Slightly worse | 23 | 3.5 (20.7) | 8.3 | 30 | 0.8 (18.9) | 5.6 | 33 | 1.4 (22.0) | 8.1 | |
| Moderately worse | 3 | −2.2 (19.0) | 2.6 | 9 | 5.9 (17.9) | 10.7 | 14 | 7.6 (32.6) | 14.4 | |
| Much worse | 2 | 20.0 (47.1) | 24.8 | 11 | 4.8 (21.0) | 11.6 | ||||
| SLP | Much improved | 8 | −29.6 (39.4) | −24.8 | 19 | −36.8 (29.8) | −30.2 | 14 | −23.8 (28.4) | −11.4 |
| Moderately improved | 22 | −24.2 (28.0) | −19.4 | 15 | −18.9 (19.9) | −12.2 | 21 | −20.6 (26.7) | −8.3 | |
| Slightly improved | 39 | −10.9 (26.7) | −6.0 | 33 | −15.5 (31.9) | −8.8 | 24 | −16.9 (25.8) | −4.6 | |
| No change | 101 | −4.8 (22.1) | 67 | −6.7 (25.7) | 48 | −12.4 (26.1) | ||||
| Slightly worse | 23 | 0.9 (19.0) | 5.7 | 30 | 0.2 (18.8) | 6.9 | 33 | 1.4 (22.0) | 13.8 | |
| Moderately worse | 3 | −5.6 (5.1) | −0.7 | 9 | 10.4 (17.0) | 17.0 | 14 | 7.6 (32.6) | 20.0 | |
| Much worse | 2 | 15.0 (21.2) | 21.7 | 11 | 4.8 (21.0) | 17.2 | ||||
| AUD | Much improved | 8 | −33.3 (29.2) | −36.3 | 19 | -14.9 (22.1) | −11.5 | 14 | −16.7 (25.1) | −14.2 |
| Moderately improved | 22 | −3.5 (18.4) | −6.4 | 15 | −23.6 (26.7) | −20.2 | 21 | −8.9 (28.0) | −6.4 | |
| Slightly improved | 39 | −9.1 (18.2) | −12.1 | 33 | 1.5 (21.0) | 4.9 | 24 | 7.5 (27.3) | 10.0 | |
| No change | 101 | 2.9 (23.7) | 67 | −3.4 (18.8) | 48 | −2.5 (17.5) | ||||
| Slightly worse | 23 | 2.9 (26.8) | 0.0 | 30 | 9.9 (24.2) | 13.3 | 33 | 9.4 (25.5) | 11.9 | |
| Moderately worse | 3 | −3.3 (10.0) | −6.3 | 9 | 19.3 (22.3) | 22.6 | 14 | 20.2 (32.7) | 22.7 | |
| Much worse | 2 | 15.0 (21.2) | 18.4 | 11 | 20.6 (22.2) | 23.1 | ||||
| REL | Much improved | 8 | −52.9 (32.3) | −45.8 | 19 | −38.9 (33.5) | −29.0 | 14 | −42.4 (24.1) | −29.3 |
| Moderately improved | 22 | −23.6 (24.5) | −16.5 | 15 | −21.1 (24.2) | −11.2 | 21 | −25.2 (31.4) | −12.2 | |
| Slightly improved | 39 | −16.2 (19.4) | −9.1 | 33 | −17.5 (25.6) | −7.6 | 24 | −17.5 (25.8) | -4.4 | |
| No change | 101 | −7.1 (23.5) | 67 | −9.9 (21.7) | 48 | −13.1 (24.8) | ||||
| Slightly worse | 23 | 3.5 (23.6) | 10.6 | 30 | −2.3 (27.8) | 7.6 | 33 | −3.7 (22.6) | 9.3 | |
