Objective Detection of Tinnitus Based on Electrophysiology
Abstract
1. Introduction
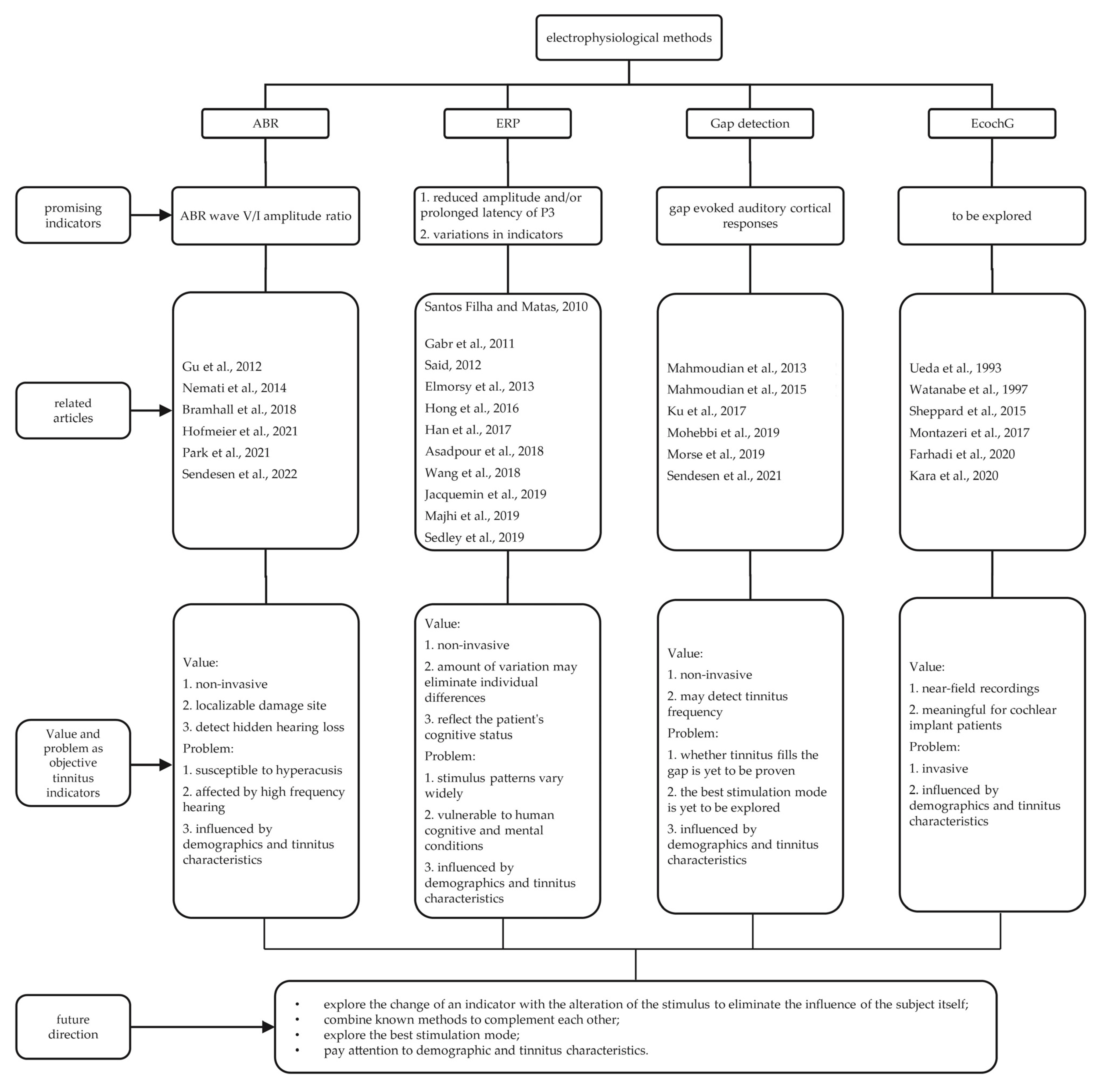
2. Electrophysiological Methods
2.1. ABR
2.1.1. Components
2.1.2. ABR Studies
2.2. ERP
2.2.1. Components
| Author, Year | Groups and Number | Etiology of Tinnitus | Mean Age | Matched | Hearing Status | Hyperacusis | Stimuli | Outcome (Tinnitus Group) |
|---|---|---|---|---|---|---|---|---|
| Schaette and McAlpine, 2011 [29] | 15 tinnitus 18 controls | not mentioned | tinnitus: 36.3 controls: 33.2 | age, sex, hearing | ≤20 dB HL (0.125–8 kHz, 12–16 kHz) | not mentioned | duration: 50 µs, 90 and 100 dB SPL, 11 clicks/s | reduced wave I amplitude and normal wave V |
| Singh et al., 2011 [21] | 25 tinnitus 20 controls | idiopathic | tinnitus: 32 controls: matched | age, sex, hearing | <25 dB HL (0.25–8 kHz) | not mentioned | not mentioned | prolonged wave I latency, shortened wave V, and I–III and I–V interpeak latencies |
| Cartocci et al., 2012 [22] | 10 tinnitus 14 controls | idiopathic | tinnitus: 43.9 controls: 45.1 | age, sex, hearing | ≤20 dB HL (0.125–8 kHz) | excluded hyperacusis by the dynamic range measure | alternating polarity, duration: 100 μs, 90 and 80 dB HL, 11 clicks/s | prolonged wave V and interpeak III–V latencies |
| Gu et al., 2012 [30] | 15 tinnitus 21 controls | not mentioned | tinnitus: 42 controls: 43 | age, sex, hearing | ≤20 dB HL (0.125–8 kHz) | not mentioned | condensation, duration: 100 μs, 30, 50, 70, and 80 dB HL, 11 clicks/s | reduced wave I amplitude and enhanced wave V amplitude |
| Nemati et al., 2014 [31] | 25 tinnitus 16 controls | idiopathic | tinnitus: 34.4 controls: matched | age, sex, hearing | <25 dB HL (0.25–8 kHz) | not mentioned | alternating polarity, duration: 90 dB SPL, 11.1 clicks/s | enhanced V/I amplitude ratio |
| Santos-Filha et al., 2014 [25] | 30 tinnitus 30 controls | noise induced | tinnitus: 41 controls: 41.6 | age, sex, hearing | <25 dB HL (0.25–8 kHz) | not mentioned | Rarefaction polarity, duration: 0.1 ms, 80 dB HL, 19 clicks/s | no significant differences in latencies |
| Gilles et al., 2016 [26] | 19 tinnitus 23 controls | noise induced | male 23.1 female 23.5 | age, sex, hearing | <25 dB HL (0.125–8 kHz, 9–16 kHz) | 3 subjects of the non-tinnitus group and 4 subjects of the tinnitus group had a score >22 on the hyperacusis questionnaire | alternating polarity, duration: 100 µs, 80 dB HL, 31 clicks/s | no significant differences |
| Konadath et al., 2016 [35] | 20 tinnitus 20 controls | idiopathic | tinnitus: 33.15 controls: 20.50 | sex, hearing | ≤20 dB HL (0.25–8 kHz) | not mentioned | duration: 100 µs, 70 dB HL, 11.1 clicks/s | reduced absolute amplitude of peaks I and V |
| Kehrle et al., 2016 [86] | 84 tinnitus 47 controls | not mentioned | tinnitus: 37.2 controls: 35.7 | age, sex, hearing | ≤25 dB HL (0.25–8 kHz) | not mentioned | negative polarity, duration: 100 ms, 80 dB HL, 21.1 clicks/s | abnormal values for the latency of wave I, wave III, wave V, the interpeak I–III, the interpeak III–V, and the interpeak I–V |
| Ravikumar et al., 2016 [24] | 50 tinnitus 50 controls | not mentioned | not mentioned | not mentioned | not mentioned | not mentioned | not mentioned | prolonged I, III, and V |
| Shim et al., 2017 [46] | 43 tinnitus 18 controls | not mentioned | tinnitus: 33.6 controls: 28.6 | age, sex, hearing | ≤20 dB HL (0.25–8 kHz) | not mentioned | duration: 90 dB HL, 13.3 clicks/s | no significant differences |
| Guest et al., 2017 [27] | 20 tinnitus 20 controls | noise induced | tinnitus: 25.7 controls: 25.5 | age, sex, hearing | ≤20 dB HL (0.25–8 kHz) | not mentioned | duration: 102 dB ppe SPL (peak-to-peak), 14.1 clicks/s | no significant differences in amplitude of wave I and V |
| Pinkl et al., 2017 [56] | 11 tinnitus with 21 tested ears 10 controls with 10 ears | idiopathic | tinnitus: 46.48 controls: 24.4 | not mentioned | <30 dB HL (0.25–20 kHz) | not mentioned | click evoked ABRs: rarefaction polarity, 85 dB HL, 21.1 stimuli/s tone burst evoked ABRs: rarefaction polarity, 85 dB HL, 21.1 stimuli/s | Click ABR: prolonged V–III IPLs for tinnitus with normal hearing and tinnitus with hearing loss and prolonged absolute V latency for tinnitus with hearing loss. Tone burst ABRs: prolonged absolute latencies and IPLs at three of the seven frequencies for tinnitus with hearing loss |
| Bramhall et al., 2018 [44] | 15 tinnitus 59 controls | noise induced | tinnitus: 26.3 controls: 26.7 | age, hearing | ≤20 dB HL (0.25–8 kHz) | pure tone loudness discomfort levelswere measured as an indicator of hyperacusis | alternating polarity, duration: 4 kHz tone burst stimuli, duration: 2 ms, 80, 90, 100, and 110 dB ppe SPL, 11.1 stimuli/s | reduced wave I amplitudes and wave I/V ratio |
| Song et al., 2018 [57] | 20 tinnitus 91 controls | not mentioned | tinnitus: 37 controls: 43 | age, sex, hearing | ≤20 dB (0.25–8 kHz) | not mentioned | duration: 90 dB click stimulus | shortened latency in wave III on the right and in wave V on the left in patients with bilateral tinnitus |
| Hofmeier et al., 2018 [53] | 17 tinnitus 17 controls | idiopathic | tinnitus: 33.2 controls: 36.5 | sex, hearing | ≤40 dB (0.125–10 kHz) | excluded by the Hyperacusis Questionnaire | duration: 100 µs, 25–75 dB SPL in 10 dB steps, 11.1 clicks/s | reduced and prolonged wave V |
| Majhi et al., 2019 [23] | 55 sensorineural hearing loss with tinnitus 51 control | idiopathic | tinnitus: 42.91 controls: 41.63 | age, sex, education level | Sensorineural hearing loss mild = 26–40 dB, moderate = 41–60 dB, severe >61 dB | not mentioned | not mentioned | prolonged latency of wave I, III, V, and interpeak latency of I–III, III–V, I–V was observed in tinnitus with sensorineural hearing loss group |
| Möhrle et al., 2019 [33] | 17 Tinnitus 17 Controls | not mentioned | not mentioned | All standard conditions | PTA ≤40 dB | excluded by the new Hyperacusis Inventory Questionnaire | duration: 0.1 ms, 25–75 dB SPL in 10 dB steps,11.1 clicks/s | prolonged latencies and reduced amplitudes of ABR wave V |
| Han et al., 2021 [48] | 10 tinnitus 6 chronic tinnitus 4 non-chronic tinnitus | idiopathic | tinnitus: 16.5 | ≤25 dB HL (0.125–8 kHz) | not mentioned | duration: 90 dB, 13.3 clicks/s | prolonged interpeak latency of III–V in tinnitus ears compared with non-tinnitus ears | |
| Hofmeier et al., 2021 [34] | 43 controls 30 tinnitus 20 tinnitus + hyperacusis | not mentioned | not mentioned | not mentioned | not mentioned | hyperacusis questionnaire | not mentioned | reduced ABR wave V amplitude, prolonged interpeak latency (IPL) I–V, reduced ABR wave V/I ratios |
| Shim et al., 2021 [47] | 27 tinnitus 27 controls | idiopathic | tinnitus: 36.7 controls: 36.0 | age, sex, hearing | ≤20 dB HL (0.25–8 kHz) | not mentioned | duration: 100 ms, 80 and 90 dB HL, 13 clicks/s | no significant differences in wave I or wave V or the wave V/I |
| Johannesen et al., 2021 [28] | 7 tinnitus 87 controls | not mentioned | not mentioned | not mentioned | ≤20 dB HL at 0.5 and 4 kHz and ≤30 dB HL at 6 and 8 kHz | not mentioned | rarefaction clicks, duration: 100 μs, intensities: from 110 down to 90 dB, peak-to-peak equivalent sound pressure level (ppe SPL), in 5-dB steps rate: 11 clicks/s | no significant differences in wave I and V amplitudes |
| Park et al., 2021 [32] | 59 tinnitus 59 controls | idiopathic | tinnitus: 42.42 controls: 41.86 | age, hearing | ≤25 dB | not mentioned | clicks, duration: 50 ms, 90 dB SPL | reduced ABR wave I amplitude and wave I/V ratio |
| Sendesen et al., 2022 [87] | 20 unilateral tinnitus | idiopathic | unilateral tinnitus: 33.55 | sex, hearing (up to 16 kHz) | <20 dB HL (0.5–4 kHz) | not mentioned | alternating polarity, duration: 80 dB HL level, 21.1 clicks/s | enhanced wave I amplitude and the ratio of III/I, V/I, and V/III wave amplitude in tinnitus ears |
2.2.2. ERP Studies
2.2.3. Main Findings
| Author, Year | Groups and Number | Etiology of Tinnitus | Mean Age | Matched | Hearing Status | Neurological and/or Psychological Disorder | Electrode | Stimuli | Outcome (Tinnitus Group) |
|---|---|---|---|---|---|---|---|---|---|
| Santos Filha and Matas, 2010 [93] | 30 tinnitus 30 controls | noise-induced | tinnitus: 41 controls: 41.6 | age | ≤25 dBHL (0.25–8 kHz) | excluded | right and left ears (A2 and A1); vertex (Cz) and forehead (Fpz) | oddball paradigm: tone bursts at 75 dB HL, in the frequencies of 1 kHz (frequent stimulus) and 1.5 kHz (rare stimulus) | prolonged latency of N1, P2, and P300 |
