A Novel Core Strengthening Intervention for Improving Trunk Function, Balance and Mobility after Stroke
Abstract
:1. Introduction
2. Materials and Methods
- Case Descriptions
2.1. Participant S1
2.2. Participant S2
2.3. Participant S3
- B.
- Core Strengthening Intervention (CSI) program
2.4. The Device
2.5. Procedure
- C.
- Outcome Measures
2.6. Trunk Impairment Scale (TIS)
2.7. Berg Balance Scale (BBS)
2.8. Timed up and Go (TUG)
2.9. 10-Meter Walk Test (10MWT)
2.10. 6-Minute Walk Test (6MWT)
2.11. Posturography
2.12. Electromyography (EMG)
2.13. Physical ACtivity Enjoyment Scale (PACES)
3. Results
3.1. Functional Outcomes
3.2. Posturography Outcomes
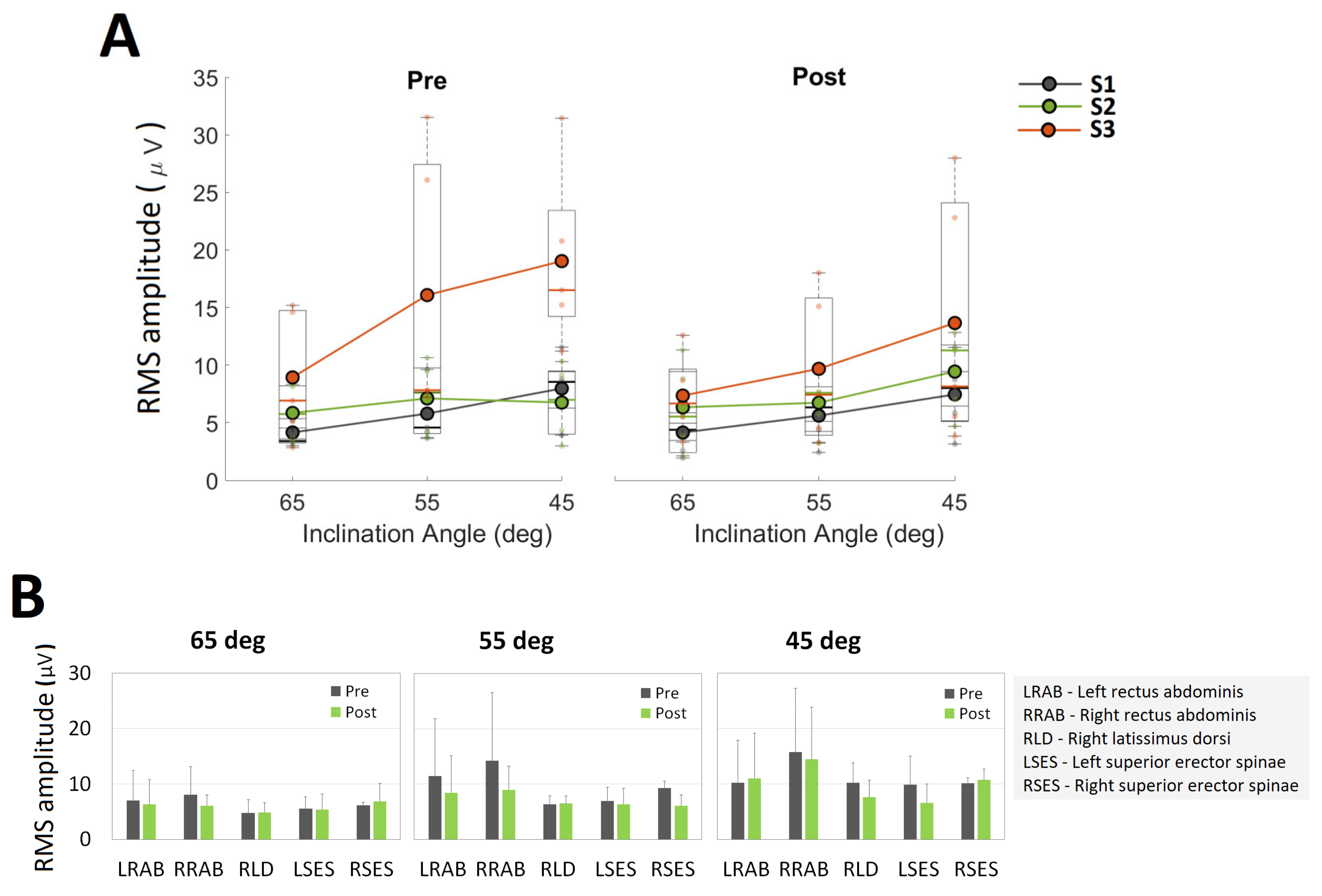
3.3. Neuromuscular Outcomes
3.4. Physical Activity Enjoyment Scale (PACES)
4. Discussion
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karthikbabu, S.; Chakrapani, M.; Ganeshan, S.; Rakshith, K.C.; Nafeez, S.; Prem, V. A review on assessment and treatment of the trunk in stroke: A need or luxury. Neural Regen. Res. 2012, 7, 1974–1977. [Google Scholar] [CrossRef] [PubMed]
- Patten, C.; Lexell, J.; Brown, H.E. Weakness and strength training in persons with poststroke hemiplegia: Rationale, method, and efficacy. J. Rehabil. Res. Dev. 2004, 41, 293. [Google Scholar] [CrossRef] [PubMed]
- Haruyama, K.; Kawakami, M.; Otsuka, T. Effect of Core Stability Training on Trunk Function, Standing Balance, and Mobility in Stroke Patients: A Randomized Controlled Trial. Neurorehabilit. Neural Repair 2016, 31, 240–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, S.; Hachisuka, K.; Ogata, H. Muscle strength of trunk flexion-extension in post-stroke hemiplegic patients1. Am. J. Phys. Med. Rehabil. 1998, 77, 288–290. [Google Scholar] [CrossRef]
- Parry, C.B.W.; Salter, M.M.K.; Voss, D.E. Proprioceptive Neuromuscular Facilitation. Rheumatology 1970, 10, 247–248. [Google Scholar] [CrossRef]