| Moderately worse | 3 | −5.6 (13.5) | 1.5 | 9 | −1.1 (8.7) | 8.8 | 14 | 4.8 (34.8) | 17.8 | |
| Much worse | 2 | 10.0 (14.1) | 19.9 | 11 | 3.9 (10.9) | 17.0 | ||||
| QOL | Much improved | 8 | −27.2 (27.6) | −23.7 | 19 | −21.7 (19.6) | −15.6 | 14 | −21.3 (20.9) | −16.9 |
| Moderately improved | 22 | −14.8 (22.8) | −11.3 | 15 | −24.6 (24.4) | −18.5 | 21 | −22.1 (27.1) | −17.7 | |
| Slightly improved | 39 | −13.6 (14.3) | −10.1 | 33 | −5.4 (17.8) | 0.7 | 24 | −9.3 (20.7) | −4.9 | |
| No change | 101 | −3.5 (19.3) | 67 | −6.1 (20.6) | 48 | −4.4 (17.6) | ||||
| Slightly worse | 23 | 8.0 (21.7) | 11.5 | 30 | 3.6 (23.5) | 9.7 | 33 | 3.7 (20.9) | 8.1 | |
| Moderately worse | 3 | −10.0 (8.7) | −6.5 | 9 | 7.2 (28.2) | 13.3 | 14 | 9.1 (24.2) | 13.5 | |
| Much worse | 2 | 13.8 (19.4) | 19.8 | 11 | 9.1 (19.0) | 13.4 | ||||
| EMO | Much improved | 8 | −30.4 (20.8) | −25.4 | 19 | −26.0 (24.1) | −20.7 | 14 | −23.8 (27.2) | −14.1 |
| Moderately improved | 22 | −17.7 (23.8) | −12.7 | 15 | −28.0 (26.6) | −22.7 | 21 | −22.4 (24.2) | −12.7 | |
| Slightly improved | 39 | −14.0 (20.8) | −9.0 | 33 | −18.2 (24.6) | −12.9 | 24 | −15.6 (21.6) | −5.8 | |
| No change | 101 | −5.1 (17.6) | 67 | −5.3 (18.8) | 48 | −9.7 (22.5) | ||||
| Slightly worse | 23 | 0.9 (20.1) | 5.9 | 30 | −0.3 (23.9) | 4.9 | 33 | −6.6 (23.5) | 3.2 | |
| Moderately worse | 3 | 7.8 (22.7) | 12.8 | 9 | 0.0 (27.1) | 5.3 | 14 | 4.0 (26.9) | 13.8 | |
| Much worse | 2 | 6.7 (33.0) | 11.9 | 11 | 7.6 (12.2) | 17.3 | ||||
| Mean (±SD) | Difference | Measurement Error | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Scale | n | T0 | T1 | T2 | Mean Diff | SD Diff | SEM | SEM × 4 | SDC |
| TFI-25 | 50 | 50.8 (±25.1) | 45.9 (±22.8) | 44.9 (±23.1) | −5.4 | 7.2 | 5.1 | 20.4 | 14.2 |
| TFI-22 | 50 | 51.3 (±25.9) | 45.7 (±23.3) | 45.0 (±23.7) | −5.9 | 7.1 | 5.0 | 20.0 | 13.9 |
| INT | 46 | 64.0 (±24.3) | 55.6 (±23.2) | 54.7 (±23.3) | −9.8 | 13.2 | 9.3 | 37.2 | 25.8 |
| SOC | 48 | 61.9 (±24.3) | 56.5 (±24.2) | 55.0 (±24.0) | −6.2 | 10.7 | 7.6 | 30.4 | 21.0 |