| Gabr et al., 2011 [99] | 40 tinnitus 40 controls | idiopathic | tinnitus: 37.3 controls: 38.5 | age, sex, hearing | ≤25 dBHL (0.25–8 kHz) | excluded | Fz (active electrode); Fpz (ground); M1 and M2 (reference) | oddball paradigm, in the frequencies of 1 kHz (standard stimulus) and 2 kHz (deviant stimulus) | prolonged P3 latency |
| Said, 2012 [104] | study group: 36 sensorineural hearingloss with tinnitus 30 sensorineural hearing loss only 24 controls | idiopathic | study group: 28.35 controls: 29.72 | age, sex | heterogeneous | excluded | Fz (active electrode); Fpz (ground); M1 and M2 (reference) | 80 dB HL, in the frequencies of 1 kHz (frequent stimulus) and 2 kHz (rare stimulus) | reduced P2 and P3 amplitude and prolonged N1, P2, P3 latency in patients with tinnitus |
| Elmorsy et al., 2013 [101] | 32 tinnitus 30 controls | idiopathic | tinnitus: 39.8 controls: 38.7 | age, sex, hearing | ≤25 dB HL (0.25–8 kHz) | excluded | Fz (active electrode); A2 and A1 (reference); forehead (ground) | 75 dB HL, in the frequencies of 1 kHz (frequent stimulus) and 2 kHz (rare stimulus) | overall reduced P3 amplitude and no significant differences for P3 latency |
| Holdefer et al., 2013 [106] | 25 tinnitus 13 controls | not mentioned | tinnitus: 49 controls: 35 | sex, hearing | tinnitus group’s mean PTA: 8 dB control group’s mean PTA: 9 dB | not mentioned | vertex (vertex electrode); behind the ears (right and left ear); the left side of the forehead (ground) | 70 dB, in the frequencies of 1 kHz (standard stimulus) and 1.1 kHz (rare stimulus) | smaller latencies in the right ear, no statistically significant differences in MMN amplitudes |
| Yang et al., 2013 [65] | 20 tinnitus 16 controls | not mentioned | tinnitus: 43.2 controls: 42.5 | age, sex | tinnitus: PTA <20 dB (n = 8) PTA: 21–40 dB (n = 12) controls: PTA <20 dB (0.5, 1, 2, 4 KHz) | not mentioned | 128 channels; Cz (reference channel); reported at Fz | oddball paradigm: pure tone at 75 dB, in the frequencies of 1500–1000 Hz (50-ms duration with a shaped 5-ms rise and fall time) | smaller mismatch negativity (MMN) and late discriminative negativity (LDN). After rTMS treatment, increased N1 response to deviant stimuli and larger MMN and LDN |
| Houdayer et al., 2015 [97] | 17 tinnitus 17 controls | not mentioned | tinnitus: 43.4 controls: 45.7 | not mentioned | <15 dB HL (0.125–8 kHz) | excluded | 29 electrodes cap, obtained from the electrode displaying the greatest ERP | oddball paradigm: tone bursts, in the frequencies of 1 kHz (frequent stimulus) and 2 kHz (rare stimulus) | shorter N1 and P2 latencies. P300 did not differ between groups |
| Hong et al., 2016 [91] | 15 tinnitus 15 controls | idiopathic | tinnitus: 30.2 controls: 28.7 | age, sex | ≤25 dB HL (0.25–8 kHz) | excluded | 32 electrodes; the tip of the nose (reference); Afz (ground) | oddball and passive listening paradigm | shorter N2, lower N200 amplitudes during the oddball task compared with the passive listening task, lower P3. Lower N1 response to the target stimuli in the oddball task |
| Konadath et al., 2016 [35] | 20 tinnitus 20 controls | idiopathic | tinnitus: 33.15 controls: 20.50 | sex, hearing | ≤20 dB HL (0.25–8 kHz) | not mentioned | 2 channels; vertical (Fpz, Cz, M1/M2) | Alternating polarity 70 dB HL 500 Hz tone bursts 1.1/s | no significant difference in the latency and amplitude except for enhanced amplitude of the P1 peak in tinnitus group |
| Gopal et al., 2017 [94] | 10 tinnitus 10 controls | heterogeneous | tinnitus: 48.9 controls: 49.8 | age, sex, hearing | varying degrees of hearing, but matched between groups | excluded | gold cup electrodes were positioned at high forehead (active electrode), right and left ear lobes (reference), and low forehead (ground) | 1000 Hz tone bursts presented at a rate of 1.1/s | enhanced N1 amplitude |
| Han et al., 2017 [108] | 33 tinnitus ears 63 control ears | idiopathic | tinnitus: 38.7 controls: 37.7 | age, sex, tinnitus ears,, hearing | ≤25 dB HL at 0.5, 1, 2, and 3 kHz, and hearing threshold ≤40 dB HL at all frequencies | excluded | 2-channel AgCI electrodes; Cz (reference); A1 and A2 (active and ground) | the first 250 ms was a 1 kHz tone followed by 250 ms of 8 kHz or 4 kHz pure tone | the normalized amplitude of the ACC of 8 KHz (tinnitus frequency) in tinnitus group was less than 4 and 8 kHz in normal control group |
| Mannarelli et al. 2017 [92] | 20 tinnitus 20 controls | idiopathic | tinnitus: 50.1 controls: 49.4 | age, sex, education | PTA <20 dB HL (up to 2000 Hz) PTA <30 dB HL (>2000 Hz) | excluded | 9 central channels; referred to linked mastoids; Fpz (ground) | auditory oddball paradigm, tone bursts at 80 dB SPL, in the frequencies of 0.5 kHz (frequent stimulus) and 1 kHz (rare stimulus) | lower P3a amplitudes, prolonged N1 latency |
| Asadpour et al., 2018 [100] | 15 tinnitus 6 controls | not mentioned | tinnitus: 39 controls: 27 | hearing | normal hearing | not mentioned | 32 EEG electrodes cap; tip of nose (reference) | auditory/visual oddball paradigm, auditory stimuli: tone bursts at 70 dB SPL, in the frequencies of 4 kHz (standard stimulus) and 6 kHz (rare stimulus) visual stimuli: 160 blue triangles as standard and 40 yellow circles as target stimuli | lower amplitude of auditory P300 peak in three EEG channels |
| Wang et al., 2018 [114] | 95 mild tinnitus group, 112 severe tinnitus | idiopathic | mild tinnitus group: 47.88, severe tinnitus group: 48.18 | not mentioned | ≤25 dB HL (0.125–8 kHz) | excluded | Electrodes recorded at Fz, Cz, and Pz; A1 (left ear) and A2 (right ear) (reference electrodes) | auditory oddball paradigm, tone bursts at 85 dB HL, in the frequencies of 2 kHz (target stimulus) and 60 dB HL in 1 kHz (non-target stimulus) | compared with mild tinnitus patients, severe tinnitus patients exhibited longer P300 and N2 latencies |
| Campbell et al., 2019 [117] | 21 tinnitus 45 controls | not mentioned | tinnitus: 21.51 (median) controls: 23.43 (median) | age, hearing | <15 dB HL (0.25–8 kHz) extended high-frequency (up to 16 kHz) was tested | tinnitus (n = 3) controls (n = 6) | 128-channel electrodes net | gating paradigmtone 50 dB HL 250 Hz | no significant differences for P1, N1 or P2 amplitude |
| Durai et al., 2019 [95] | 16 tinnitus 14 controls | idiopathic | tinnitus: 53.44 controls: 50.25 | age, sex, hearing | matched (0.25–8 kHz) | excluded | 66 active surface electrodes | ABA streaming paradigm, prediction paradigm | enhanced N1c, decreased P2 waveforms for frequency-4, and enhanced P2 waveforms for frequency-7 conditions |
| Jacquemin et al., 2019 [60] | 22 tinnitus | heterogeneous | tinnitus: 51 | heterogeneous | not mentioned | 31 electrodes cap; chin (reference); right mastoid (ground); recorded at the right eye | auditory oddball paradigm, in the frequencies of 1 kHz (frequent stimulus) and 2 kHz (rare stimulus) | shortening of the N1, P2, N2, and P3 latencies after HD-tDCS treatment, the amplitude of N2 being significantly larger after HD-tDCS | |
| Majhi et al., 2019 [103] | 55 tinnitus 51 controls | idiopathic | tinnitus: 42.91 controls: 41.63 | age, sex, education | Sensorineural hearing loss was classified into mild: 26–40 dB, moderate: 41–60 dB, severe: >61 dB | excluded | not mentioned | oddball paradigm | increased P300 latency and decreased P300 amplitude were found in sensorineural hearing loss with tinnitus cases. Increasing severity of tinnitus and degree of hearing loss |
| Sedley et al., 2019 [109] | 26 chronic tinnitus, 26 non-tinnitus controls, 15 acute tinnitus) | not mentioned | chronic tinnitus: 55.4, controls: 59.7, acute tinnitus: 53.8 | age, hearing | matched | not mentioned | 64 channels | MMN paradigm | tinnitus subjects had larger responses to upward deviants and less responses to downward deviants than matched controls |
| Vasudevan et al., 2019 [96] | 10 tinnitus 10 controls | not mentioned | tinnitus: 38.8 controls: 37.9 | age, sex, hearing | ≤40 dB HL (500 Hz, 1 kHz, and 2 kHz) | excluded | 32-channel EazyCap; combined mastoid (reference) | auditory oddball paradigm, tone bursts at 75 dB SPL, in the frequencies of 1 kHz (standard stimulus) and 1.5 kHz (deviant stimulus) | larger N1 and P3 amplitudes along with prolonged P3 latency |
