Preconditioned MSCs Alleviate Cerebral Ischemia-Reperfusion Injury in Rats by Improving the Neurological Function and the Inhibition of Apoptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Ethics Statement
2.2. Rat Middle Cerebral Artery Occlusion (MCAO)
2.3. Design of Experimental and Animal Groups
2.4. Behavioral Tests
2.4.1. mNSS
2.4.2. The Adhesive Removal Tests
2.4.3. The Rotarod Test
2.5. 2,3,5-Triphenyltetrazolium Chloride (TTC) Staining
2.6. Histopathological Evaluation (HE) Staining
2.7. Formation of Neurons in the Infarct Area
2.8. Immunohistochemistry
2.9. TUNEL Staining
2.10. MSC Culture
2.11. Preconditioning MSCs with Ischemic Brain Tissue and Grouping
2.12. MTT Analysis
2.13. Flow Cytometry Detection
2.14. Enzyme-Linked Immunosorbent Assay
2.15. Immunofluorescence Detection of Cells
2.16. Western Blotting
2.17. Statistical Analysis
3. Results
3.1. Characteristics of MSCs
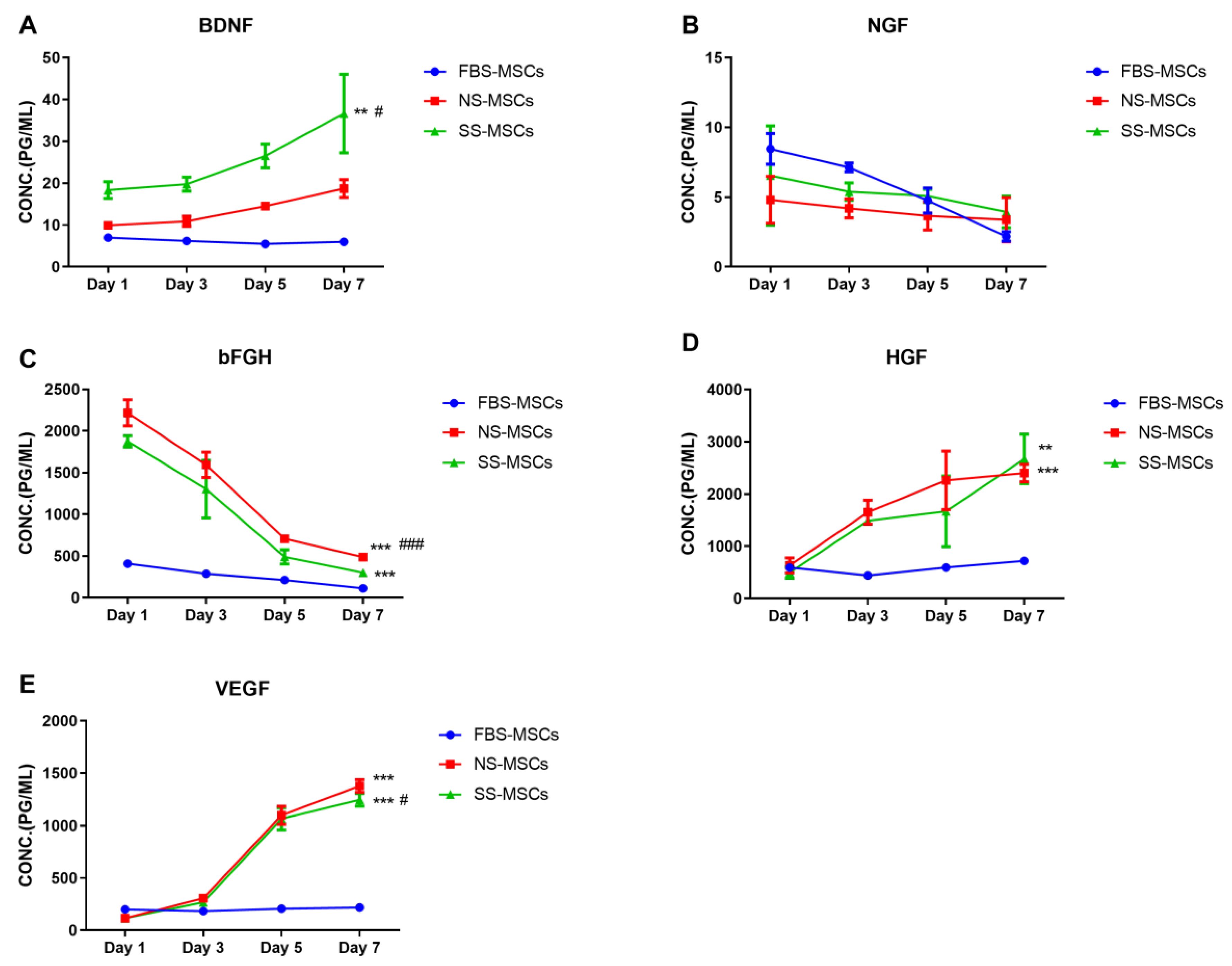
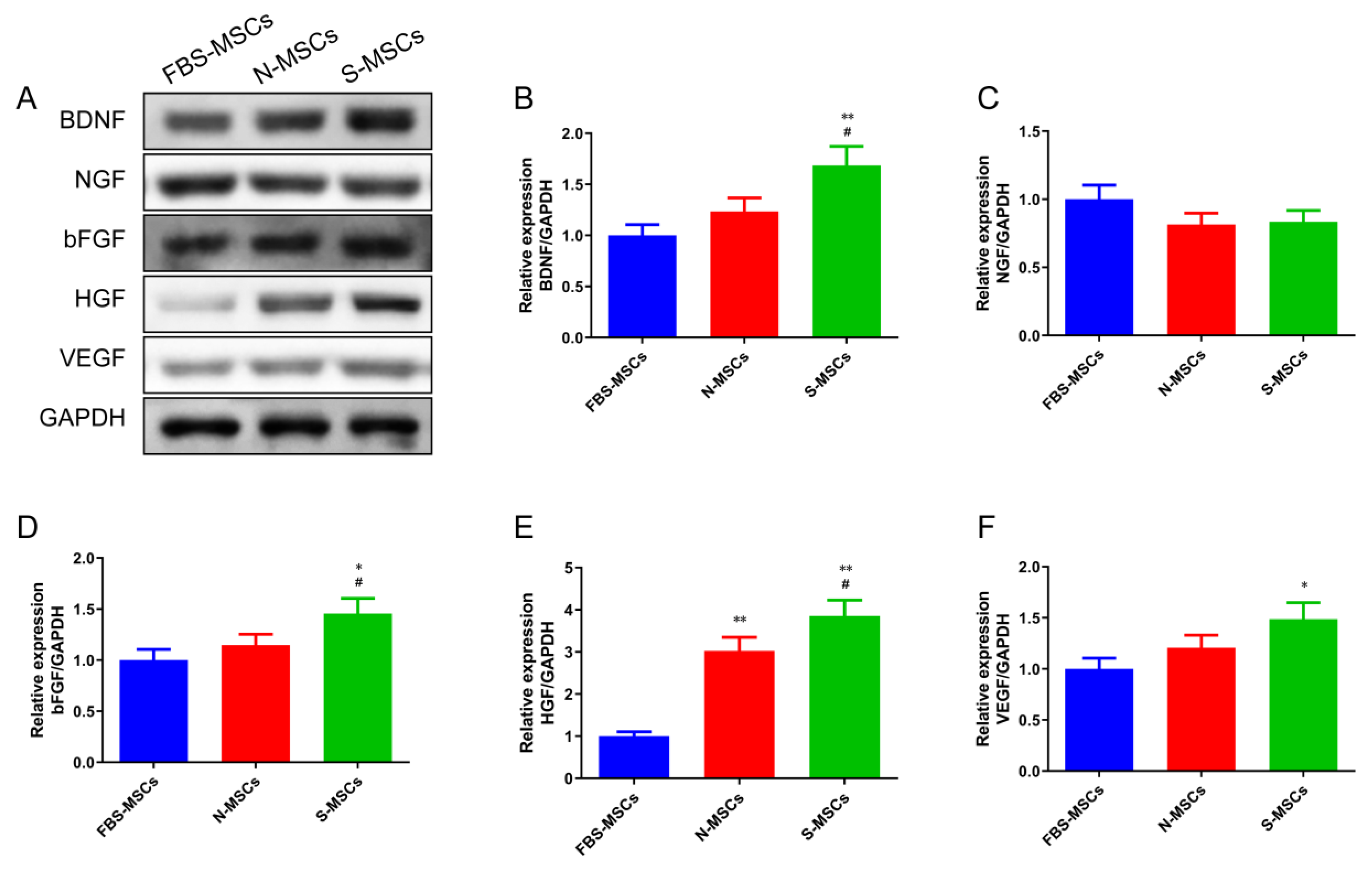
3.2. Comparison of Growth Factor Levels Secreted by MSCs
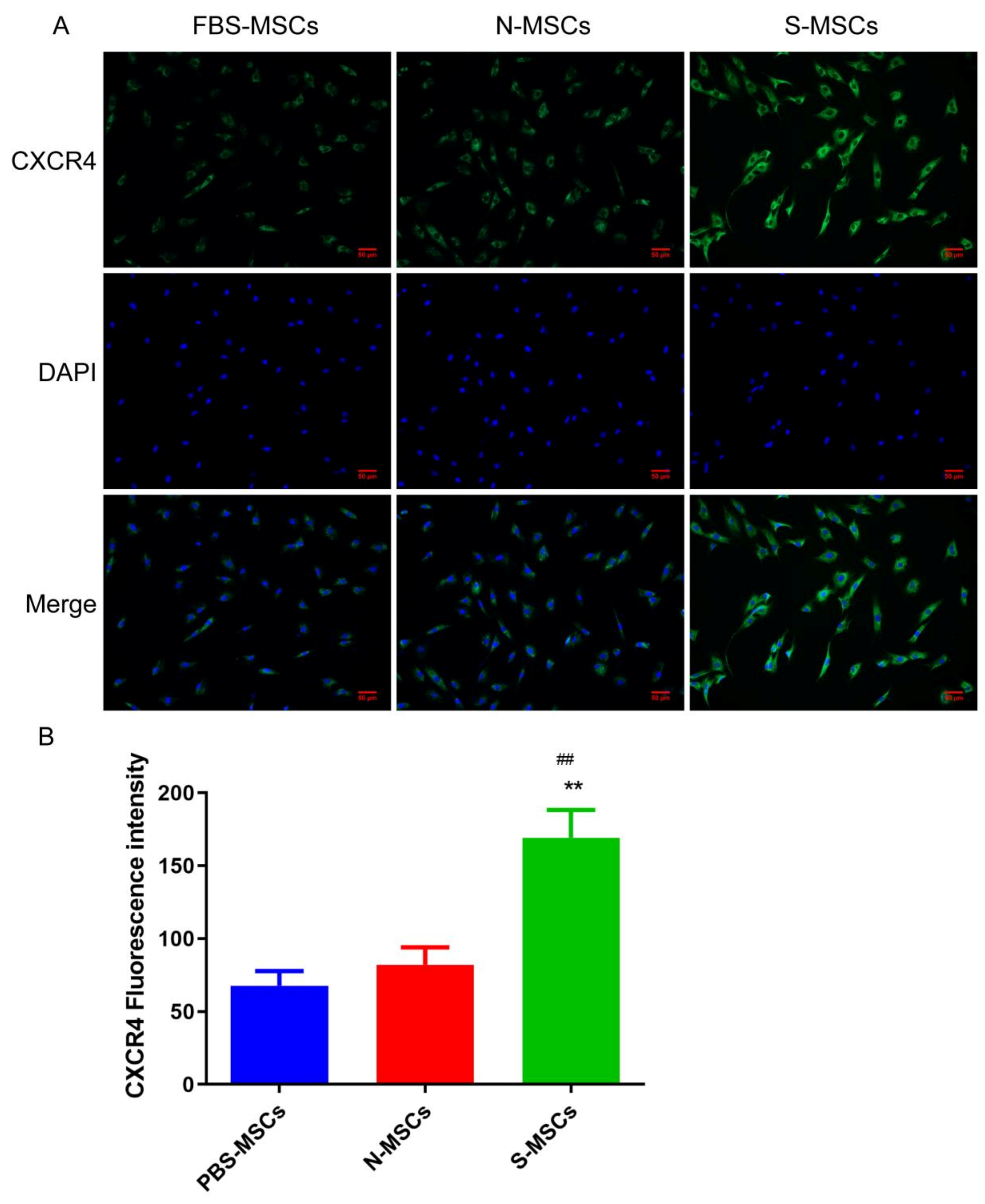
3.3. Comparison of Chemokines of Ischemic Brain Tissue Pretreatment MSCs
3.4. S-MSCs Reduced Cerebral Infarction Volume and Attenuates Neurologic Injury in I/R Rats
3.5. S-MSCs Has Neuroprotective Effects in I/R Rats
3.6. S-MSCs Relief Neuronal and Axonal Injury after MCAO
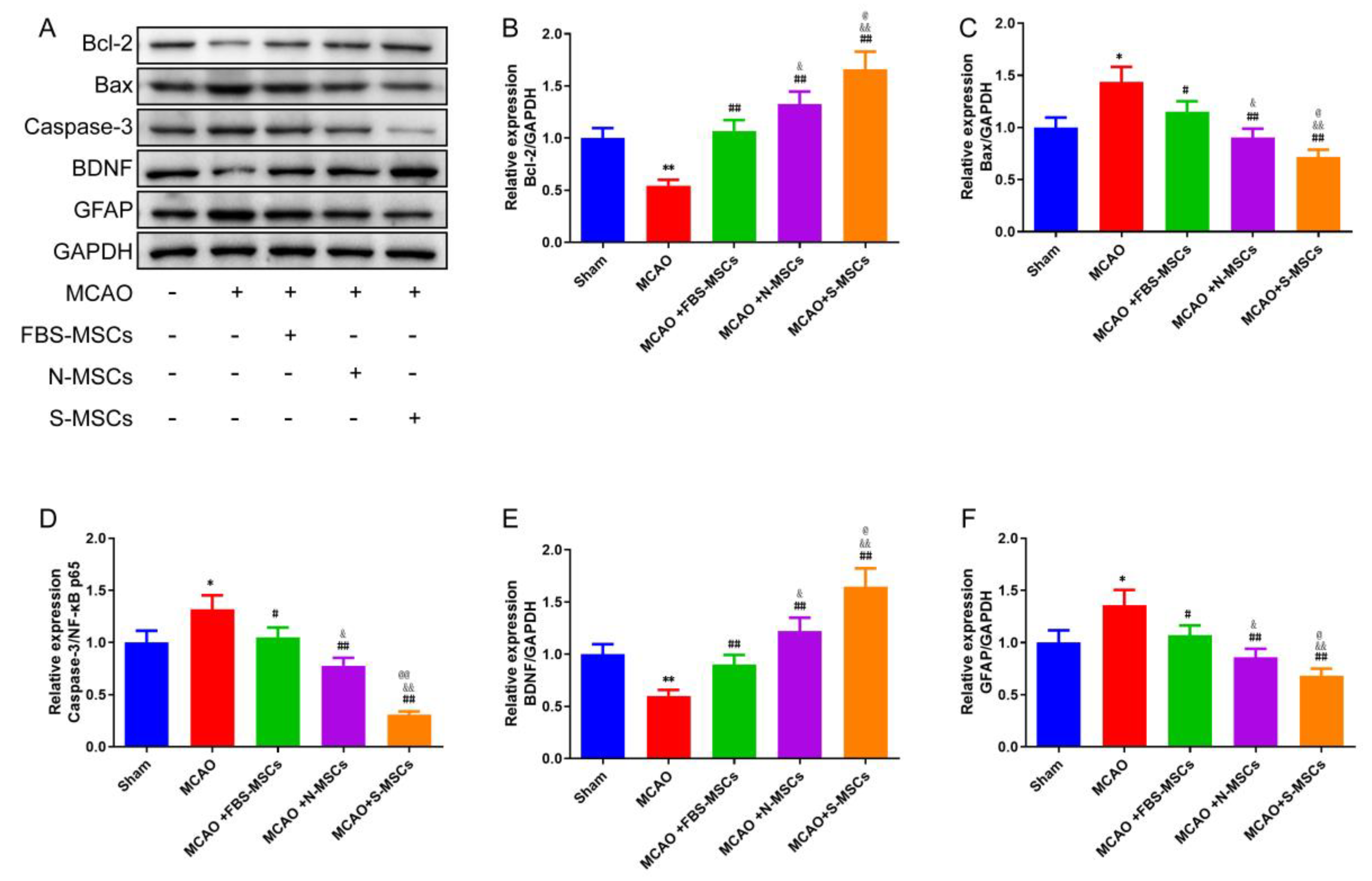
3.7. S-MSCs Preconditioning Attenuates Cerebral Apoptosis after MCAO