| COG | 49 | 40.9 (±28.4) | 39.3 (±27.2) | 38.0 (±26.0) | −2.2 | 13.5 | 9.6 | 38.4 | 26.5 |
| SLP | 48 | 52.6 (±32.0) | 46.4 (±29.2) | 47.1 (±29.4) | −4.8 | 13.3 | 9.4 | 37.6 | 25.9 |
| AUD | 50 | 47.7 (±30.1) | 47.5 (±28.8) | 44.4 (±28.2) | −1.7 | 16.2 | 10.7 | 42.8 | 29.6 |
| REL | 50 | 62.0 (±29.3) | 55.9 (±26.4) | 53.5 (±27.4) | −7.4 | 14.4 | 10.2 | 40.8 | 28.3 |
| QOL | 49 | 38.6 (±32.4) | 34.7 (±28.9) | 33.3 (±29.0) | −4.6 | 11.3 | 8.0 | 32.0 | 22.2 |
| EMO | 50 | 42.8 (±31.5) | 35.7 (±29.7) | 38.4 (±30.2) | −5.7 | 14.6 | 10.3 | 41.2 | 28.6 |
| TFI Grading Categories | CGI-3 | T1 | T2 | T3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | MCID | n | Mean (SD) | MCID | n | Mean (SD) | MCID | |||
| TFI | Improved | 8 | −5.3 (9.6) | 11 | −5.7 (6.9) | 11 | −3.3 (8.1) | ||||
| Small problem | No change | 22 | 0.2 (9.1) | −5.5 | 13 | −1.7 (7.1) | −4.0 | 10 | −5.2 (8.9) | −4.0 | |
| Worsened | 3 | 9.2 (14.3) | 5 | 5.4 (18.9) | 8 | 12.7 (21.9) | |||||
| Improved | 20 | −14.3 (10.3) | 18 | −17.3 (12.8) | 15 | −21.9 (12.0) | |||||
| Moderate problem | No change | 30 | −2.3 (10.2) | −12.0 | 20 | −1.5 (7.9) | −15.7 | 15 | −5.4 (12.0) | −15.7 | |
| Worsened | 4 | 8.9 (14.1) | 11 | 9.3 (12.5) | 16 | 7.3 (17.0) | |||||
| Improved | 21 | −16.7 (15.9) | 23 | −23.1 (16.1) | 22 | −20.4 (20.2) | |||||
| Big problem | No change | 22 | −2.8 (11.8) | −13.9 | 13 | −8.9 (10.2) | −14.2 | 9 | −8.2 (15.0) | −14.2 | |
| Worsened | 10 | −1.8 (9.8) | 11 | 2.8 (14.6) | 14 | −1.0 (16.1) | |||||
| Improved | 20 | −24.1 (19.6) | 15 | −26.6 (18.9) | 11 | −32.8 (22.8) | |||||
| Very big problem | No change | 27 | −10.5 (13.8) | −13.6 | 21 | −14.7 (18.4) | −11.9 | 14 | −16.4 (12.1) | −11.9 | |
| Worsened | 9 | 0.5 (11.9) | 14 | −2.9 (14.2) | 20 | −1.8 (12.7) | |||||
| TFI-22 | Improved | 8 | −5.3 (9.2) | 11 | −6.4 (6.9) | 11 | −5.7 (6.7) | ||||
| Small problem | No change | 22 | 0.2 (10.1) | −5.6 | 13 | −1.8 (7.1) | −4.6 | 10 | −4.6 (8.3) | −4.6 | |
| Worsened | 3 | 9.9 (14.9) | 5 | 3.8 (22.2) | 8 | 11.3 (22.4) | |||||