| Mohan et al., 2022 [105] | 10 tinnitus 10 controls | idiopathic | tinnitus: 25.9 controls: 27 | age, hearing | ≤30 dB HL (0.25–8 kHz) | excluded | 64-channel EazyCap; Cz (reference) | auditory oddball paradigm | increased P300 amplitude |
2.3. Gap Detection
2.3.1. Background
2.3.2. Gap Detection Studies
2.3.3. Main Findings
2.4. EcochG
2.4.1. Components
2.4.2. Previous Studies
2.4.3. Main Findings
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trevis, K.J.; McLachlan, N.M.; Wilson, S.J. A systematic review and meta-analysis of psychological functioning in chronic tinnitus. Clin. Psychol. Rev. 2018, 60, 62–86. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.A.; Reavis, K.M.; Griest, S.E.; Thielman, E.J.; Theodoroff, S.M.; Grush, L.D.; Carlson, K.F. Tinnitus: An Epidemiologic Perspective. Otolaryngol. Clin. N. Am. 2020, 53, 481–499. [Google Scholar] [CrossRef] [PubMed]
- Baguley, D.; McFerran, D.; Hall, D. Tinnitus. Lancet 2013, 382, 1600–1607. [Google Scholar] [CrossRef]
- Riga, M.; Komis, A.; Maragoudakis, P.; Korres, G.; Ferekidis, E.; Danielides, V. Objective assessment of subjective tinnitus through contralateral suppression of otoacoustic emissions by white noise: Effects of frequency, gender, tinnitus bilaterality and age. Acta Otorhinolaryngol. Ital. 2018, 38, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Duda, V.; Scully, O.; Baillargeon, M.S.; Hébert, S. Does Tinnitus Fill in the Gap Using Electrophysiology? A Scoping Review. Otolaryngol. Clin. N. Am. 2020, 53, 563–582. [Google Scholar] [CrossRef]
- Eggermont, J.J.; Roberts, L.E. The neuroscience of tinnitus. Trends Neurosci. 2004, 27, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, J.J.; Roberts, L.E. Tinnitus: Animal models and findings in humans. Cell Tissue Res. 2015, 361, 311–336. [Google Scholar] [CrossRef]
- Schaette, R.; Kempter, R. Development of tinnitus-related neuronal hyperactivity through homeostatic plasticity after hearing loss: A computational model. Eur. J. Neurosci. 2006, 23, 3124–3138. [Google Scholar] [CrossRef]
- Rauschecker, J.P.; Leaver, A.M.; Mühlau, M. Tuning out the noise: Limbic-auditory interactions in tinnitus. Neuron 2010, 66, 819–826. [Google Scholar] [CrossRef]
- Llinás, R.R.; Ribary, U.; Jeanmonod, D.; Kronberg, E.; Mitra, P.P. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc. Natl. Acad. Sci. USA 1999, 96, 15222–15227. [Google Scholar] [CrossRef]
- De Ridder, D.; Vanneste, S.; Freeman, W. The Bayesian brain: Phantom percepts resolve sensory uncertainty. Neurosci. Biobehav. Rev. 2014, 44, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Ciorba, A.; Hatzopoulos, S.; Corazzi, V.; Cogliandolo, C.; Aimoni, C.; Bianchini, C.; Stomeo, F.; Pelucchi, S. Newborn hearing screening at the Neonatal Intensive Care Unit and Auditory Brainstem Maturation in preterm infants. Int. J. Pediatr. Otorhinolaryngol. 2019, 123, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Markand, O.N. Brainstem auditory evoked potentials. J. Clin. Neurophysiol. 1994, 11, 319–342. [Google Scholar] [CrossRef] [PubMed]
- Rupa, V.; Job, A.; George, M.; Rajshekhar, V. Cost-effective initial screening for vestibular schwannoma: Auditory brainstem response or magnetic resonance imaging? Otolaryngol. Head Neck Surg. 2003, 128, 823–828. [Google Scholar] [CrossRef]
- Kotlarz, J.P.; Eby, T.L.; Borton, T.E. Analysis of the efficiency of retrocochlear screening. Laryngoscope 1992, 102, 1108–1112. [Google Scholar] [CrossRef]
- Melcher, J.R.; Kiang, N.Y. Generators of the brainstem auditory evoked potential in cat. III: Identified cell populations. Hear. Res. 1996, 93, 52–71. [Google Scholar] [CrossRef]
- Simpson, G.V.; Knight, R.T.; Brailowsky, S.; Prospero-Garcia, O.; Scabini, D. Altered peripheral and brainstem auditory function in aged rats. Brain Res. 1985, 348, 28–35. [Google Scholar] [CrossRef]
- Chen, T.J.; Chen, S.S. Generator study of brainstem auditory evoked potentials by a radiofrequency lesion method in rats. Exp. Brain Res. 1991, 85, 537–542. [Google Scholar] [CrossRef]
- Reichmuth, C.; Mulsow, J.; Finneran, J.J.; Houser, D.S.; Supin, A.Y. Measurement and response characteristics of auditory brainstem responses in pinnipeds. Aquat. Mamm. 2007, 33, 132–150. [Google Scholar] [CrossRef]
- Liberman, M.C.; Kujawa, S.G. Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear. Res. 2017, 349, 138–147. [Google Scholar] [CrossRef]
- Singh, S.; Munjal, S.K.; Panda, N.K. Comparison of auditory electrophysiological responses in normal-hearing patients with and without tinnitus. J. Laryngol. Otol. 2011, 125, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Cartocci, G.; Attanasio, G.; Fattapposta, F.; Locuratolo, N.; Mannarelli, D.; Filipo, R. An electrophysiological approach to tinnitus interpretation. Int. Tinnitus. J. 2012, 17, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.K.; Khandelwal, K.; Shareef, M. Auditory Brainstem Response in Patients of Tinnitus with Sensorineural Hearing Loss. Indian J. Otolaryngol. Head Neck Surg. 2019, 71 (Suppl. 2), 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, G.; Ashok Murthy, V. A Study of Brainstem Auditory Evoked Responses in Normal Hearing Patients with Tinnitus. Indian J. Otolaryngol. Head Neck Surg. 2016, 68, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Santos-Filha, V.A.; Samelli, A.G.; Matas, C.G. Noise-induced tinnitus: Auditory evoked potential in symptomatic and asymptomatic patients. Clinics 2014, 69, 487–490. [Google Scholar] [CrossRef]
- Gilles, A.; Schlee, W.; Rabau, S.; Wouters, K.; Fransen, E.; Van de Heyning, P. Decreased Speech-In-Noise Understanding in Young Adults with Tinnitus. Front. Neurosci. 2016, 10, 288. [Google Scholar] [CrossRef]
- Guest, H.; Munro, K.J.; Prendergast, G.; Howe, S.; Plack, C.J. Tinnitus with a normal audiogram: Relation to noise exposure but no evidence for cochlear synaptopathy. Hear. Res. 2017, 344, 265–274. [Google Scholar] [CrossRef]
- Johannesen, P.T.; Lopez-Poveda, E.A. Age-related central gain compensation for reduced auditory nerve output for people with normal audiograms, with and without tinnitus. iScience 2021, 24, 102658. [Google Scholar] [CrossRef]
- Schaette, R.; McAlpine, D. Tinnitus with a normal audiogram: Physiological evidence for hidden hearing loss and computational model. J. Neurosci. 2011, 31, 13452–13457. [Google Scholar] [CrossRef]
- Gu, J.W.; Herrmann, B.S.; Levine, R.A.; Melcher, J.R. Brainstem auditory evoked potentials suggest a role for the ventral cochlear nucleus in tinnitus. J. Assoc. Res. Otolaryngol. 2012, 13, 819–833. [Google Scholar] [CrossRef]
- Nemati, S.; Faghih Habibi, A.; Panahi, R.; Pastadast, M. Cochlear and brainstem audiologic findings in normal hearing tinnitus subjects in comparison with non-tinnitus control group. Acta Med. Iran. 2014, 52, 822–826. [Google Scholar] [PubMed]
- Park, E.; Song, I.; Jeong, Y.J.; Im, G.J.; Jung, H.H.; Choi, J.; Rah, Y.C. Evidence of Cochlear Synaptopathy and the Effect of Systemic Steroid in Acute Idiopathic Tinnitus With Normal Hearing. Otol. Neurotol. 2021, 42, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Möhrle, D.; Hofmeier, B.; Amend, M.; Wolpert, S.; Ni, K.; Bing, D.; Klose, U.; Pichler, B.; Knipper, M.; Rüttiger, L. Enhanced Central Neural Gain Compensates Acoustic Trauma-induced Cochlear Impairment, but Unlikely Correlates with Tinnitus and Hyperacusis. Neuroscience 2019, 407, 146–169. [Google Scholar] [CrossRef] [PubMed]
- Hofmeier, B.; Wertz, J.; Refat, F.; Hinrichs, P.; Saemisch, J.; Singer, W.; Rüttiger, L.; Klose, U.; Knipper, M.; Wolpert, S. Functional biomarkers that distinguish between tinnitus with and without hyperacusis. Clin. Transl. Med. 2021, 11, e378. [Google Scholar] [CrossRef] [PubMed]
- Konadath, S.; Manjula, P. Auditory brainstem response and late latency response in individuals with tinnitus having normal hearing. Intractable Rare Dis. Res. 2016, 5, 262–268. [Google Scholar] [CrossRef][Green Version]