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics—2019 update: A report from the American heart association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Pourmohammadi-Bejarpasi, Z.; Roushandeh, A.M.; Saberi, A.; Rostami, M.K.; Toosi, S.M.R.; Jahanian-Najafabadi, A.; Roudkenar, M.H. Mesenchymal stem cells-derived mitochondria transplantation mitigates I/R-induced injury, abolishes I/R-induced apoptosis, and restores motor function in acute ischemia stroke rat model. Brain Res. Bull. 2020, 165, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.K.; Sung, B.; Ahn, K.S.; Kunnumakkara, A.B.; Chaturvedi, M.M.; Aggarwal, B.B. Gambogic acid, a novel ligand for transferrin receptor, potentiates TNF-induced apoptosis through modulation of the nuclear factor-kappaB signaling pathway. Blood 2007, 110, 3517–3525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bang, O.Y.; Lee, J.S.; Lee, P.H.; Lee, G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 2005, 57, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J. Cell Mol. Med. 2018, 22, 1428–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, S.S.; Xu, X.P.; Chang, W.; Lu, Z.H.; Huang, L.L.; Xu, J.Y.; Guo, F.M. LincRNA-p21 promotes mesenchymal stem cell migration capacity and survival through hypoxic preconditioning. Stem Cell Res. Ther. 2018, 9, 280. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.H.; Li, Y.; Chen, J.; Zacharek, A.; Gao, Q.; Kapke, A.; Lu, M.; Raginski, K.; Vanguri, P.; Smith, A.; et al. Therapeutic Benefit of Bone Marrow Stromal Cells Administered 1 Month after Stroke. J. Cereb. Blood Flow Metab. 2006, 27, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Li, W.Y.; Choi, Y.J.; Lee, P.H.; Huh, K.; Kang, Y.M.; Kim, H.S.; Ahn, Y.H.; Lee, G.; Bang, O.Y. Mesenchymal Stem Cells for Ischemic Stroke: Changes in Effects after Ex Vivo Culturing. Cell Transplant. 2008, 17, 1045–1059. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Ho, T.Y.; Hsiang, C.Y.; Tang, N.Y.; Hsieh, C.L.; Kao, S.T.; Lee, Y.C. Angelica sinensis Exerts Angiogenic and Anti-apoptotic Effects Against Cerebral Ischemia-Reperfusion Injury by Activating p38MAPK/HIF-1[Formula: See text]/VEGF-A Signaling in Rats. Am. J. Chin. Med. 2017, 45, 1683–1708. [Google Scholar] [CrossRef]
- Chen, J.L.; Li, Y.; Wang, L.; Zhang, Z.; Lu, D.; Lu, M.; Chopp, M. Therapeutic Benefit of Intravenous Administration of Bone Marrow Stromal Cells After Cerebral Ischemia in Rats. Stroke 2001, 32, 1005–1011. [Google Scholar] [CrossRef] [Green Version]
- Balkaya, M.; Kröber, J.; Gertz, K.; Peruzzaro, S.; Endres, M. Characterization of long-term functional outcome in a murine model of mild brain ischemia. J. Neurosci. Methods 2013, 213, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Shiotsuki, H.; Yoshimi, K.; Shimo, Y.; Funayama, M.; Takamatsu, Y.; Ikeda, K.; Takahashi, R.; Kitazawa, S.; Hattori, N. A rotarod test for evaluation of motor skill learning. J. Neurosci. Methods 2010, 189, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Zha, Y.Y.; Yang, B.; Tang, M.L.; Guo, Q.C.; Chen, J.T.; Wen, L.P.; Wang, M. Concentration-dependent effects of fullerenol on cultured hippocampal neuron viability. Int. J. Nanomed. 2012, 7, 3099–3109. [Google Scholar]
- He, J.; Liu, J.; Huang, Y.; Zhuo, Y.; Chen, W.; Duan, D.; Tang, X.; Lu, M.; Hu, Z. Olfactory Mucosa Mesenchymal Stem Cells Alleviate Cerebral Ischemia/Reperfusion Injury Via Golgi Apparatus Secretory Pathway Ca2+ -ATPase Isoform1. Front. Cell Dev. Biol. 2020, 8, 586541. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Fernández, M.; Rodríguez-Frutos, B.; Álvarez-Grech, J.; Vallejo-Cremades, M.; Expósito-Alcaide, M.; Merino, J.; Roda, J.; Díez-Tejedor, E. Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats. Neuroscience 2010, 175, 394–405. [Google Scholar] [CrossRef]