- Van Criekinge, T.; Truijen, S.; Schröder, J.; Maebe, Z.; Blanckaert, K.; Van Der Waal, C.; Vink, M.; Saeys, W. The effectiveness of trunk training on trunk control, sitting and standing balance and mobility post-stroke: A systematic review and meta-analysis. Clin. Rehabil. 2019, 33, 992–1002. [Google Scholar] [CrossRef]
- Van Nes, I.J.; Nienhuis, B.; Latour, H.; Geurts, A.C. Posturographic assessment of sitting balance recovery in the subacute phase of stroke. Gait Posture 2008, 28, 507–512. [Google Scholar] [CrossRef]
- Verheyden, G.; Nieuwboer, A.; De Wit, L.; Feys, H.; Schuback, B.; Baert, I.; Jenni, W.; Schupp, W.; Thijs, V.; De Weerdt, W. Trunk performance after stroke: An eye catching predictor of functional outcome. J. Neurol. Neurosurg. Psychiatry 2006, 78, 694–698. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.-L.; Sheu, C.-F.; Hsueh, I.-P.; Wang, C.-H. Trunk Control as an Early Predictor of Comprehensive Activities of Daily Living Function in Stroke Patients. Stroke 2002, 33, 2626–2630. [Google Scholar] [CrossRef] [Green Version]
- Duarte, E.; Marco, E.; Muniesa, J.M.; Belmonte, R.; Diaz, P.; Tejero, M.; Escalada, F. Trunk control test as a functional predictor in stroke patients. J. Rehabil. Med. 2002, 34, 267–272. [Google Scholar] [CrossRef] [Green Version]
- Karatas, M.; Çetin, N.; Bayramoglu, M.; Dilek, A. Trunk Muscle Strength in Relation to Balance and Functional Disability in Unihemispheric Stroke Patients. Am. J. Phys. Med. Rehabil. 2004, 83, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-Y.; Huang, J.-C.; Tseng, H.-Y.; Yang, Y.-C.; Lin, S.-I. Effects of Trunk Exercise on Unstable Surfaces in Persons with Stroke: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 9135. [Google Scholar] [CrossRef] [PubMed]
- Cabanas-Valdés, R.; Boix-Sala, L.; Grau-Pellicer, M.; Guzmán-Bernal, J.; Caballero-Gómez, F.; Urrútia, G. The Effectiveness of Additional Core Stability Exercises in Improving Dynamic Sitting Balance, Gait and Functional Rehabilitation for Subacute Stroke Patients (CORE-Trial): Study Protocol for a Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 6615. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, U.; Karimi, H.; Amir, S.; Ahmed, A. Effects of intensive multiplanar trunk training coupled with dual-task exercises on balance, mobility, and fall risk in patients with stroke: A randomized controlled trial. J. Int. Med Res. 2021, 49, 03000605211059413. [Google Scholar] [CrossRef]
- Androwis, G.J.; Pilkar, R.; Ramanujam, A.; Nolan, K.J. Electromyography Assessment during Gait in a Robotic Exoskeleton for Acute Stroke. Front. Neurol. 2018, 9, 630. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, I.; Sajin, A.; Fisher, I.; Neeb, M.; Shochina, M.; Katz-Leurer, M.; Meiner, Z. The Effectiveness of Locomotor Therapy Using Robotic-Assisted Gait Training in Subacute Stroke Patients: A Randomized Controlled Trial. PM&R 2009, 1, 516–523. [Google Scholar] [CrossRef]
- Nolan, K.J.; Karunakaran, K.K.; Roberts, P.; Tefertiller, C.; Walter, A.M.; Zhang, J.; Leslie, D.; Jayaraman, A.; Francisco, G.E. Utilization of Robotic Exoskeleton for Overground Walking in Acute and Chronic Stroke. Front. Neurorobot. 2021, 15, 689363. [Google Scholar] [CrossRef]
- Molteni, F.; Gasperini, G.; Gaffuri, M.; Colombo, M.; Giovanzana, C.; Lorenzon, C.; Farina, N.; Cannaviello, G.; Scarano, S.; Proserpio, D.; et al. Wearable robotic exoskeleton for overground gait training in sub-acute and chronic hemiparetic stroke patients: Preliminary results. Eur. J. Phys. Rehabil. Med. 2017, 53, 676–684. [Google Scholar] [CrossRef]
- Cho, D.Y.; Park, S.-W.; Lee, M.J.; Park, D.S.; Kim, E.J. Effects of robot-assisted gait training on the balance and gait of chronic stroke patients: Focus on dependent ambulators. J. Phys. Ther. Sci. 2015, 27, 3053–3057. [Google Scholar] [CrossRef] [Green Version]
- Min, J.H.; Seong, H.Y.; Ko, S.-H.; Jo, W.-R.; Sohn, H.-J.; Ahn, Y.H.; Son, J.H.; Seo, H.-Y.; Son, Y.-R.; Mun, S.-J.; et al. Effects of trunk stabilization training robot on postural control and gait in patients with chronic stroke: A randomized controlled trial. Int. J. Rehabil. Res. 2020, 43, 159–166. [Google Scholar] [CrossRef]