- Joo, J.W.; Jeong, Y.J.; Han, M.S.; Chang, Y.S.; Rah, Y.C.; Choi, J. Analysis of Auditory Brainstem Response Change, according to Tinnitus Duration, in Patients with Tinnitus with Normal Hearing. J. Int. Adv. Otol. 2020, 16, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Jerger, J.; Hall, J. Effects of age and sex on auditory brainstem response. Arch. Otolaryngol. 1980, 106, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.R. The effects of cochlear hearing loss, age and sex on the auditory brainstem response. Audiology 1996, 35, 246–258. [Google Scholar] [CrossRef]
- Hwang, J.H.; Chao, J.C.; Ho, H.C.; Hsiao, S.H. Effects of sex, age and hearing asymmetry on the interaural differences of auditory brainstem responses. Audiol. Neurootol. 2008, 13, 29–33. [Google Scholar] [CrossRef]
- Vielsmeier, V.; Lehner, A.; Strutz, J.; Steffens, T.; Kreuzer, P.M.; Schecklmann, M.; Landgrebe, M.; Langguth, B.; Kleinjung, T. The Relevance of the High Frequency Audiometry in Tinnitus Patients with Normal Hearing in Conventional Pure-Tone Audiometry. Biomed. Res. Int. 2015, 2015, 302515. [Google Scholar] [CrossRef]
- Kim, D.K.; Park, S.N.; Kim, H.M.; Son, H.R.; Kim, N.G.; Park, K.H.; Yeo, S.W. Prevalence and significance of high-frequency hearing loss in subjectively normal-hearing patients with tinnitus. Ann. Otol. Rhinol. Laryngol. 2011, 120, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Guest, H.; Munro, K.J.; Plack, C.J. Tinnitus with a normal audiogram: Role of high-frequency sensitivity and reanalysis of brainstem-response measures to avoid audiometric over-matching. Hear. Res. 2017, 356, 116–117. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Kim, M.G.; Kim, S.S.; Byun, J.Y.; Park, M.S.; Yeo, S.G. Evaluation of tinnitus patients by audiometric configuration. Am. J. Otolaryngol. 2016, 37, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bramhall, N.F.; Konrad-Martin, D.; McMillan, G.P. Tinnitus and Auditory Perception After a History of Noise Exposure: Relationship to Auditory Brainstem Response Measures. Ear Hear. 2018, 39, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Lauter, J.L.; Loomis, R.L. Individual differences in auditory electric responses: Comparisons of between-subject and within-subject variability. II. Amplitude of brainstem Vertex-positive peaks. Scand. Audiol. 1988, 17, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.J.; An, Y.H.; Kim, D.H.; Yoon, J.E.; Yoon, J.H. Comparisons of auditory brainstem response and sound level tolerance in tinnitus ears and non-tinnitus ears in unilateral tinnitus patients with normal audiograms. PLoS ONE 2017, 12, e0189157. [Google Scholar] [CrossRef]
- Shim, H.J.; Cho, Y.T.; Oh, H.S.; An, Y.H.; Kim, D.H.; Kang, Y.S. Within-Subject Comparisons of the Auditory Brainstem Response and Uncomfortable Loudness Levels in Ears With and Without Tinnitus in Unilateral Tinnitus Subjects With Normal Audiograms. Otol. Neurotol. 2021, 42, 10–17. [Google Scholar] [CrossRef]
- Han, M.S.; Jeong, Y.J.; Im, G.J.; Song, J.J.; Chae, S.W.; Chan Rah, Y.; Choi, J. Auditory brainstem response test results in normal hearing adolescents with subjective tinnitus. Int. J. Pediatr. Otorhinolaryngol. 2021, 146, 110775. [Google Scholar] [CrossRef]
- Hickox, A.E.; Liberman, M.C. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J. Neurophysiol. 2014, 111, 552–564. [Google Scholar] [CrossRef]
- Barbee, C.M.; James, J.A.; Park, J.H.; Smith, E.M.; Johnson, C.E.; Clifton, S.; Danhauer, J.L. Effectiveness of Auditory Measures for Detecting Hidden Hearing Loss and/or Cochlear Synaptopathy: A Systematic Review. Semin. Hear. 2018, 39, 172–209. [Google Scholar]
- Lu, J.; West, M.B.; Du, X.; Cai, Q.; Ewert, D.L.; Cheng, W.; Nakmali, D.; Li, W.; Huang, X.; Kopke, R.D. Electrophysiological assessment and pharmacological treatment of blast-induced tinnitus. PLoS ONE 2021, 16, e0243903. [Google Scholar] [CrossRef] [PubMed]
- Schecklmann, M.; Landgrebe, M.; Langguth, B. Phenotypic characteristics of hyperacusis in tinnitus. PLoS ONE 2014, 9, e86944. [Google Scholar]
- Hofmeier, B.; Wolpert, S.; Aldamer, E.S.; Walter, M.; Thiericke, J.; Braun, C.; Zelle, D.; Rüttiger, L.; Klose, U.; Knipper, M. Reduced sound-evoked and resting-state BOLD fMRI connectivity in tinnitus. Neuroimage Clin. 2018, 20, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Singer, W.; Zuccotti, A.; Jaumann, M.; Lee, S.C.; Panford-Walsh, R.; Xiong, H.; Zimmermann, U.; Franz, C.; Geisler, H.S.; Köpschall, I.; et al. Noise-induced inner hair cell ribbon loss disturbs central arc mobilization: A novel molecular paradigm for understanding tinnitus. Mol. Neurobiol. 2013, 47, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.; Moshtaghi, O.; Saez, N.; Richardson, M.; Djalilian, H.; Zeng, F.G.; Lin, H. Auditory Brainstem Response Wave I Amplitude Has Limited Clinical Utility in Diagnosing Tinnitus in Humans. Brain Sci. 2022, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Pinkl, J.; Wilson, M.J.; Billingsly, D.; Munguia-Vazquez, R. Detailed Analysis of High Frequency Auditory Brainstem Response in Patients with Tinnitus: A Preliminary Study. Int. Tinnitus J. 2017, 21, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Shin, S.A.; Chang, D.S.; Lee, H.Y. Audiometric Profiles in Patients With Normal Hearing and Bilateral or Unilateral Tinnitus. Otol. Neurotol. 2018, 39, e416–e421. [Google Scholar] [CrossRef]
- Makar, S.K.; Mukundan, G.; Gore, G. Auditory System Synchronization and Cochlear Function in Patients with Normal Hearing With Tinnitus: Comparison of Multiple Feature with Longer Duration and Single Feature with Shorter Duration Tinnitus. Int. Tinnitus J. 2017, 21, 133–138. [Google Scholar] [CrossRef]
- Hausler, R.; Levine, R.A. Brain stem auditory evoked potentials are related to interaural time discrimination in patients with multiple sclerosis. Brain Res. 1980, 191, 589–594. [Google Scholar] [CrossRef]
- Jacquemin, L.; Mertens, G.; Van de Heyning, P.; Vanderveken, O.M.; Topsakal, V.; De Hertogh, W.; Michiels, S.; Beyers, J.; Moyaert, J.; Van Rompaey, V.; et al. An Exploratory Study on the Use of Event-Related Potentials as an Objective Measure of Auditory Processing and Therapy Effect in Patients With Tinnitus: A Transcranial Direct Current Stimulation Study. Otol. Neurotol. 2019, 40, e868–e875. [Google Scholar] [CrossRef]
- Duncan, C.C.; Barry, R.J.; Connolly, J.F.; Fischer, C.; Michie, P.T.; Näätänen, R.; Polich, J.; Reinvang, I.; Van Petten, C. Event-related potentials in clinical research: Guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin. Neurophysiol. 2009, 120, 1883–1908. [Google Scholar] [CrossRef]
- Escera, C.; Alho, K.; Winkler, I.; Näätänen, R. Neural mechanisms of involuntary attention to acoustic novelty and change. J. Cogn. Neurosci. 1998, 10, 590–604. [Google Scholar] [CrossRef]
- Näätänen, R.; Picton, T. The N1 wave of the human electric and magnetic response to sound: A review and an analysis of the component structure. Psychophysiology 1987, 24, 375–425. [Google Scholar] [CrossRef] [PubMed]
- Rushby, J.A.; Barry, R.J.; Doherty, R.J. Separation of the components of the late positive complex in an ERP dishabituation paradigm. Clin. Neurophysiol. 2005, 116, 2363–2380. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xiong, H.; Yu, R.; Wang, C.; Zheng, Y.; Zhang, X. The characteristic and changes of the event-related potentials (ERP) and brain topographic maps before and after treatment with rTMS in subjective tinnitus patients. PLoS ONE 2013, 8, e70831. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo, A.A.; Penido, N.O.; Figueiredo, R.R. Event related potentials (ERPs) to assess the tinnitus complaint during drug treatment. Prog. Brain Res. 2021, 262, 175–187. [Google Scholar]
- Kiss, I.; Dashieff, R.M.; Lordeon, P. A parieto-occipital generator for P300: Evidence from human intracranial recordings. Int. J. Neurosci. 1989, 49, 133–139. [Google Scholar] [CrossRef]
- Smith, M.E.; Halgren, E.; Sokolik, M.; Baudena, P.; Musolino, A.; Liegeois-Chauvel, C.; Chauvel, P. The intracranial topography of the P3 event-related potential elicited during auditory oddball. Electroencephalogr. Clin. Neurophysiol. 1990, 76, 235–248. [Google Scholar] [CrossRef]
- Halgren, E.; Baudena, P.; Clarke, J.M.; Heit, G.; Marinkovic, K.; Devaux, B.; Vignal, J.P.; Biraben, A. Intracerebral potentials to rare target and distractor auditory and visual stimuli. II. Medial, lateral and posterior temporal lobe. Electroencephalogr. Clin. Neurophysiol. 1995, 94, 229–250. [Google Scholar] [CrossRef]