- Bunnell, B.A.; Betancourt, A.M.; Sullivan, D.E. New concepts on the immune modulation mediated by mesenchymal stem cells. Stem Cell Res. Ther. 2010, 1, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyama, K.; Honmou, O.; Harada, K.; Suzuki, J.; Houkin, K.; Hamada, H.; Kocsis, J.D. Therapeutic benefits of angiogenetic gene-modified human mesenchymal stem cells after cerebral ischemia. Exp. Neurol. 2009, 216, 47–55. [Google Scholar] [CrossRef]
- Coppin, L.; Sokal, E.; Stéphenne, X. Thrombogenic Risk Induced by Intravascular Mesenchymal Stem Cell Therapy: Current Status and Future Perspectives. Cells 2019, 8, 1160. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Li, Y.; Wang, L.; Katakowski, M.; Zhang, L.; Chen, J.; Xu, Y.; Gautam, S.C.; Chopp, M. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology 2002, 22, 275–279. [Google Scholar] [CrossRef]
- Li, X.B.; Huang, M.; Zhao, R.; Zhao, C.; Liu, Y.; Zou, H.; Zhang, Y.A. Intravenously Delivered Allogeneic Mesenchymal Stem Cells Bidirectionally Regulate Inflammation and Induce Neurotrophic Effects in Distal Middle Cerebral Artery Occlusion Rats Within the First 7 Days After Stroke. Cell. Physiol. Biochem. 2018, 46, 1951–1970. [Google Scholar] [CrossRef]
- Zhong, Q.; Zhou, Y.; Ye, W.; Cai, T.; Zhang, X.; Deng, D.Y. Hypoxia-Inducible Factor 1-alpha-AA-Modified Bone Marrow Stem Cells Protect PC12 Cells from Hypoxia-Induced Apoptosis, Partially Through VEGF/PI3K/Akt/FoxO1 Pathway. Stem Cells Dev. 2012, 21, 2703–2717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagenaar, N.; de