| Improved | 20 | −15.6 (11.6) | 18 | −18.6 (14.0) | 15 | −24.3 (11.9) | |||||
| Moderate problem | No change | 30 | −3.3 (10.2) | −12.3 | 20 | −2.0 (9.3) | −16.6 | 15 | −6.8 (12.7) | −16.6 | |
| Worsened | 4 | 10.6 (14.4) | 11 | 7.9 (12.2) | 16 | 5.8 (17.7) | |||||
| Improved | 21 | −18.2 (17.6) | 23 | −25.6 (16.0) | 22 | −23.5 (20.6) | |||||
| Big problem | No change | 22 | −4.3 (11.1) | −14.0 | 13 | −9.4 (12.4) | −16.2 | 9 | −9.2 (17.2) | −16.2 | |
| Worsened | 10 | −2.7 (13.6) | 11 | 2.3 (15.6) | 14 | −2.9 (18.6) | |||||
| Improved | 20 | −24.8 (19.7) | 15 | −27.3 (18.6) | 11 | −33.8 (22.4) | |||||
| Very big problem | No change | 27 | −11.2 (13.8) | −13.6 | 21 | −15.5 (19.1) | −11.8 | 14 | −17.8 (12.9) | −11.8 | |
| Worsened | 9 | 0.1 (12.6) | 14 | −4.1 (13.4) | 20 | −3.0 (12.5) | |||||
| T0–T1 | T0–T2 | T0–T3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
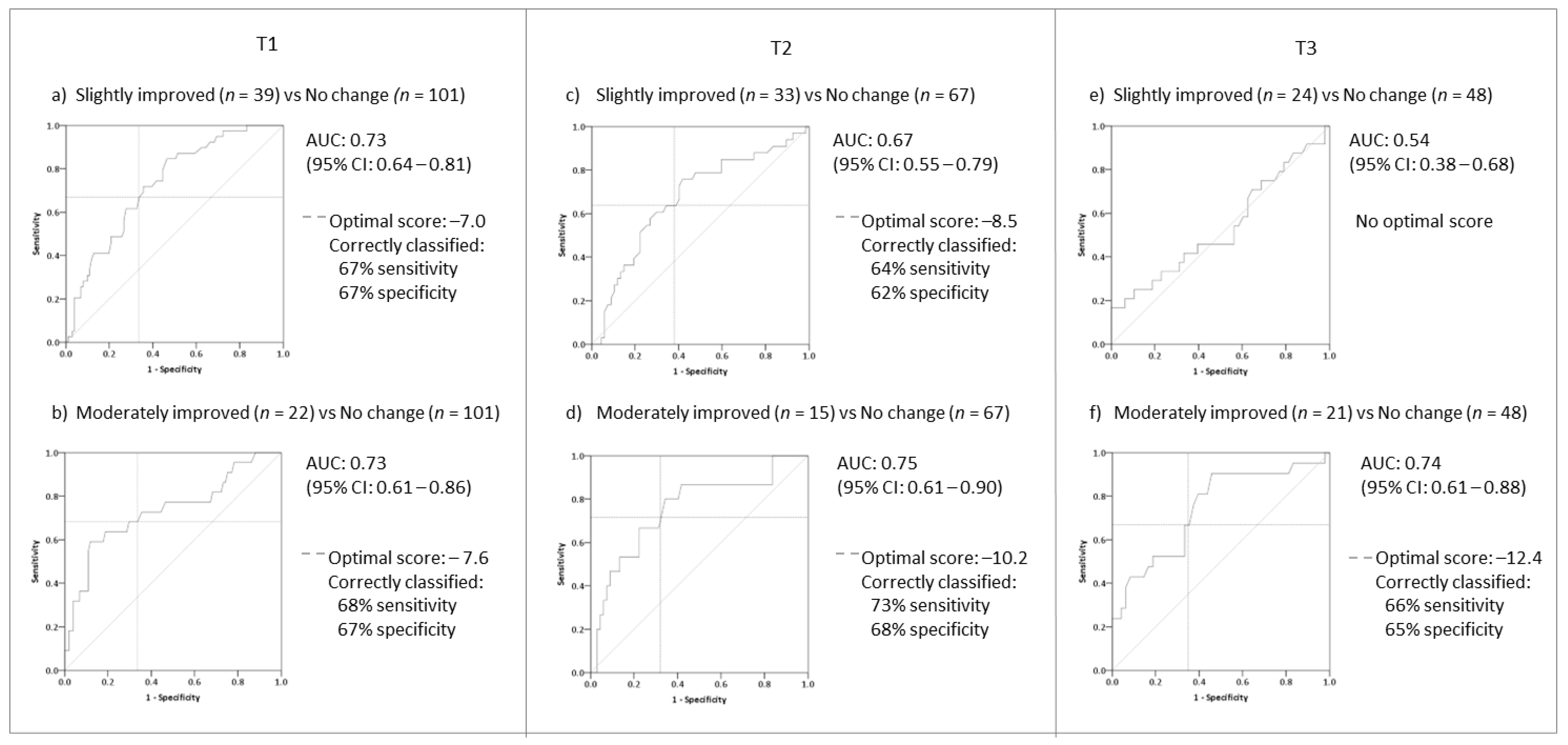
| Improved (69) vs. No Change (101) | Improved (67) vs. No Change (67) | Improved (59) vs. No Change (48) | ||||||||||
| Scale | AUC (95%CI) | Optimal Value | Sens % | Spec % | AUC (95%CI) | Optimal Value | Sens % | Spec % | AUC (95%CI) | Optimal Value | Sens % | Spec % |
| TFI | 0.75 (0.68–0.82) | −7.60 | 65 | 67 | 0.74 (0.66–0.83) | −10.2 | 68 | 67 | 0.70 (0.57–0.77) | −11.8 | 63 | 61 |
| TFI-22 | 0.74 (0.67–0.82) | −7.50 | 68 | 66 | 0.75 (0.67–0.84) | −10.6 | 73 | 70 | 0.70 (0.59–0.79) | −12.0 | 61 | 57 |
| INT | 0.69 (0.60–0.77) | −11.66 | 63 | 69 | 0.68 (0.59–0.77) | −13.34 | 60 | 58 | 0.63 (0.52–0.74) | −18.34 | 59 | 56 |
| SOC | 0.69 (0.61–0.77) | −8.34 | 66 | 63 | 0.73 (0.64–0.82) | −13.34 | 67 | 66 | 0.64 (0.54–0.75) | −13.34 | 63 | 63 |
| COG | 0.61 (0.52–0.69) | −6.66 | 57 | 56 | 0.66 (0.56–0.75) | −8.34 | 60 | 61 | 0.65 (0.55–0.75) | −8.34 | 62 | 60 |
| SLP | 0.64 (0.56–0.73) | −8.34 | 57 | 57 | 0.67 (0.57–0.76) | −11.66 | 64 | 67 | 0.63 (0.53–0.74) | −16.65 | 64 | 65 |
| AUD | 0.64 (0.56–0.73) | −1.66 | 53 | 59 | 0.56 (0.46–066) | −1.66 | 54 | 54 | 0.54 (0.43–0.65) | −1.66 | 54 | 56 |