- Halgren, E.; Marinkovic, K.; Chauvel, P. Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalogr. Clin. Neurophysiol. 1998, 106, 156–164. [Google Scholar] [CrossRef]
- Tarkka, I.M.; Stokić, D.S.; Basile, L.F.; Papanicolaou, A.C. Electric source localization of the auditory P300 agrees with magnetic source localization. Electroencephalogr. Clin. Neurophysiol. 1995, 96, 538–545. [Google Scholar] [CrossRef]
- Hyde, M. The N1 response and its applications. Audiol. Neurootol. 1997, 2, 281–307. [Google Scholar] [CrossRef] [PubMed]
- Joos, K.; Gilles, A.; Van de Heyning, P.; De Ridder, D.; Vanneste, S. From sensation to percept: The neural signature of auditory event-related potentials. Neurosci. Biobehav. Rev. 2014, 42, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Crowley, K.E.; Colrain, I.M. A review of the evidence for P2 being an independent component process: Age, sleep and modality. Clin. Neurophysiol. 2004, 115, 732–744. [Google Scholar] [CrossRef]
- Golob, E.J.; Starr, A. Age-related qualitative differences in auditory cortical responses during short-term memory. Clin. Neurophysiol. 2000, 111, 2234–2244. [Google Scholar] [CrossRef]
- Parasuraman, R.; Beatty, J. Brain events underlying detection and recognition of weak sensory signals. Science 1980, 210, 80–83. [Google Scholar] [CrossRef]
- Winkler, I.; Tervaniemi, M.; Näätänen, R. Two separate codes for missing-fundamental pitch in the human auditory cortex. J. Acoust. Soc. Am. 1997, 102 Pt 1, 1072–1082. [Google Scholar] [CrossRef]
- Huotilainen, M.; Winkler, I.; Alho, K.; Escera, C.; Virtanen, J.; Ilmoniemi, R.J.; Jääskeläinen, I.P.; Pekkonen, E.; Näätänen, R. Combined mapping of human auditory EEG and MEG responses. Electroencephalogr. Clin. Neurophysiol. 1998, 108, 370–379. [Google Scholar] [CrossRef]
- Lü, Z.L.; Williamson, S.J.; Kaufman, L. Human auditory primary and association cortex have differing lifetimes for activation traces. Brain Res. 1992, 572, 236–241. [Google Scholar] [CrossRef]
- Arnott, S.R.; Bardouille, T.; Ross, B.; Alain, C. Neural generators underlying concurrent sound segregation. Brain Res. 2011, 1387, 116–124. [Google Scholar] [CrossRef]
- Knyazev, G.G.; Levin, E.A.; Savostyanov, A.N. A failure to stop and attention fluctuations: An evoked oscillations study of the stop-signal paradigm. Clin. Neurophysiol. 2008, 119, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Bokura, H.; Yamaguchi, S.; Kobayashi, S. Electrophysiological correlates for response inhibition in a Go/NoGo task. Clin. Neurophysiol. 2001, 112, 2224–2232. [Google Scholar] [CrossRef]
- Falkenstein, M.; Hoormann, J.; Hohnsbein, J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol. 1999, 101, 267–291. [Google Scholar] [CrossRef]
- Johnson, R., Jr.; Donchin, E. On how P300 amplitude varies with the utility of the eliciting stimuli. Electroencephalogr. Clin. Neurophysiol. 1978, 44, 424–437. [Google Scholar] [CrossRef]
- Kramer, A.F.; Wickens, C.D.; Donchin, E. An analysis of the processing requirements of a complex perceptual-motor task. Hum. Factors 1983, 25, 597–621. [Google Scholar] [CrossRef]
- Kehrle, H.M.; Sampaio, A.L.; Granjeiro, R.C.; de Oliveira, T.S.; Oliveira, C.A. Tinnitus Annoyance in Normal-Hearing Individuals: Correlation With Depression and Anxiety. Ann. Otol. Rhinol. Laryngol. 2016, 125, 185–194. [Google Scholar] [CrossRef]
- Sendesen, E.; Kaynakoglu, B.; Veziroglu, L.B.; Türkyılmaz, M.D. Auditory brainstem response in unilateral tinnitus patients: Does symmetrical hearing thresholds and within-subject comparison affect responses? Eur. Arch. Otorhinolaryngol. 2022. [Google Scholar] [CrossRef]
- Dornhoffer, J.; Danner, C.; Mennemeier, M.; Blake, D.; Garcia-Rill, E. Arousal and attention deficits in patients with tinnitus. Int. Tinnitus J. 2006, 12, 9–16. [Google Scholar]
- Attias, J.; Urbach, D.; Gold, S.; Shemesh, Z. Auditory event related potentials in chronic tinnitus patients with noise induced hearing loss. Hear. Res. 1993, 71, 106–113. [Google Scholar] [CrossRef]
- Jacobson, G.P.; McCaslin, D.L. A reexamination of the long latency N1 response in patients with tinnitus. J. Am. Acad. Audiol. 2003, 14, 393–400. [Google Scholar] [CrossRef]
- Hong, S.K.; Park, S.; Ahn, M.H.; Min, B.K. Top-down and bottom-up neurodynamic evidence in patients with tinnitus. Hear. Res. 2016, 342, 86–100. [Google Scholar] [CrossRef]
- Mannarelli, D.; Pauletti, C.; Mancini, P.; Fioretti, A.; Greco, A.; De Vincentiis, M.; Fattapposta, F. Selective attentional impairment in chronic tinnitus: Evidence from an event-related potentials study. Clin. Neurophysiol. 2017, 128, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Santos Filha, V.A.; Matas, C.G. Late Auditory evoked potentials in individuals with tinnitus. Braz. J. Otorhinolaryngol. 2010, 76, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Gopal, K.V.; Thomas, B.P.; Nandy, R.; Mao, D.; Lu, H. Potential Audiological and MRI Markers of Tinnitus. J. Am. Acad. Audiol. 2017, 28, 742–757. [Google Scholar] [CrossRef] [PubMed]
- Durai, M.; Sanders, M.; Kobayashi, K.; Searchfield, G.D. Auditory Streaming and Prediction in Tinnitus Sufferers. Ear Hear. 2019, 40, 345–357. [Google Scholar] [CrossRef]
- Vasudevan, H.; Palaniswamy, H.P.; Balakrishnan, R. Sensory and Cognitive Components of Auditory Processing in Individuals With Tinnitus. Am. J. Audiol. 2019, 28, 834–842. [Google Scholar] [CrossRef]
- Houdayer, E.; Teggi, R.; Velikova, S.; Gonzalez-Rosa, J.J.; Bussi, M.; Comi, G.; Leocani, L. Involvement of cortico-subcortical circuits in normoacousic chronic tinnitus: A source localization EEG study. Clin. Neurophysiol. 2015, 126, 2356–2365. [Google Scholar] [CrossRef]
- Attias, J.; Furman, V.; Shemesh, Z.; Bresloff, I. Impaired brain processing in noise-induced tinnitus patients as measured by auditory and visual event-related potentials. Ear Hear. 1996, 17, 327–333. [Google Scholar] [CrossRef]
- Gabr, T.A.; El-Hay, M.A.; Badawy, A. Electrophysiological and psychological studies in tinnitus. Auris Nasus Larynx 2011, 38, 678–683. [Google Scholar] [CrossRef]
- Asadpour, A.; Alavi, A.; Jahed, M.; Mahmoudian, S. Cognitive Memory Comparison Between Tinnitus and Normal Cases Using Event-Related Potentials. Front. Integr. Neurosci. 2018, 12, 48. [Google Scholar] [CrossRef]
- Elmorsy, S.M.; Abdeltawwab, M.M. Auditory P300: Selective attention to 2 KHZ tone-bursts in patients with idiopathic subjective tinnitus. Int. J. 2013, 1, 7. [Google Scholar]
- Araneda, R.; De Volder, A.G.; Deggouj, N.; Philippot, P.; Heeren, A.; Lacroix, E.; Decat, M.; Rombaux, P.; Renier, L. Altered top-down cognitive control and auditory processing in tinnitus: Evidences from auditory and visual spatial stroop. Restor. Neurol. Neurosci. 2015, 33, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.K.; Khandelwal, K.; Shrivastava, M.K. Tinnitus and Cognition: Linked? Indian J. Otolaryngol. Head Neck Surg. 2019, 71 (Suppl. 2), 1426–1430. [Google Scholar] [CrossRef]
- Said, E.A. Electrophysiological differences in sensorineural hearing loss patients with and without problem-tinnitus. Egypt. J. Otolaryngol. 2012, 28, 22–34. [Google Scholar]
- Mohan, A.; Luckey, A.; Weisz, N.; Vanneste, S. Predisposition to domain-wide maladaptive changes in predictive coding in auditory phantom perception. Neuroimage 2022, 248, 118813. [Google Scholar] [CrossRef]
- Holdefer, L.; Oliveira, C.A.; Venosa, A.R. The mismatch negativity test in ears with and without tinnitus-a path to the objectification of tinnitus. Int. Tinnitus J. 2013, 18, 168–174. [Google Scholar] [CrossRef]
- Picton, T.W. The P300 wave of the human event-related potential. J. Clin. Neurophysiol. 1992, 9, 456–479. [Google Scholar] [CrossRef]
- Han, J.H.; Won, J.Y.; Hong, S.K.; Kim, J.H.; Kim, E.S.; Kim, H.J.; Lee, H.J. Objective measurement of subjective tinnitus using the acoustic change complex. PLoS ONE 2017, 12, e0188268. [Google Scholar] [CrossRef]
- Sedley, W.; Alter, K.; Gander, P.E.; Berger, J.; Griffiths, T.D. Exposing Pathological Sensory Predictions in Tinnitus Using Auditory Intensity Deviant Evoked Responses. J. Neurosci. 2019, 39, 10096–10103. [Google Scholar] [CrossRef]
- Pfefferbaum, A.; Ford, J.M.; White, P.M.; Roth, W.T. P3 in schizophrenia is affected by stimulus modality, response requirements, medication status, and negative symptoms. Arch. Gen. Psychiatry 1989, 46, 1035–1044. [Google Scholar] [CrossRef]