Theije, C.; De Vries, L.S.; Groenendaal, F.; Benders, M.J.N.L.; Nijboer, C.H.A. Promoting neuroregeneration after perinatal arterial ischemic stroke: Neurotrophic factors and mesenchymal stem cells. Pediatr. Res. 2017, 83, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Li, F.; Zhu, L.; Wang, J.; Yang, Z.; Pan, Y. Update on Therapeutic Mechanism for Bone Marrow Stromal Cells in Ischemic Stroke. J. Mol. Neurosci. 2013, 52, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 2013, 33, 1711–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okazaki, T.; Magaki, T.; Takeda, M.; Kajiwara, Y.; Hanaya, R.; Sugiyama, K.; Arita, K.; Nishimura, M.; Kato, Y.; Kurisu, K. Intravenous administration of bone marrow stromal cells increases survivin and Bcl-2 protein expression and improves sensorimotor function following ischemia in rats. Neurosci. Lett. 2008, 430, 109–114. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, L.; Xie, W.; Meng, X.; Feng, Y.; Sun, G.; Sun, X. Notoginsenoside R1 Improves Cerebral Ischemia/Reperfusion Injury by Promoting Neurogenesis via the BDNF/Akt/CREB Pathway. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Li, Y.; Xiang, L.; Wang, C.; Song, Y.; Miao, J.; Miao, M. Protection against acute cerebral ischemia/reperfusion injury by Leonuri Herba Total Alkali via modulation of BDNF-TrKB-PI3K/Akt signaling pathway in rats. Biomed Pharmacother. 2021, 133, 111021. [Google Scholar] [CrossRef]
- Xiao, B.; Chai, Y.; Lv, S.; Ye, M.; Wu, M.; Xie, L.; Fan, Y.; Zhu, X.; Gao, Z. Endothelial cell-derived exosomes protect SH-SY5Y nerve cells against ischemia/reperfusion injury. Int. J. Mol. Med. 2017, 40, 1201–1209. [Google Scholar] [CrossRef]