- Saglia, J.A.; De Luca, A.; Squeri, V.; Ciaccia, L.; Sanfilippo, C.; Ungaro, S.; De Michieli, L. Design and development of a novel core, balance and lower limb rehabilitation robot: Hunova®. In Proceedings of the 2019 IEEE 16th International Conference on Rehabilitation Robotics (ICORR), Toronto, ON, Canada, 24–28 June 2019; pp. 417–422. [Google Scholar] [CrossRef]
- De Luca, A.; Squeri, V.; Barone, L.M.; Mansin, H.V.; Ricci, S.; Pisu, I.; Cassiano, C.; Capra, C.; Lentino, C.; De Michieli, L.; et al. Dynamic Stability and Trunk Control Improvements Following Robotic Balance and Core Stability Training in Chronic Stroke Survivors: A Pilot Study. Front. Neurol. 2020, 11, 494. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-H.; Park, S.-D. The effects of core stability strength exercise on muscle activity and trunk impairment scale in stroke patients. J. Exerc. Rehabil. 2013, 9, 362–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiengkaew, V.; Jitaree, K.; Chaiyawat, P. Minimal Detectable Changes of the Berg Balance Scale, Fugl-Meyer Assessment Scale, Timed “Up & Go” Test, Gait Speeds, and 2-Minute Walk Test in Individuals With Chronic Stroke With Different Degrees of Ankle Plantarflexor Tone. Arch. Phys. Med. Rehabil. 2012, 93, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.L.; Stistrup, R.D.; Schjøtt, C.S.; Madsen, J.; Vinther, A. Absolute and Relative Reliability of the Timed ‘Up & Go’ Test and ‘30second Chair-Stand’ Test in Hospitalised Patients with Stroke. PLoS ONE 2016, 11, e0165663. [Google Scholar] [CrossRef]
- Perera, S.; Mody, S.H.; Woodman, R.C.; Studenski, S.A. Meaningful Change and Responsiveness in Common Physical Performance Measures in Older Adults. J. Am. Geriatr. Soc. 2006, 54, 743–749. [Google Scholar] [CrossRef]
- Ballester, B.R.; Maier, M.; Duff, A.; Cameirão, M.; Bermúdez, S.; Duarte, E.; Cuxart, A.; Rodriguez, S.; Mozo, R.M.S.S.; Verschure, P.F.M.J. A critical time window for recovery extends beyond one-year post-stroke. J. Neurophysiol. 2019, 122, 350–357. [Google Scholar] [CrossRef] [Green Version]
