T1w/T2w Ratio and Cognition in 9-to-11-Year-Old Children
Abstract
:1. Introduction
2. Materials and Methods
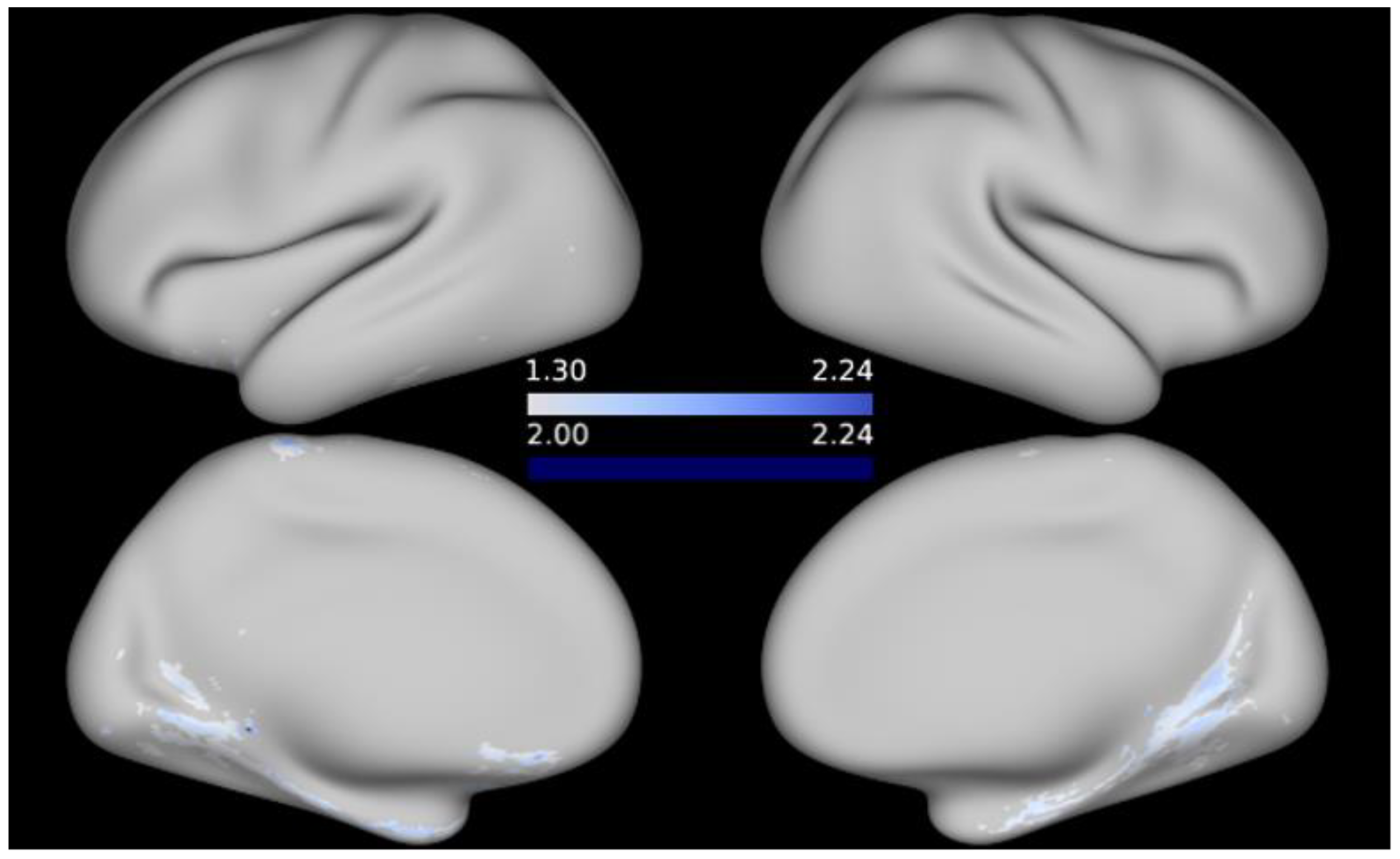
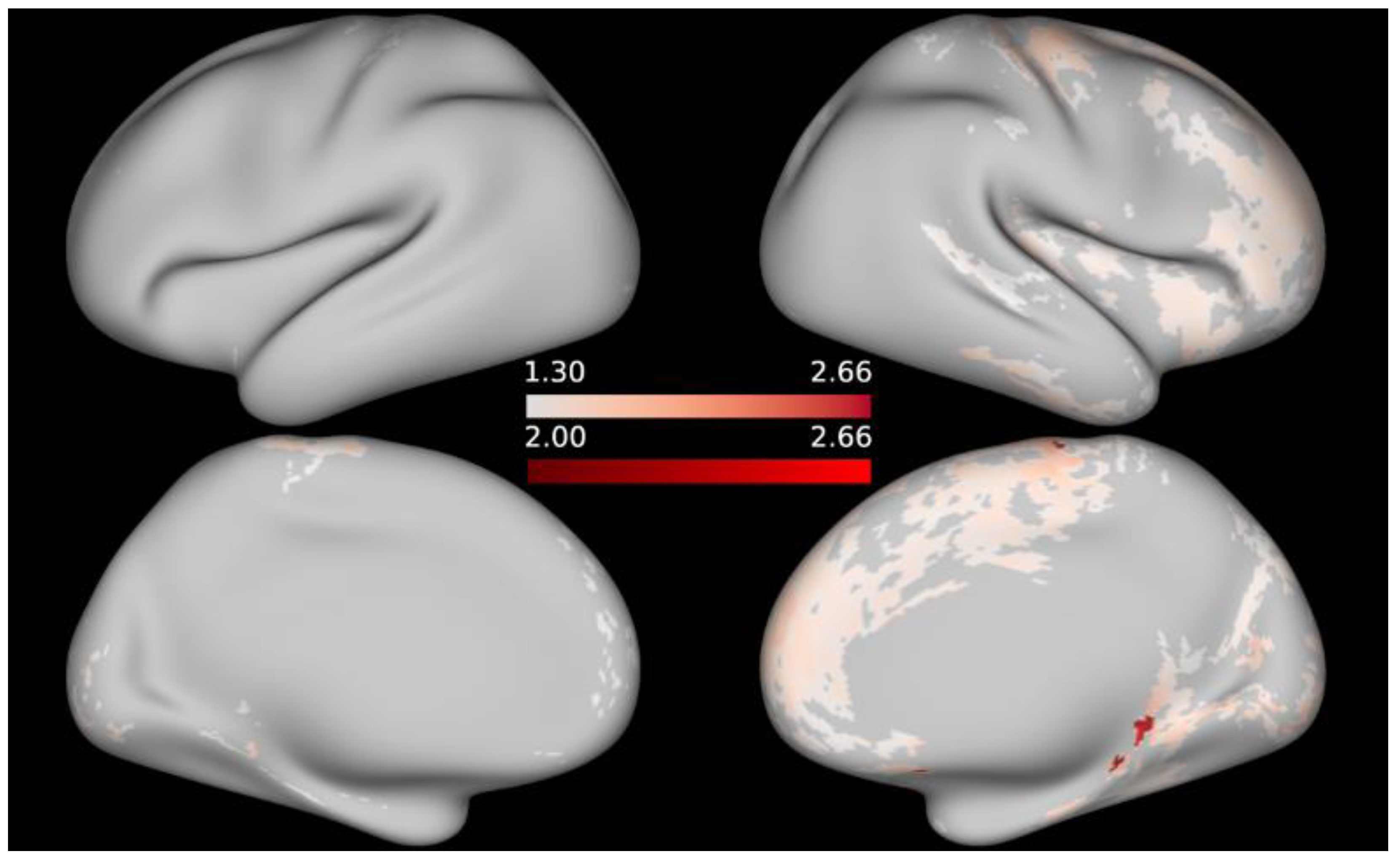
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rakic, P. Evolution of the Neocortex: A Perspective from Developmental Biology. Nat. Rev. Neurosci. 2009, 10, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Norbom, L.B.; Ferschmann, L.; Parker, N.; Agartz, I.; Andreassen, O.A.; Paus, T.; Westlye, L.T.; Tamnes, C.K. New Insights into the Dynamic Development of the Cerebral Cortex in Childhood and Adolescence: Integrating Macro- and Microstructural MRI Findings. Prog. Neurobiol. 2021, 204, 102109. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, J.H.; Knickmeyer, R.C.; Gao, W. Imaging Structural and Functional Brain Development in Early Childhood. Nat. Rev. Neurosci. 2018, 19, 123–137. [Google Scholar] [CrossRef]
- Mills, K.L.; Goddings, A.L.; Herting, M.M.; Meuwese, R.; Blakemore, S.J.; Crone, E.A.; Dahl, R.E.; Güroğlu, B.; Raznahan, A.; Sowell, E.R.; et al. Structural Brain Development between Childhood and Adulthood: Convergence across Four Longitudinal Samples. Neuroimage 2016, 141, 273–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgaleta, M.; Johnson, W.; Waber, D.P.; Colom, R.; Karama, S. Cognitive Ability Changes and Dynamics of Cortical Thickness Development in Healthy Children and Adolescents. Neuroimage 2014, 84, 810–819. [Google Scholar] [CrossRef] [Green Version]
- Shaw, P.; Greenstein, D.; Lerch, J.; Clasen, R.; Lenroot, N.; Gogtay, A.; Giedd, J. Intellectual Ability and Cortical Development in Children and Adolescents. Nature 2006, 440, 676–679. [Google Scholar] [CrossRef]
- Walhovd, K.B.; Krogsrud, S.K.; Amlien, I.K.; Bartsch, H.; Bjørnerud, A.; Due-Tønnessen, P.; Grydeland, H.; Hagler, D.J.; Håberg, A.K.; Kremen, W.S.; et al. Neurodevelopmental Origins of Lifespan Changes in Brain and Cognition. Proc. Natl. Acad. Sci. USA 2016, 113, 9357–9362. [Google Scholar] [CrossRef] [Green Version]
- Corrigan, N.M.; Yarnykh, V.L.; Hippe, D.S.; Owen, J.P.; Huber, E.; Zhao, T.C.; Kuhl, P.K. Myelin Development in Cerebral Gray and White Matter during Adolescence and Late Childhood. Neuroimage 2021, 227, 117678. [Google Scholar] [CrossRef]
- Heath, F.; Hurley, S.A.; Johansen-Berg, H.; Sampaio-Baptista, C. Advances in Noninvasive Myelin Imaging. Dev. Neurobiol. 2018, 78, 136–151. [Google Scholar] [CrossRef] [Green Version]