- Salgari, G.C.; Potts, G.F.; Schmidt, J.; Chan, C.C.; Spencer, C.C.; Bedwell, J.S. Event-related potentials to rare visual targets and negative symptom severity in a transdiagnostic psychiatric sample. Clin. Neurophysiol. 2021, 132, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Deniz, M.; Bayazit, Y.A.; Celenk, F.; Karabulut, H.; Yilmaz, A.; Gunduz, B.; Saridogan, C.; Dagli, M.; Erdal, E.; Menevse, A. Significance of serotonin transporter gene polymorphism in tinnitus. Otol. Neurotol. 2010, 31, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Rufener, K.S.; Liem, F.; Meyer, M. Age-related differences in auditory evoked potentials as a function of task modulation during speech-nonspeech processing. Brain Behav. 2014, 4, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.N.; Hu, W.; Li, J.J.; Zhou, J.X.; Zhang, J.P.; Shi, G.F.; He, P.; Li, Z.W.; Li, M. The characteristics of cognitive impairment in subjective chronic tinnitus. Brain Behav. 2018, 8, e00918. [Google Scholar] [CrossRef] [PubMed]
- Schlee, W.; Hartmann, T.; Langguth, B.; Weisz, N. Abnormal resting-state cortical coupling in chronic tinnitus. BMC Neurosci. 2009, 10, 11. [Google Scholar] [CrossRef]
- Heeren, A.; Maurage, P.; Perrot, H.; De Volder, A.; Renier, L.; Araneda, R.; Lacroix, E.; Decat, M.; Deggouj, N.; Philippot, P. Tinnitus specifically alters the top-down executive control sub-component of attention: Evidence from the Attention Network Task. Behav. Brain Res. 2014, 269, 147–154. [Google Scholar] [CrossRef]
- Campbell, J.; LaBrec, A.; Bean, C.; Nielsen, M.; So, W. Auditory Gating and Extended High-Frequency Thresholds in Normal-Hearing Adults With Minimal Tinnitus. Am. J. Audiol. 2019, 28, 209–224. [Google Scholar] [CrossRef]
- Galazyuk, A.; Hébert, S. Gap-Prepulse Inhibition of the Acoustic Startle Reflex (GPIAS) for Tinnitus Assessment: Current Status and Future Directions. Front. Neurol. 2015, 6, 88. [Google Scholar] [CrossRef]
- Lobarinas, E.; Blair, C.; Spankovich, C.; Le Prell, C. Partial to complete suppression of unilateral noise-induced tinnitus in rats after cyclobenzaprine treatment. J. Assoc. Res. Otolaryngol. 2015, 16, 263–272. [Google Scholar] [CrossRef]
- Berger, J.I.; Coomber, B.; Shackleton, T.M.; Palmer, A.R.; Wallace, M.N. A novel behavioural approach to detecting tinnitus in the guinea pig. J. Neurosci. Methods 2013, 213, 188–195. [Google Scholar] [CrossRef]
- Turner, J.G.; Brozoski, T.J.; Bauer, C.A.; Parrish, J.L.; Myers, K.; Hughes, L.F.; Caspary, D.M. Gap detection deficits in rats with tinnitus: A potential novel screening tool. Behav. Neurosci. 2006, 120, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.I.; Owen, W.; Wilson, C.A.; Hockley, A.; Coomber, B.; Palmer, A.R.; Wallace, M.N. Gap-induced reductions of evoked potentials in the auditory cortex: A possible objective marker for the presence of tinnitus in animals. Brain Res. 2018, 1679, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.; Hébert, S. The gap-startle paradigm to assess auditory temporal processing: Bridging animal and human research. Psychophysiology 2016, 53, 759–766. [Google Scholar] [CrossRef]
- Fournier, P.; Hébert, S. Gap detection deficits in humans with tinnitus as assessed with the acoustic startle paradigm: Does tinnitus fill in the gap? Hear. Res. 2013, 295, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.A.; Berger, J.I.; de Boer, J.; Sereda, M.; Palmer, A.R.; Hall, D.A.; Wallace, M.N. Gap-induced inhibition of the post-auricular muscle response in humans and guinea pigs. Hear. Res. 2019, 374, 13–23. [Google Scholar] [CrossRef]
- Campolo, J.; Lobarinas, E.; Salvi, R. Does tinnitus “fill in” the silent gaps? Noise Health 2013, 15, 398–405. [Google Scholar]
- Boyen, K.; Başkent, D.; van Dijk, P. The Gap Detection Test: Can It Be Used to Diagnose Tinnitus? Ear Hear. 2015, 36, e138–e145. [Google Scholar] [CrossRef]
- Mehdizade Gilani, V.; Ruzbahani, M.; Mahdi, P.; Amali, A.; Nilforush Khoshk, M.H.; Sameni, J.; Karimi Yazdi, A.; Emami, H. Temporal processing evaluation in tinnitus patients: Results on analysis of gap in noise and duration pattern test. Iran. J. Otorhinolaryngol. 2013, 25, 221–226. [Google Scholar]
- Raj-Koziak, D.; Gos, E.; Szkiełkowska, A.; Panasiewicz, A.; Karpiesz, L.; Kutyba, J.; Skarzynski, H.; Skarzynski, P.H. Auditory processing in normally hearing individuals with and without tinnitus: Assessment with four psychoacoustic tests. Eur. Arch. Otorhinolaryngol. 2022, 279, 275–283. [Google Scholar] [CrossRef]
- Sanches, S.G.; Sanchez, T.G.; Carvallo, R.M. Influence of cochlear function on auditory temporal resolution in tinnitus patients. Audiol. Neurootol. 2010, 15, 273–281. [Google Scholar] [CrossRef]
- Jain, C.; Sahoo, J.P. The effect of tinnitus on some psychoacoustical abilities in individuals with normal hearing sensitivity. Int. Tinnitus J. 2014, 19, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Shadwick, K.; Sun, W. Acoustic startle reflex and pre-pulse inhibition in tinnitus patients. J. Otol. 2014, 9, 141–145. [Google Scholar] [CrossRef]
- Radziwon, K.E.; Stolzberg, D.J.; Urban, M.E.; Bowler, R.A.; Salvi, R.J. Salicylate-induced hearing loss and gap detection deficits in rats. Front. Neurol. 2015, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Morse, K.; Vander Werff, K.R. Comparison of Silent Gap in Noise Cortical Auditory Evoked Potentials in Matched Tinnitus and No-Tinnitus Control Subjects. Am. J. Audiol. 2019, 28, 260–273. [Google Scholar] [CrossRef]
- Paul, B.T.; Schoenwiesner, M.; Hébert, S. Towards an objective test of chronic tinnitus: Properties of auditory cortical potentials evoked by silent gaps in tinnitus-like sounds. Hear. Res. 2018, 366, 90–98. [Google Scholar] [CrossRef]
- Fournier, P.; Hébert, S. The gap prepulse inhibition of the acoustic startle (GPIAS) paradigm to assess auditory temporal processing: Monaural versus binaural presentation. Psychophysiology 2021, 58, e13755. [Google Scholar] [CrossRef]
- Mahmoudian, S.; Farhadi, M.; Najafi-Koopaie, M.; Darestani-Farahani, E.; Mohebbi, M.; Dengler, R.; Esser, K.H.; Sadjedi, H.; Salamat, B.; Danesh, A.A.; et al. Central auditory processing during chronic tinnitus as indexed by topographical maps of the mismatch negativity obtained with the multi-feature paradigm. Brain Res. 2013, 1527, 161–173. [Google Scholar] [CrossRef]
- Mahmoudian, S.; Farhadi, M.; Mohebbi, M.; Alaeddini, F.; Najafi-Koopaie, M.; Farahani, E.D.; Mojallal, H.; Omrani, R.; Daneshi, A.; Lenarz, T. Alterations in auditory change detection associated with tinnitus residual inhibition induced by auditory electrical stimulation. J. Am. Acad. Audiol. 2015, 26, 408–422. [Google Scholar] [CrossRef]
- Ku, Y.; Ahn, J.W.; Kwon, C.; Kim, D.Y.; Suh, M.W.; Park, M.K.; Lee, J.H.; Oh, S.H.; Kim, H.C. The gap-prepulse inhibition deficit of the cortical N1-P2 complex in patients with tinnitus: The effect of gap duration. Hear. Res. 2017, 348, 120–128. [Google Scholar] [CrossRef]
- Mohebbi, M.; Daneshi, A.; Asadpour, A.; Mohsen, S.; Farhadi, M.; Mahmoudian, S. The potential role of auditory prediction error in decompensated tinnitus: An auditory mismatch negativity study. Brain Behav. 2019, 9, e01242. [Google Scholar] [CrossRef]
- Sendesen, E.; Erbil, N.; Türkyılmaz, M.D. The mismatch negativity responses of individuals with tinnitus with normal extended high-frequency hearing-is it possible to use mismatch negativity in the evaluation of tinnitus? Eur. Arch. Otorhinolaryngol. 2021, 279, 3425–3434. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.W.; Kim, K.W.; Park, I.Y.; Oh, S.H. Parameter optimization for applying the prepulse gap paradigm to humans. Korean J. Audiol. 2013, 17, 118–123. [Google Scholar] [CrossRef][Green Version]
- Musiek, F.E.; Shinn, J.B.; Jirsa, R.; Bamiou, D.E.; Baran, J.A.; Zaida, E. GIN (Gaps-In-Noise) test performance in subjects with confirmed central auditory nervous system involvement. Ear Hear. 2005, 26, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Pratt, H.; Starr, A.; Michalewski, H.J.; Bleich, N.; Mittelman, N. The N1 complex to gaps in noise: Effects of preceding noise duration and intensity. Clin. Neurophysiol. 2007, 118, 1078–1087. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ku, Y.; Kim, D.Y.; Kwon, C.; Noh, T.S.; Park, M.K.; Lee, J.H.; Oh, S.H.; Kim, H.C.; Suh, M.W. Effect of age on the gap-prepulse inhibition of the cortical N1-P2 complex in humans as a step towards an objective measure of tinnitus. PLoS ONE 2020, 15, e0241136. [Google Scholar] [CrossRef]