- Pilkar, R.; Arzouni, N.; Nolan, K.J. Postural stability during long duration quiet standing in post stroke hemiplegia. Biomed. Signal Process. Control 2018, 39, 162–168. [Google Scholar] [CrossRef]
- Marigold, D.S.; Eng, J.J. The relationship of asymmetric weight-bearing with postural sway and visual reliance in stroke. Gait Posture 2006, 23, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.-F.; Liaw, L.-J.; Wang, R.-Y.; Su, F.-C.; Hsu, A.-T. Electromyography of symmetrical trunk movements and trunk position sense in chronic stroke patients. J. Phys. Ther. Sci. 2015, 27, 2675–2681. [Google Scholar] [CrossRef] [Green Version]
- Palevo, G.; Walsh, D.J.; Park, E.; Polascik, M.; Pace, J.; Edwards, B. Physiological Responses to Allcore360o Core Training System. J. Exerc. Physiol. 2021, 24, 67–73. [Google Scholar]
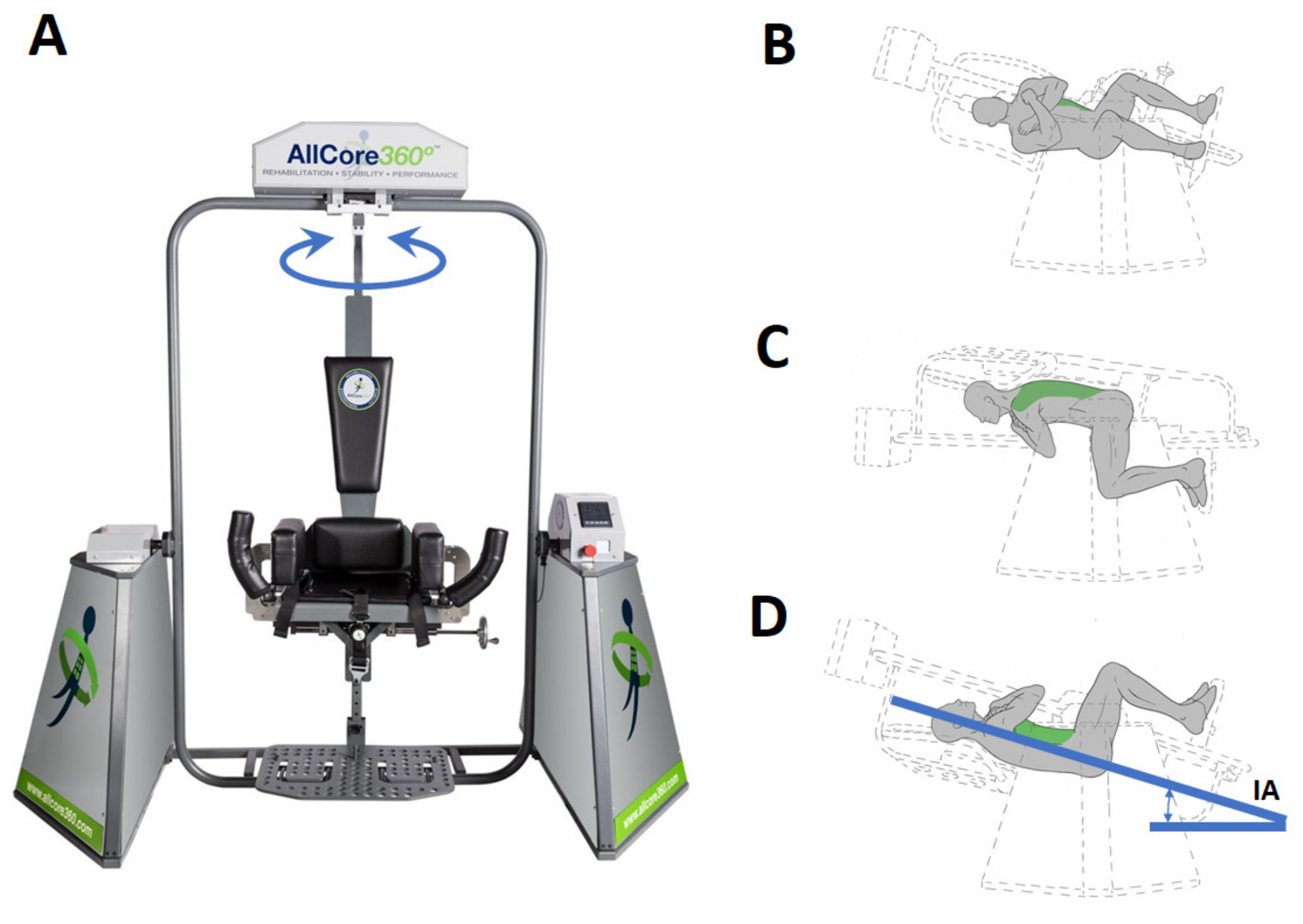

| ID | TSI (Years) | Age (Years) | Sex | Height (cm) | Weight (kg) | BMI |
|---|---|---|---|---|---|---|
| S1 | 18 | 58 | Male | 170.2 | 95 | 33 |
| S2 | 3.7 | 64 | Female | 167.6 | 78 | 28 |
| S3 | 2.7 | 63 | Male | 182.9 | 88 | 27 |
| Participant | Direction | Inclination Angle (Deg) | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 65 | 60 | 55 | 50 | 45 | 40 | 35 | |||
| S1 | CW | 1 | 0 | 20 | 0 | 34 | 16 | 0 | 71 |
| CCW | 1 | 0 | 20 | 0 | 34 | 16 | 0 | 71 | |
| Total | 2 | 0 | 40 | 0 | 68 | 32 | 0 | 142 | |
| S2 | CW | 11 | 3 | 16 | 3 | 17 | 9 | 1 | 60 |
| CCW | 11 | 3 | 16 | 3 | 17 | 9 | 1 | 60 | |
| Total | 22 | 6 | 32 | 6 | 34 | 18 | 2 | 120 | |
| S3 | CW | 10 | 3 | 16 | 6 | 21 | 4 | 0 | 60 |
| CCW | 10 | 3 | 16 | 6 | 21 | 4 | 0 | 60 | |
| Total | 20 | 6 | 32 | 12 | 42 | 8 | 0 | 120 | |
| Assessments | Participant | Baseline | Follow Up | Change (Difference, %Change) | Reference Values for Meaningful Changes |
|---|---|---|---|---|---|
| Trunk Impairment Scale | S1 | 11 | 19 | (8, 72.7%) | |