- Spear, L.P. Adolescent Neurodevelopment. J. Adolesc. Health 2013, 52 (Suppl. 2), S7–S13. [Google Scholar] [CrossRef] [Green Version]
- Shafee, R.; Buckner, R.L.; Fischl, B. Gray Matter Myelination of 1555 Human Brains Using Partial Volume Corrected MRI Images. Neuroimage 2015, 105, 473–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pujol, J.; Soriano-Mas, C.; Ortiz, H.; Sebastián-Gallés, N.; Losilla, J.M.; Deus, J. Myelination of Language-Related Areas in the Developing Brain. Neurology 2006, 66, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Deoni, S.C.L.; O’Muircheartaigh, J.; Elison, J.T.; Walker, L.; Doernberg, E.; Waskiewicz, N.; Dirks, H.; Piryatinsky, I.; Dean, D.C.; Jumbe, N.L. White Matter Maturation Profiles through Early Childhood Predict General Cognitive Ability. Brain Struct. Funct. 2016, 221, 1189–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, J.M.; Lyons, D.A. Myelin Dynamics throughout Life: An Ever-Changing Landscape? Front. Cell. Neurosci. 2018, 12, 424. [Google Scholar] [CrossRef] [PubMed]
- de Faria, O.; Pivonkova, H.; Varga, B.; Timmler, S.; Evans, K.A.; Káradóttir, R.T. Periods of Synchronized Myelin Changes Shape Brain Function and Plasticity. Nat. Neurosci. 2021, 24, 1508–1521. [Google Scholar] [CrossRef]
- Douglas Fields, R. White Matter in Learning, Cognition and Psychiatric Disorders. Trends Neurosci. 2008, 31, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Ganzetti, M.; Wenderoth, N.; Mantini, D. Whole Brain Myelin Mapping Using T1- and T2-Weighted MR Imaging Data. Front. Hum. Neurosci. 2014, 8, 671. [Google Scholar] [CrossRef]
- Laule, C.; Vavasour, I.M.; Kolind, S.H.; Li, D.K.B.; Traboulsee, T.L.; Moore, G.R.W.; Mackay, A.L. Magnetic Resonance Imaging of Myelin. Neurotherapeutics 2007, 4, 460–484. [Google Scholar] [CrossRef] [Green Version]
- Piredda, G.F.; Hilbert, T.; Thiran, J.P.; Kober, T. Probing Myelin Content of the Human Brain with MRI: A Review. Magn. Reson. Med. 2021, 85, 627–652. [Google Scholar] [CrossRef]
- Xiao, L.; Ohayon, D.; Mckenzie, I.A.; Sinclair-Wilson, A.; Wright, J.L.; Fudge, A.D.; Emery, B.; Li, H.; Richardson, W.D. Rapid Production of New Oligodendrocytes Is Required in the Earliest Stages of Motor-Skill Learning. Nat. Neurosci. 2016, 19, 1210–1217. [Google Scholar] [CrossRef]