| REL | 0.69 (0.60–0.77) | −13.33 | 61 | 69 | 0.64 (0.55–0.74) | −16.67 | 61 | 61 | 0.64 (0.54–0.75) | −15 | 61 | 60 |
| QOL | 0.69 (0.61–0.77) | −6.25 | 65 | 65 | 0.62 (0.53–0.72) | −6.25 | 60 | 58 | 0.66 (0.56–0.77) | −6.25 | 61 | 63 |
| EMO | 0.66 (0.57–0.74) | −8.34 | 62 | 68 | 0.73 (0.64–0.82) | −8.34 | 65 | 70 | 0.63 (0.52–0.73) | −11.67 | 55 | 65 |
| Worsened (26) vs. No change (101) | Worsened (41) vs. No change (67) | Worsened (58) vs. No change (48) | ||||||||||
| Scale | AUC (95% CI) | Optimal value | Sens % | Spec % | AUC (95% CI) | Optimal value | Sens % | Spec % | AUC (95%CI) | Optimal value | Sens % | Spec % |
| TFI | 0.63 (0.50–0.75) | 1.4 | 62 | 58 | 0.66 (0.56–0.77) | 4.0 | 61 | 59 | 0.70 (0.61–0.80) | 2.8 | 64 | 62 |
| TFI-22 | 0.64 (0.51–0.78) | 1.6 | 59 | 58 | 0.64 (0.53–0.75) | 3.9 | 56 | 56 | 0.70 (0.60–0.80) | 4.1 | 64 | 64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fackrell, K.; Hall, D.A.; Barry, J.; Hoare, D.J. Integrating Distribution-Based and Anchor-Based Techniques to Identify Minimal Important Change for the Tinnitus Functional Index (TFI) Questionnaire. Brain Sci. 2022, 12, 726. https://doi.org/10.3390/brainsci12060726
Fackrell K, Hall DA, Barry J, Hoare DJ. Integrating Distribution-Based and Anchor-Based Techniques to Identify Minimal Important Change for the Tinnitus Functional Index (TFI) Questionnaire. Brain Sciences. 2022; 12(6):726. https://doi.org/10.3390/brainsci12060726
Chicago/Turabian StyleFackrell, Kathryn, Deborah Ann Hall, Johanna Barry, and Derek James Hoare. 2022. "Integrating Distribution-Based and Anchor-Based Techniques to Identify Minimal Important Change for the Tinnitus Functional Index (TFI) Questionnaire" Brain Sciences 12, no. 6: 726. https://doi.org/10.3390/brainsci12060726
APA StyleFackrell, K., Hall, D. A., Barry, J., & Hoare, D. J. (2022). Integrating Distribution-Based and Anchor-Based Techniques to Identify Minimal Important Change for the Tinnitus Functional Index (TFI) Questionnaire. Brain Sciences, 12(6), 726. https://doi.org/10.3390/brainsci12060726