- Deng, Q.J.; Xu, X.F.; Ren, J. Effects of SDF-1/CXCR4 on the Repair of Traumatic Brain Injury in Rats by Mediating Bone Marrow Derived Mesenchymal Stem Cells. Cell Mol. Neurobiol. 2018, 38, 467–477. [Google Scholar] [CrossRef]
- Cheng, X.; Yeung, P.K.; Zhong, K.; Zilundu, P.L.; Zhou, L.; Chung, S.K. Astrocytic endothelin-1 overexpression promotes neural progenitor cells proliferation and differentiation into astrocytes via the Jak2/Stat3 pathway after stroke. J. Neuroinflamm. 2019, 16, 227. [Google Scholar] [CrossRef]
- Hofmeijer, J.; van Putten, M.J.A.M. Ischemic Cerebral Damage An Appraisal of Synaptic Failure. Stroke 2012, 43, 607–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, S.; Lin, H.; Liu, H.; Lu, Z.; Wang, H.; Fan, S.; Li, N. Honokiol Attenuates Sepsis-Associated Acute Kidney Injury via the Inhibition of Oxidative Stress and Inflammation. Inflammation 2019, 42, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Leu, S.; Lin, Y.-C.; Yuen, C.-M.; Yen, C.-H.; Kao, Y.-H.; Sun, C.-K.; Yip, H.-K. Adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. J. Transl. Med. 2010, 8, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofroniew, M.V. Reactive Astrocytes in Neural Repair and Protection. Neurosci. 2005, 11, 400–407. [Google Scholar] [CrossRef]
- Ogata, N.; Yonekawa, Y.; Taki, W.; Kannagi, R.; Murachi, T.; Hamakubo, T.; Kikuchi, H. Degradation of neurofilament protein in cerebral ischemia. J. Neurosurg. 1989, 70, 103–107. [Google Scholar] [CrossRef]
- Mages, B.; Aleithe, S.; Altmann, S.; Blietz, A.; Nitzsche, B.; Barthel, H.; Horn, A.K.E.; Hobusch, C.; Härtig, W.; Krueger, M.; et al. Impaired Neurofilament Integrity and Neuronal Morphology in Different Models of Focal Cerebral Ischemia and Human Stroke Tissue. Front. Cell. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [Green Version]