- McKenzie, I.A.; Ohayon, D.; Li, H.; De Faria, J.P.; Emery, B.; Tohyama, K.; Richardson, W.D. Motor Skill Learning Requires Active Central Myelination. Science 2014, 346, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Mayoral, S.R.; Choi, H.S.; Chan, J.R.; Kheirbek, M.A. Preservation of a Remote Fear Memory Requires New Myelin Formation. Nat. Neurosci. 2020, 23, 487–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stüber, C.; Morawski, M.; Schäfer, A.; Labadie, C.; Wähnert, M.; Leuze, C.; Streicher, M.; Barapatre, N.; Reimann, K.; Geyer, S.; et al. Myelin and Iron Concentration in the Human Brain: A Quantitative Study of MRI Contrast. Neuroimage 2014, 93, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Glasser, M.F.; van Essen, D.C. Mapping Human Cortical Areas In Vivo Based on Myelin Content as Revealed by T1- and T2-Weighted MRI. J. Neurosci. 2011, 31, 11597–11616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenig, S.H. Cholesterol of Myelin Is the Determinant of Gray-white Contrast in MRI of Brain. Magn. Reson. Med. 1991, 20, 285–291. [Google Scholar] [CrossRef]
- Bock, N.A.; Kocharyan, A.; Liu, J.V.; Silva, A.C. Visualizing the Entire Cortical Myelination Pattern in Marmosets with Magnetic Resonance Imaging. J. Neurosci. Methods 2009, 185, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Yoshiura, T.; Higano, S.; Rubio, A.; Shrier, D.A.; Kwok, W.E.; Iwanaga, S.; Numaguchi, Y. Heschl and Superior Temporal Gyri: Low Signal Intensity of Cortex on T2- Weighted MR Images of the Normal Brain. Radiology 2000, 214, 217–221. [Google Scholar] [CrossRef]
- Arshad, M.; Stanley, J.A.; Raz, N. Test–Retest Reliability and Concurrent Validity of In Vivo Myelin Content Indices: Myelin Water Fraction and Calibrated T1w/T2w Image Ratio. Hum. Brain Mapp. 2017, 38, 1780–1790. [Google Scholar] [CrossRef] [Green Version]
- Hagiwara, A.; Hori, M.; Kamagata, K.; Warntjes, M.; Matsuyoshi, D.; Nakazawa, M.; Ueda, R.; Andica, C.; Koshino, S.; Maekawa, T.; et al. Myelin Measurement: Comparison between Simultaneous Tissue Relaxometry, Magnetization Transfer Saturation Index, and T1w/T2w Ratio Methods. Sci. Rep. 2018, 8, 10554. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.N.; Figley, T.D.; Solar, K.G.; Shatil, A.S.; Figley, C.R. Comparisons between Multi-Component Myelin Water Fraction, T1w/T2w Ratio, and Diffusion Tensor Imaging Measures in Healthy Human Brain Structures. Sci. Rep. 2019, 9, 2500. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Cherel, M.; Budin, F.; Gilmore, J.; Zaldarriaga Consing, K.; Rasmussen, J.; Wadhwa, P.D.; Entringer, S.; Glasser, M.F.; Van Essen, D.C.; et al. Early Postnatal Myelin Content Estimate of White Matter via T1w/T2w Ratio. Med. Imaging 2015 Biomed. Appl. Mol. Struct. Funct. Imaging 2015, 9417, 94171R. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Budin, F.; Noel, J.; Prieto, J.C.; Gilmore, J.; Rasmussen, J.; Wadhwa, P.D.; Entringer, S.; Buss, C.; Styner, M. White Matter Fiber-Based Analysis of T1w/T2w Ratio Map. Proc. SPIE 2017, 10133, 101330P. [Google Scholar] [CrossRef] [Green Version]