- Threlkeld, S.W.; Penley, S.C.; Rosen, G.D.; Fitch, R.H. Detection of silent gaps in white noise following cortical deactivation in rats. Neuroreport 2008, 19, 893–898. [Google Scholar] [CrossRef]
- Weible, A.P.; Moore, A.K.; Liu, C.; DeBlander, L.; Wu, H.; Kentros, C.; Wehr, M. Perceptual gap detection is mediated by gap termination responses in auditory cortex. Curr. Biol. 2014, 24, 1447–1455. [Google Scholar] [CrossRef]
- Eggermont, J.J. Hearing loss, hyperacusis, or tinnitus: What is modeled in animal research? Hear. Res. 2013, 295, 140–149. [Google Scholar] [CrossRef]
- Aasen, I.; Kolli, L.; Kumari, V. Sex effects in prepulse inhibition and facilitation of the acoustic startle response: Implications for pharmacological and treatment studies. J. Psychopharmacol. 2005, 19, 39–45. [Google Scholar] [CrossRef]
- Lister, J.J.; Maxfield, N.D.; Pitt, G.J.; Gonzalez, V.B. Auditory evoked response to gaps in noise: Older adults. Int. J. Audiol. 2011, 50, 211–225. [Google Scholar] [CrossRef]
- Florentine, M.; Buus, S. Temporal gap detection in sensorineural and simulated hearing impairments. J. Speech Hear. Res. 1984, 27, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, T.D.; Berg, W.K. Stimulus rise time, intensity, and bandwidth effects on acoustic startle amplitude and probability. Psychophysiology 1986, 23, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Wynn, J.K.; Dawson, M.E.; Schell, A.M. Discrete and continuous prepulses have differential effects on startle prepulse inhibition and skin conductance orienting. Psychophysiology 2000, 37, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, J.J. Ups and Downs in 75 Years of Electrocochleography. Front. Syst. Neurosci. 2017, 11, 2. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef]
- Dallos, P.; Wang, C.Y. Bioelectric correlates of kanamycin intoxication. Audiology 1974, 13, 277–289. [Google Scholar] [CrossRef]
- Dallos, P.; Santos-Sacchi, J.; Flock, A. Intracellular recordings from cochlear outer hair cells. Science 1982, 218, 582–584. [Google Scholar] [CrossRef]
- Withnell, R.H. Brief report: The cochlear microphonic as an indication of outer hair cell function. Ear Hear. 2001, 22, 75–77. [Google Scholar] [CrossRef]
- Russell, I.J. Chapter 3.20-Cochlear receptor potentials. Senses A Compr. Ref. 2008, 11, 320–358. [Google Scholar]
- Gibson, W.P. The Clinical Uses of Electrocochleography. Front. Neurosci. 2017, 11, 274. [Google Scholar] [CrossRef]
- Ferraro, J.A.; Blackwell, W.L.; Mediavilla, S.J.; Thedinger, B.S. Normal summating potential to tone bursts recorded from the tympanic membrane in humans. J. Am. Acad. Audiol. 1994, 5, 17–23. [Google Scholar] [PubMed]
- Durrant, J.D.; Wang, J.; Ding, D.L.; Salvi, R.J. Are inner or outer hair cells the source of summating potentials recorded from the round window? J. Acoust. Soc. Am. 1998, 104, 370–377. [Google Scholar] [CrossRef]
- Salvi, R.; Sun, W.; Ding, D.; Chen, G.D.; Lobarinas, E.; Wang, J.; Radziwon, K.; Auerbach, B.D. Inner Hair Cell Loss Disrupts Hearing and Cochlear Function Leading to Sensory Deprivation and Enhanced Central Auditory Gain. Front. Neurosci. 2016, 10, 621. [Google Scholar] [CrossRef] [PubMed]
- Pappa, A.K.; Hutson, K.A.; Scott, W.C.; Wilson, J.D.; Fox, K.E.; Masood, M.M.; Giardina, C.K.; Pulver, S.H.; Grana, G.D.; Askew, C.; et al. Hair cell and neural contributions to the cochlear summating potential. J. Neurophysiol. 2019, 121, 2163–2180. [Google Scholar] [CrossRef] [PubMed]
- Meenderink, S.W.F.; Lin, X.; Dong, W. Using electrocochleography to detect sensory and neural damages in a gerbil model. Sci. Rep. 2021, 11, 19557. [Google Scholar] [CrossRef]
- Portmann, M.; Harrison, R.V.; Negrevergne, M.; Dauman, R.; Aran, J.M. Electrocochleographic measures of cochlear frequency selectivity in hearing loss of cochlear origin. Acta Otolaryngol. 1983, 95, 657–663. [Google Scholar] [CrossRef]
- Snyder, R.L.; Schreiner, C.E. The auditory neurophonic: Basic properties. Hear. Res. 1984, 15, 261–280. [Google Scholar] [CrossRef]
- Choudhury, B.; Fitzpatrick, D.C.; Buchman, C.A.; Wei, B.P.; Dillon, M.T.; He, S.; Adunka, O.F. Intraoperative round window recordings to acoustic stimuli from cochlear implant patients. Otol. Neurotol. 2012, 33, 1507–1515. [Google Scholar] [CrossRef]
- Fontenot, T.E.; Giardina, C.K.; Fitzpatrick, D.C. A Model-Based Approach for Separating the Cochlear Microphonic from the Auditory Nerve Neurophonic in the Ongoing Response Using Electrocochleography. Front. Neurosci. 2017, 11, 592. [Google Scholar] [CrossRef]
- Chertoff, M.E. Analytic treatment of the compound action potential: Estimating the summed post-stimulus time histogram and unit response. J. Acoust. Soc. Am. 2004, 116, 3022–3030. [Google Scholar] [CrossRef]
- Mori, N.; Saeki, K.; Matsunaga, T.; Asai, H. Comparison between AP and SP parameters in trans- and extratympanic electrocochleography. Audiology 1982, 21, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, Y.; Komatsuzaki, A.; Nishida, H. Cochlear microphonics for hearing preservation in vestibular schwannoma surgery. Laryngoscope 1999, 109, 1982–1987. [Google Scholar] [CrossRef] [PubMed]
- Roland, P.S.; Yellin, M.W.; Meyerhoff, W.L.; Frank, T. Simultaneous comparison between transtympanic and extratympanic electrocochleography. Am. J. Otol. 1995, 16, 444–450. [Google Scholar]
- Schoonhoven, R.; Fabius, M.A.; Grote, J.J. Input/output curves to tone bursts and clicks in extratympanic and transtympanic electrocochleography. Ear Hear. 1995, 16, 619–630. [Google Scholar] [CrossRef]
- Zakaria, M.N.; Nik Othman, N.A.; Musa, Z. Does the location of electrode on tympanic membrane matter when recording electrocochleography? Acta Otolaryngol. 2021, 141, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, J.J.; Odenthal, D.W. Methods in electrocochleography. Acta Otolaryngol. Suppl. 1974, 316, 17–24. [Google Scholar] [CrossRef]
- Kara, E.; Aydın, K.; Akbulut, A.A.; Karakol, S.N.; Durmaz, S.; Yener, H.M.; Gözen, E.D.; Kara, H. Assessment of Hidden Hearing Loss in Normal Hearing Individuals with and Without Tinnitus. J. Int. Adv. Otol. 2020, 16, 87–92. [Google Scholar] [CrossRef]
- Weisz, N.; Hartmann, T.; Dohrmann, K.; Schlee, W.; Norena, A. High-frequency tinnitus without hearing loss does not mean absence of deafferentation. Hear. Res. 2006, 222, 108–114. [Google Scholar] [CrossRef]
- Farhadi, M.; Salem, M.M.; Asghari, A.; Daneshi, A.; Mirsalehi, M.; Mahmoudian, S. Impact of Acamprosate on Chronic Tinnitus: A Randomized-Controlled Trial. Ann. Otol. Rhinol. Laryngol. 2020, 129, 1110–1119. [Google Scholar] [CrossRef]
- Sheppard, A.M.; Chen, G.D.; Salvi, R. Potassium ion channel openers, Maxipost and Retigabine, protect against peripheral salicylate ototoxicity in rats. Hear. Res. 2015, 327, 1–8. [Google Scholar] [CrossRef]
- Montazeri, K.; Mahmoudian, S.; Razaghi, Z.; Farhadi, M. Alterations in Auditory Electrophysiological Responses Associated With Temporary Suppression of Tinnitus Induced by Low-Level Laser Therapy: A Before-After Case Series. J. Lasers Med. Sci. 2017, 8 (Suppl. 1), S38–S45. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Aso, S.; Watanabe, Y.; Mizukoshi, K. Changes in auditory evoked responses during intravenous lidocaine. Acta Otolaryngol. Suppl. 1993, 504, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Mulders, W.H.; Ding, D.; Salvi, R.; Robertson, D. Relationship between auditory thresholds, central spontaneous activity, and hair cell loss after acoustic trauma. J. Comp. Neurol. 2011, 519, 2637–2647. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Okawara, D.; Baba, S.; Yagi, T. Electrocochleographic analysis of the suppression of tinnitus by electrical promontory stimulation. Audiology 1997, 36, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Melcher, J.R.; Levine, R.A.; Bergevin, C.; Norris, B. The auditory midbrain of people with tinnitus: Abnormal sound-evoked activity revisited. Hear. Res. 2009, 257, 63–74. [Google Scholar] [CrossRef]