| S2 | 20 | 20 | 0 | 4 a | |
| S3 | 17 | 17 | 0 | ||
| Berg Balance Scale | S1 | 31 | 45 | (14, 45.2%) | |
| S2 | 47 | 49 | (2, 4.3%) | 2.5 to 4.6 b | |
| S3 | 50 | 52 | (2, 4%) | ||
| Timed-Up and Go (s) | S1 | 22.6 | 17.5 | (−5.1, −22.7) | |
| S2 | 13.6 | 14.3 | (0.7, 5.2%) | 2.9 c | |
| S3 | 12.7 | 12.1 | (−0.6, −4.9) | ||
| 10-m walk test (m/s) | S1 | 0.8 | 0.76 | (−0.04, −5.3%) | |
| S2 | 0.88 | 0.78 | (−0.1, −11.6%) | 0.05 to 0.1 d | |
| S3 | 0.91 | 0.94 | (0.04, 2.5%) | ||
| 6-min walk test (m) | S1 | 251.8 | 240.4 | (−11.4, −4.5%) | |
| S2 | 287.5 | 291.9 | (4.4, 1.5%) | 20 m to 50 m e | |
| S3 | 250.8 | 257.2 | (6.4, 2.6%) |
| % Change | ||||
|---|---|---|---|---|
| APCoP Range | APCoP RMS | MLCoP Range | MLCoP RMS | |
| S01 | −1.49 | 10.98 | −29.24 | −32.30 |
| S02 | −5.18 | 17.83 | −68.55 | −72.10 |
| S03 | 52.12 | 26.23 | 7.63 | −1.75 |
| mean | 15.15 | 18.35 | −30.05 | −35.38 |
| sd | 32.07 | 7.63 | 38.10 | 35.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilkar, R.; Veerubhotla, A.; Ibironke, O.; Ehrenberg, N. A Novel Core Strengthening Intervention for Improving Trunk Function, Balance and Mobility after Stroke. Brain Sci. 2022, 12, 668. https://doi.org/10.3390/brainsci12050668
Pilkar R, Veerubhotla A, Ibironke O, Ehrenberg N. A Novel Core Strengthening Intervention for Improving Trunk Function, Balance and Mobility after Stroke. Brain Sciences. 2022; 12(5):668. https://doi.org/10.3390/brainsci12050668
Chicago/Turabian StylePilkar, Rakesh, Akhila Veerubhotla, Oluwaseun Ibironke, and Naphtaly Ehrenberg. 2022. "A Novel Core Strengthening Intervention for Improving Trunk Function, Balance and Mobility after Stroke" Brain Sciences 12, no. 5: 668. https://doi.org/10.3390/brainsci12050668
APA StylePilkar, R., Veerubhotla, A., Ibironke, O., & Ehrenberg, N. (2022). A Novel Core Strengthening Intervention for Improving Trunk Function, Balance and Mobility after Stroke. Brain Sciences, 12(5), 668. https://doi.org/10.3390/brainsci12050668