- Shams, Z.; Norris, D.G.; Marques, J.P. A Comparison of In Vivo MRI Based Cortical Myelin Mapping Using T1w/T2w and R1 Mapping at 3T. PLoS ONE 2019, 14, e0218089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nerland, S.; Jørgensen, K.N.; Nordhøy, W.; Maximov, I.I.; Bugge, R.A.B.; Westlye, L.T.; Andreassen, O.A.; Geier, O.M.; Agartz, I. Multisite Reproducibility and Test-Retest Reliability of the T1w/T2w-Ratio: A Comparison of Processing Methods. Neuroimage 2021, 245, 118709. [Google Scholar] [CrossRef] [PubMed]
- Grydeland, H.; Walhovd, K.B.; Tamnes, C.K.; Westlye, L.T.; Fjell, A.M. Intracortical Myelin Links with Performance Variability across the Human Lifespan: Results from T1- and T2- Weighted MRI Myelin Mapping and Diffusion Tensor Imaging. J. Neurosci. 2013, 33, 18618–18630. [Google Scholar] [CrossRef] [Green Version]
- Norbom, L.B.; Rokicki, J.; Alnæs, D.; Kaufmann, T.; Doan, N.T.; Andreassen, O.A.; Westlye, L.T.; Tamnes, C.K. Maturation of Cortical Microstructure and Cognitive Development in Childhood and Adolescence: A T1w/T2w Ratio MRI Study. Hum. Brain Mapp. 2020, 41, 4676–4690. [Google Scholar] [CrossRef]
- Vandewouw, M.M.; Young, J.M.; Shroff, M.M.; Taylor, M.J.; Sled, J.G. Altered Myelin Maturation in Four Year Old Children Born Very Preterm. NeuroImage Clin. 2019, 21, 101635. [Google Scholar] [CrossRef]
- Schmitt, J.E.; Raznahan, A.; Liu, S.; Neale, M.C. The Genetics of Cortical Myelination in Young Adults and Its Relationships to Cerebral Surface Area, Cortical Thickness, and Intelligence: A Magnetic Resonance Imaging Study of Twins and Families. Neuroimage 2020, 20, 116319. [Google Scholar] [CrossRef]
- Patel, Y.; Parker, N.; Salum, G.A.; Pausova, Z.; Paus, T. General Psychopathology, Cognition, and the Cerebral Cortex in 10-Year-Old Children: Insights From the Adolescent Brain Cognitive Development Study. Front. Hum. Neurosci. 2022, 15, 781554. [Google Scholar] [CrossRef]
- Bischoff-Grethe, A.; Fennema-Notestine, C.; Kaye, W.; Wierenga, C. Adolescent Anorexia Nervosa Is Associated with Lower Myelin Content in Cortical Cognitive Control Regions. Biol. Psychiatry 2021, 89, S364–S365. [Google Scholar] [CrossRef]