- Lowe, A.S.; Walton, J.P. Alterations in peripheral and central components of the auditory brainstem response: A neural assay of tinnitus. PLoS ONE 2015, 10, e0117228. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Groups and Number | Etiology of Tinnitus | Mean Age | Matched | Hearing Status | Stimuli | Measuring Method | Outcome |
|---|---|---|---|---|---|---|---|---|
| Sanches et al., 2010 [130] | 20 tinnitus 28 controls | not mentioned | tinnitus: 33.8 controls: 28.8 | not mentioned | ≤25 dB HL (0.25–8 kHz) | 2, 3, 4, 5, 6, 8, 10, 12, 15 or 20 ms gap embedded in 50 dB SL white noise | threshold and number of correct responses | lower percentage of correct responses and longer time interval required to detect gap in the tinnitus group |
| Campolo et al., 2013 [126] | 13 tinnitus 13 controls | not mentioned | tinnitus: 50 controls: 24 | not mentioned | tinnitus: varying degree of HLcontrols: ≤20 dB HL (0.25–8 kHz), ≤35 dB HL above 8 kHz | tinnitus: 50 ms silent gaps embedded in one-third octave bands of noise located 1-octave below, 1-octave above, and at the pitch of the subject’s tinnitus controls: 50 ms gaps embedded in NBN located at 1.2, 8, and 12.6 kHz | press the response button within 2s if they detect a gap | both tinnitus and controls could detect the 50 ms gaps |
| Fournier et al., 2013 [136] | test: 15 tinnitus 17 controls retest: 10 tinnitus 9 controls | not mentioned | test-tinnitus: 28.5 controls: 23 retest- tinnitus: 29.3 controls: 4.5 | sex, education | <35 dB HL (0.25–4 kHz) | Startle noises were 50 ms broadband noise bursts (20 Hz–20 kHz) set at 105 dB SPL backgroundnoise set at 65 dB SPL The low-frequency background noise was centered at 500 Hz (200–1200 Hz) and high-frequency background noise at 4 kHz (3.5–4.5 kHz), 50 ms silent gap presented 120 ms before the startle sound | eyeblink: % inhibition = [(pulse-alone) − (gap/prepulse)]/(pulse-alone) × 100. | normal prepulse inhibition but higher reactivity to the startle sounds in the tinnitus group, the tinnitus group displayed a consistent deficit in gap processing at both low- and high-background noise frequencies |
| Mahmoudian et al., 2013 [137] | 28 tinnitus 33 controls | idiopathic | tinnitus: 33.78 controls: 35.21 | age, sex | ≤20 dB HL (0.25–2 kHz), ≤40 dB HL (4–8 kHz) | 7 ms silent gap embedded in standard stimuli presented at an intensity of 65 dB SPL pure tones of 0.5, 1, and 1.5 kHz | electroencephalogram (EEG) | reduced MMN amplitude and area under the curve for the silent gap in tinnitus group |
| Mehdizade et al., 2013 [128] | 20 tinnitus 20 controls | not mentioned | tinnitus: 30.31 controls: 27.8 | age, sex | ≤20 dB HL (0.25–8.0 KHz for air conduction and 0.25–4.0 KHz for bone conduction) | 0 to 3 silence gaps of different durations (2–6, 8, 10, 12, 15, 20 ms) embedded in 6s 50 dB SL white noise stimuli | identify the silence gaps | tinnitus patients needed a longer duration of gap to detect than those of the non-tinnitus subjects |
| Jain and Sahoo, 2014 [131] | 10 mild tinnitus 10 moderate tinnitus20 controls | idiopathic | mild tinnitus: 36.8 moderate tinnitus: 39.4 controls: 36.1 | age | ≤25 dB HL (0.25–8 kHz) | a temporal gap embedded in 500 ms broadband noise | three-interval, alternate forced-choice (3-AFC) method | individuals with moderate tinnitus need larger silent intervals to detect a gap within a noise than individuals with mild tinnitus as well as those without complaints of tinnitus |
| Shadwick& Sun, 2014 [132] | 7 tinnitus 9 controls | not mentioned | range: 20–55 | age | controls: ≤20 dB HL (0.25–8.0 KHz) | The background noise was a narrowband noise with a 100 Hz bandwidth presented at 38–40 dB SPL centered at a frequency of the patient’s tinnitus The startle noise was a broadband signal at 100 dB SPL The gap duration was 100 ms and the duration of startle stimulus was 50 ms (rise/fall time 1 ms) ten trials at each frequency (0.5–8 kHz) | eye-blink amplitude | The amplitude of the startle response in the tinnitus group with normal hearing thresholds was significantly higher than the control group and those with tinnitus and hearing loss |
| Boyen et al., 2015 [127] | 22 tinnitus 20 nontinnitus 10 controls with normal hearing | not mentioned | tinnitus: 53 non-tinnitus: 52controls: 23 | tinnitus and non-tinnitus: age, gender, and hearing characteristics matched | Threshold differences between ears were 20 dB or less for at least five of the six test frequencies (0.25–8 kHz) controls: ≤20 dB HL (0.25–8 kHz) | Four 300 ms narrow-band noise (4–8, 4–5, 5–6.3, 6.3–8 kHz) served as stimuli and were presented at 5, 10, and 25 dB SPL above their respective hearing thresholds The gap size at the start of the test was 30 ms | two-down/one-up adaptive procedure (2D1U) | tinnitus group did not display elevated gap thresholds |
| Mahmoudian et al., 2015 [138] | 28 tinnitus | idiopathic | tinnitus: 35.33 | ≤20 dB HL (0.25–2 kHz), ≤40 dB HL (4–8 kHz) | 7 ms silent gap embedded in standard stimuli presented at an intensity of 65 dB SPL pure tones of 0.5, 1, and 1.5 kHz | EEG | No statistically significant differences in MMN amplitude and AUC of gap after AES treatment | |
| Ku et al., 2017 [139] | 16 tinnitus 18 controls | not mentioned | tinnitus: 59.2 controls: 59.2 | age, hearing | <70, 30, and 70 dB HL at 0.5, 1, and 8 kHz frequencies, respectively | 20 dB SL continuous pure tone (8 kHz or 600 Hz) background noise and a 65 dB SL intense sound stimulus (1-kHz tone burst of 20-ms duration) 100-, 50- or 20-ms temporal gaps | the peak-to-peak amplitude of the N1–P2 complex in response to the gap-intense sound stimuli/peak-to-peak amplitude of the N1–P2 complex in response to the no-gap-intense sound stimuli | GPI deficit of patients with tinnitus was found on the N1–P2 complex with the tinnitus-pitch-matched frequency background noise and 20-ms gap duration |
| Mohebbi et al., 2019 [140] | 20 compensated tinnitus 20 decompensated tinntitus 20 controls | not mentioned | compensated tinnitus: 44.35 decompensated tinntitus: 42.35 controls: 40.05 | age, hearing | ≤20 dB HL (0.25–2 kHz), ≤40 dB HL (4–8 kHz) | 7 ms silent gap embedded in standard stimuli presented at an intensity of 85 dB SPL pure tones of 7, 8, and 8.5 kHz | EEG | reduced MMN amplitude and area under the curve for the silent gap deviant in decompensated tinnitus group compared with normal control and compensated tinnitus group |
| Morse et al., 2019 [134] | 13 tinnitus 13 controls | not mentioned | tinnitus: 52.85 controls: 54.54 | age, sex, hearing | mean PTA tinnitus: 18.87 controls: 20.15 (0.25–8 kHz) | 6-s white noise segments with one to three silent gaps embedded. gaps ranging in duration between 2 and 20 ms | behavioral gap detection threshold: pressing a button each time a silent gap was perceived. Gap evoked P1–N1–P2 amplitude, latency, and area | no significant difference in silent gap evoked P1–N1–P2 amplitude, latency or area differences between groups |
| Sendesen et al., 2021 [141] | 16 tinnitus 20 controls | idiopathic | tinnitus: 28.5 controls: 27.9 | age, sex, hearing | <20 dB HL (0.125–16 kHz) | 15 ms silent gap embedded in standard stimuli presented at an intensity of 65 dB SPL pure tones of 0.5, 1, and 1.5 kHz | EEG | reduced MMN amplitude for the silent gap in tinnitus group. no statistically significant differences for MMN latencies between the groups |
| Raj-Koziak et al., 2022 [129] | 54 tinnitus 43 controls | not mentioned | tinnitus: 37.1 controls: 35.5 | hearing | ≤20 dB HL (0.125–8 kHz) High-frequency (9–16 kHz) was tested | gap with a duration of 10 ms and decreased or increased by 50% embedded in 50 dB HL white noise | detect gap | tinnitus patients needed a longer duration of gap to detect than those of the non-tinnitus subjects |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.; Li, S. Objective Detection of Tinnitus Based on Electrophysiology. Brain Sci. 2022, 12, 1086. https://doi.org/10.3390/brainsci12081086
Fan S, Li S. Objective Detection of Tinnitus Based on Electrophysiology. Brain Sciences. 2022; 12(8):1086. https://doi.org/10.3390/brainsci12081086
Chicago/Turabian StyleFan, Shuwen, and Shufeng Li. 2022. "Objective Detection of Tinnitus Based on Electrophysiology" Brain Sciences 12, no. 8: 1086. https://doi.org/10.3390/brainsci12081086
APA StyleFan, S., & Li, S. (2022). Objective Detection of Tinnitus Based on Electrophysiology. Brain Sciences, 12(8), 1086. https://doi.org/10.3390/brainsci12081086