- Ruan, L.; Wang, B.; ZhuGe, Q.; Jin, K. Coupling of neurogenesis and angiogenesis after ischemic stroke. Brain Res. 2015, 1623, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Rosenblum, S.; Smith, T.N.; Wang, N.; Chua, J.Y.; Westbroek, E.; Wang, K.; Guzman, R. BDNF Pretreatment of Human Embryonic-Derived Neural Stem Cells Improves Cell Survival and Functional Recovery After Transplantation in Hypoxic-Ischemic Stroke. Cell Transpl. 2015, 24, 2449–2461. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Mao, X.; Wang, D.; Xia, S. Preconditioned MSCs Alleviate Cerebral Ischemia-Reperfusion Injury in Rats by Improving the Neurological Function and the Inhibition of Apoptosis. Brain Sci. 2022, 12, 631. https://doi.org/10.3390/brainsci12050631
Zheng J, Mao X, Wang D, Xia S. Preconditioned MSCs Alleviate Cerebral Ischemia-Reperfusion Injury in Rats by Improving the Neurological Function and the Inhibition of Apoptosis. Brain Sciences. 2022; 12(5):631. https://doi.org/10.3390/brainsci12050631
Chicago/Turabian StyleZheng, Jin, Xueyu Mao, Delong Wang, and Shiliang Xia. 2022. "Preconditioned MSCs Alleviate Cerebral Ischemia-Reperfusion Injury in Rats by Improving the Neurological Function and the Inhibition of Apoptosis" Brain Sciences 12, no. 5: 631. https://doi.org/10.3390/brainsci12050631
APA StyleZheng, J., Mao, X., Wang, D., & Xia, S. (2022). Preconditioned MSCs Alleviate Cerebral Ischemia-Reperfusion Injury in Rats by Improving the Neurological Function and the Inhibition of Apoptosis. Brain Sciences, 12(5), 631. https://doi.org/10.3390/brainsci12050631