- Qiu, Y.; She, S.; Zhang, S.; Wu, F.; Liang, Q.; Peng, Y.; Yuan, H.; Ning, Y.; Wu, H.; Huang, R. Cortical Myelin Content Mediates Differences in Affective Temperaments. J. Affect. Disord. 2021, 282, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhang, Y.; Li, Y.; Meng, Y.; Li, M.; Wang, Q.; Deng, W.; Ma, X.; Palaniyappan, L.; Zhang, N.; et al. Depth-Dependent Abnormal Cortical Myelination in First-Episode Treatment-Naïve Schizophrenia. Hum. Brain Mapp. 2020, 41, 2782–2793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuno, F.; Kudo, T.; Yamamoto, A.; Matsuoka, K.; Takahashi, M.; Iida, H.; Ihara, M.; Nagatsuka, K.; Kishimoto, T. Significant Correlation between Openness Personality in Normal Subjects and Brain Myelin Mapping with T1/T2-Weighted MR Imaging. Heliyon 2017, 3, e00411. [Google Scholar] [CrossRef] [PubMed]
- Toschi, N.; Passamonti, L.; Bellesi, M. Sleep Quality Relates to Emotional Reactivity via Intracortical Myelination. Sleep 2021, 44, zsaa146. [Google Scholar] [CrossRef] [PubMed]
- Darki, F.; Nyström, P.; Mcalonan, G.; Bölte, S.; Falck-Ytter, T. T1-Weighted/T2-Weighted Ratio Mapping at 5 Months Captures Individual Differences in Behavioral Development and Differentiates Infants at Familial Risk for Autism from Controls. Cereb. Cortex 2021, 31, 4068–4077. [Google Scholar] [CrossRef]
- Ponticorvo, S.; Manara, R.; Russillo, M.C.; Erro, R.; Picillo, M.; Di Salle, G.; Di Salle, F.; Barone, P.; Esposito, F.; Pellecchia, M.T. Magnetic Resonance T1w/T2w Ratio and Voxel-Based Morphometry in Multiple System Atrophy. Sci. Rep. 2021, 11, 21683. [Google Scholar] [CrossRef]
- Glasser, M.F.; Coalson, T.S.; Harms, M.P.; Baum, G.L.; Autio, J.A.; Auerbach, E.J.; Xu, J.; Greve, D.N.; Yacoub, E.; Van Essen, D.C.; et al. Transmit Field Bias Correction of T1w/T2w Myelin Maps. bioRxiv 2021. [Google Scholar]
- Masouleh, S.K.; Eickhoff, S.B.; Hoffstaedter, F.; Genon, S. Empirical Examination of the Replicability of Associations between Brain Structure and Psychological Variables. eLife 2019, 8, e43464. [Google Scholar] [CrossRef]
- Brito, N.H.; Noble, K.G. Socioeconomic Status and Structural Brain Development. Front. Neurosci. 2014, 8, 276. [Google Scholar] [CrossRef] [Green Version]
- Norbom, L.B.; Hanson, J.L.; van der Meer, D.; Ferschmann, L.; Røysamb, E.; von Soest, T.; Andreassen, O.A.; Agartz, I.; Westlye, L.T.; Tamnes, C.K. Parental Socioeconomic Status Is Linked to Cortical Microstructure and Language Abilities in Children and Adolescents. Available online: https://psyarxiv.com/tv2fc/ (accessed on 2 May 2022).
- Deoni, S.C.L.; Dean, D.C.; Remer, J.; Dirks, H.; O’Muircheartaigh, J. Cortical Maturation and Myelination in Healthy Toddlers and Young Children. Neuroimage 2015, 115, 147–161. [Google Scholar] [CrossRef] [Green Version]
- Grydeland, H.; Vértes, P.E.; Váša, F.; Romero-Garcia, R.; Whitaker, K.; Alexander-Bloch, A.F.; Bjørnerud, A.; Patel, A.X.; Sederevičius, D.; Tamnes, C.K.; et al. Waves of Maturation and Senescence in Micro-Structural MRI Markers of Human Cortical Myelination over the Lifespan. Cereb. Cortex 2019, 29, 1369–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, P.; Kuan, C.C.; Kaga, K.; Sano, M.; Mima, K. Myelination Progression in Language-Correlated Regions in Brain of Normal Children Determined by Quantitative MRI Assessment. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 1751–1763. [Google Scholar] [CrossRef] [PubMed]
- Baum, G.L.; Flournoy, J.C.; Glasser, M.F.; Harms, M.P.; Mair, P.; Sanders, A.; Barch, D.; Buckner, R.L.; Bookheimer, S.; Dapretto, M.; et al. Graded Variation in Cortical T1w/T2w Myelination during Adolescence. bioRxiv 2021. [Google Scholar]
- Casey, B.J.; Cannonier, T.; Conley, M.I.; Cohen, A.O.; Barch, D.M.; Heitzeg, M.M.; Soules, M.E.; Teslovich, T.; Dellarco, D.V.; Garavan, H.; et al. The Adolescent Brain Cognitive Development (ABCD) Study: Imaging Acquisition across 21 Sites. Dev. Cogn. Neurosci. 2018, 32, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Auchter, A.M.; Hernandez Mejia, M.; Heyser, C.J.; Shilling, P.D.; Jernigan, T.L.; Brown, S.A.; Tapert, S.F.; Dowling, G.J. A Description of the ABCD Organizational Structure and Communication Framework. Dev. Cogn. Neurosci. 2018, 32, 8–15. [Google Scholar] [CrossRef]
- Charness, M.E. The Adolescent Brain Cognitive Development Study External Advisory Board. Dev. Cogn. Neurosci. 2018, 32, 155–160. [Google Scholar] [CrossRef]
- Clark, D.B.; Fisher, C.B.; Bookheimer, S.; Brown, S.A.; Evans, J.H.; Hopfer, C.; Hudziak, J.; Montoya, I.; Murray, M.; Pfefferbaum, A.; et al. Biomedical Ethics and Clinical Oversight in Multisite Observational Neuroimaging Studies with Children and Adolescents: The ABCD Experience. Dev. Cogn. Neurosci. 2018, 32, 143–154. [Google Scholar] [CrossRef]
- Szaflarski, J.P.; Binder, J.R.; Possing, E.T.; McKiernan, K.A.; Ward, B.D.; Hammeke, T.A. Language Lateralization in Left-Handed and Ambidextrous People: FMRI Data. Neurology 2002, 59, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Weintraub, S.; Dikmen, S.S.; Heaton, R.K.; Tulsky, D.S.; Zelazo, P.D.; Bauer, P.J.; Carlozzi, N.E.; Slotkin, J.; Blitz, D.; Wallner-Allen, K.; et al. Cognition Assessment Using the NIH Toolbox. Neurology 2013, 80 (Suppl. 3), S54–S64. [Google Scholar] [CrossRef] [Green Version]
- Luciana, M.; Bjork, J.M.; Nagel, B.J.; Barch, D.M.; Gonzalez, R.; Nixon, S.J.; Banich, M.T. Adolescent Neurocognitive Development and Impacts of Substance Use: Overview of the Adolescent Brain Cognitive Development (ABCD) Baseline Neurocognition Battery. Dev. Cogn. Neurosci. 2018, 32, 67–79. [Google Scholar] [CrossRef]
- Hagler, D.J.; Hatton, S.; Cornejo, M.D.; Makowski, C.; Fair, D.A.; Dick, A.S.; Sutherland, M.T.; Casey, B.J.; Barch, D.M.; Harms, M.P.; et al. Image Processing and Analysis Methods for the Adolescent Brain Cognitive Development Study. Neuroimage 2019, 202, 116091. [Google Scholar] [CrossRef] [PubMed]
- Glasser, M.F.; Sotiropoulos, S.N.; Wilson, J.A.; Coalson, T.S.; Fischl, B.; Andersson, J.L.; Xu, J.; Jbabdi, S.; Webster, M.; Polimeni, J.R.; et al. The Minimal Preprocessing Pipelines for the Human Connectome Project. Neuroimage 2013, 80, 105–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischl, B. FreeSurfer. Neuroimage 2012, 62, 774–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.J.; Woolrich, M.W.; Smith, S.M. FSL. Neuroimage 2012, 2, 782–790. [Google Scholar] [CrossRef] [Green Version]
- Glasser, M.F.; Goyal, M.S.; Preuss, T.M.; Raichle, M.E.; Van Essen, D.C. Trends and Properties of Human Cerebral Cortex: Correlations with Cortical Myelin Content. Neuroimage 2014, 93, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Winkler, A.M.; Ridgway, G.R.; Webster, M.A.; Smith, S.M.; Nichols, T.E. Permutation Inference for the General Linear Model. Neuroimage 2014, 92, 381–397. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.M.; Nichols, T.E. Threshold-Free Cluster Enhancement: Addressing Problems of Smoothing, Threshold Dependence and Localisation in Cluster Inference. Neuroimage 2009, 44, 83–98. [Google Scholar] [CrossRef] [Green Version]
- Samara, A.; Feng, K.; Pivik, R.T.; Jarratt, K.P.; Badger, T.M.; Ou, X. White Matter Microstructure Correlates with Memory Performance in Healthy Children: A Diffusion Tensor Imaging Study. J. Neuroimaging 2019, 29, 233–241. [Google Scholar] [CrossRef]
- Simmonds, D.J.; Hallquist, M.N.; Asato, M.; Luna, B. Developmental Stages and Sex Differences of White Matter and Behavioral Development through Adolescence: A Longitudinal Diffusion Tensor Imaging (DTI) Study. Neuroimage 2014, 92, 356–368. [Google Scholar] [CrossRef] [Green Version]
- Vestergaard, M.; Skakmadsen, K.; Baaré, W.F.C.; Skimminge, A.; Ejersbo, L.R.; Ramsøy, T.Z.; Gerlach, C.; Åkeson, P.; Paulson, O.B.; Jernigan, T.L. White Matter Microstructure in Superior Longitudinal Fasciculus Associated with Spatial Working Memory Performance in Children. J. Cogn. Neurosci. 2011, 23, 2135–2146. [Google Scholar] [CrossRef] [Green Version]
- Dougherty, R.F.; Ben-Shachar, M.; Deutsch, G.K.; Hernandez, A.; Fox, G.R.; Wandell, B.A. Temporal-Callosal Pathway Diffusivity Predicts Phonological Skills in Children. Proc. Natl. Acad. Sci. USA 2007, 104, 8556–8561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fields, R.D. A New Mechanism of Nervous System Plasticity: Activity-Dependent Myelination. Nat. Rev. Neurosci. 2015, 16, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.J.; Kirilina, E.; Mohammadi, S.; Weiskopf, N. Microstructural Imaging of Human Neocortex In Vivo. Neuroimage 2018, 182, 184–206. [Google Scholar] [CrossRef]
- Du, G.; Lewis, M.M.; Sica, C.; Kong, L.; Huang, X. Magnetic Resonance T1w/T2w Ratio: A Parsimonious Marker for Parkinson Disease. Ann. Neurol. 2019, 85, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, K.; Zeng, Q.; Huang, P.; Jiaerken, Y.; Wang, S.; Shen, Z.; Xu, X.; Xu, J.; Wang, C.; et al. Application of T1-/T2-Weighted Ratio Mapping to Elucidate Intracortical Demyelination Process in the Alzheimer’s Disease Continuum. Front. Neurosci. 2019, 13, 904. [Google Scholar] [CrossRef]
- Pelkmans, W.; Dicks, E.; Barkhof, F.; Vrenken, H.; Scheltens, P.; van der Flier, W.M.; Tijms, B.M. Gray Matter T1-w/T2-w Ratios Are Higher in Alzheimer’s Disease. Hum. Brain Mapp. 2019, 40, 3900–3909. [Google Scholar] [CrossRef]
- Fischl, B.; Dale, A.M. Measuring the Thickness of the Human Cerebral Cortex from Magnetic Resonance Images. Proc. Natl. Acad. Sci. USA 2000, 97, 11050–11055. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.N.; Figley, T.D.; Marrie, R.A.; Figley, C.R. Can T1w/T2w Ratio Be Used as a Myelin-Specific Measure in Subcortical Structures? Comparisons between FSE-Based T1w/T2w Ratios, GRASE-Based T1w/T2w Ratios and Multi-Echo GRASE-Based Myelin Water Fractions. NMR Biomed. 2018, 31, e3868. [Google Scholar] [CrossRef]
- Sowell, E.R.; Thompson, P.M.; Leonard, C.M.; Welcome, S.E.; Kan, E.; Toga, A.W. Longitudinal Mapping of Cortical Thickness and Brain Growth in Normal Children. J. Neurosci. 2004, 24, 8223–8231. [Google Scholar] [CrossRef]


| Demographic Variable | Females N = 449 | Males N = 511 |
|---|---|---|
| Age (in years) | M = 9.97 (SD = 0.61) | M = 10.04 (SD = 0.62) |
| Race | White = 347 | White = 417 |
| African American = 79 | African American = 66 | |
| Native American = 15 | Native American = 7 | |
| Asian = 25 | Asian = 29 | |
| Other = 37 | Other = 33 | |
| Household income (in the past 12 months) | USD 200 k and greater = 46 | USD 200 k and greater = 53 |
| USD 100 k–199 k = 141 | USD 100 k–199 k = 158 | |
| USD 75 k–99,999 = 73 | USD 75 k–99,999 = 72 | |
| USD 50 k–74,999 = 43 | USD 50 k–74,999 = 69 | |
| USD 35 k–49,999 = 47 | USD 35 k–49,999 = 43 | |
| USD 25 k–34,999 = 21 | USD 25 k–34,999 = 23 | |
| USD 16 k–24,999 = 19 | USD 16 k–24,999 = 30 | |
| USD 12 k–15,999 = 8 | USD 12 k–15,999 = 8 | |
| USD 5 k–11,999 = 17 | USD 5 k–11,999 = 10 | |
| Less than USD 5000 = 5 | Less than USD 5000 = 7 | |
| Parental Education 2 | Bachelor’s degree or higher = 264 Some form of post-high school education = 129 | Bachelor’s degree or higher = 287 Some form of post-high school education = 156 |
| High school degree = 32 | High school degree = 39 | |
| No high school degree = 23 | No high school degree = 28 |
| Task | Cognitive Domains |
|---|---|
| Oral Reading Recognition Test | Language Reading Decoding |
| Picture Vocabulary Test | Language Receptive vocabulary |
| Flanker Inhibitory Control and Attention Test | Executive functioning Attention Inhibitory control |
| Dimensional Change Card Sort Test | Executive functioning Cognitive flexibility |
| Picture Sequence Memory Test | Visuospatial sequencing |
| Episodic memory | |
| List Sorting Working Memory Test | Working memory |
| Information processing | |
| Pattern Comparison Processing Speed Test | Processing speed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langensee, L.; Rumetshofer, T.; Behjat, H.; Novén, M.; Li, P.; Mårtensson, J. T1w/T2w Ratio and Cognition in 9-to-11-Year-Old Children. Brain Sci. 2022, 12, 599. https://doi.org/10.3390/brainsci12050599
Langensee L, Rumetshofer T, Behjat H, Novén M, Li P, Mårtensson J. T1w/T2w Ratio and Cognition in 9-to-11-Year-Old Children. Brain Sciences. 2022; 12(5):599. https://doi.org/10.3390/brainsci12050599
Chicago/Turabian StyleLangensee, Lara, Theodor Rumetshofer, Hamid Behjat, Mikael Novén, Ping Li, and Johan Mårtensson. 2022. "T1w/T2w Ratio and Cognition in 9-to-11-Year-Old Children" Brain Sciences 12, no. 5: 599. https://doi.org/10.3390/brainsci12050599
APA StyleLangensee, L., Rumetshofer, T., Behjat, H., Novén, M., Li, P., & Mårtensson, J. (2022). T1w/T2w Ratio and Cognition in 9-to-11-Year-Old Children. Brain Sciences, 12(5), 599. https://doi.org/10.3390/brainsci12050599





