The Role of Systemic Physiology in Individual Hemodynamic Responses Measured on the Head Due to Long-Term Stimulation Involving Colored Light Exposure and a Cognitive Task: An SPA-fNIRS Study
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Subjects
2.2. Measurement Setup
2.3. Experimental Protocol
2.4. Signal Processing and Statistical Analysis
3. Results
3.1. The Visual Cortex Shows Stronger Response to Blue Than to Red Light Exposure
3.2. Systemic Physiological Reactions Are Not Different for Red Compared to Blue Light Exposure
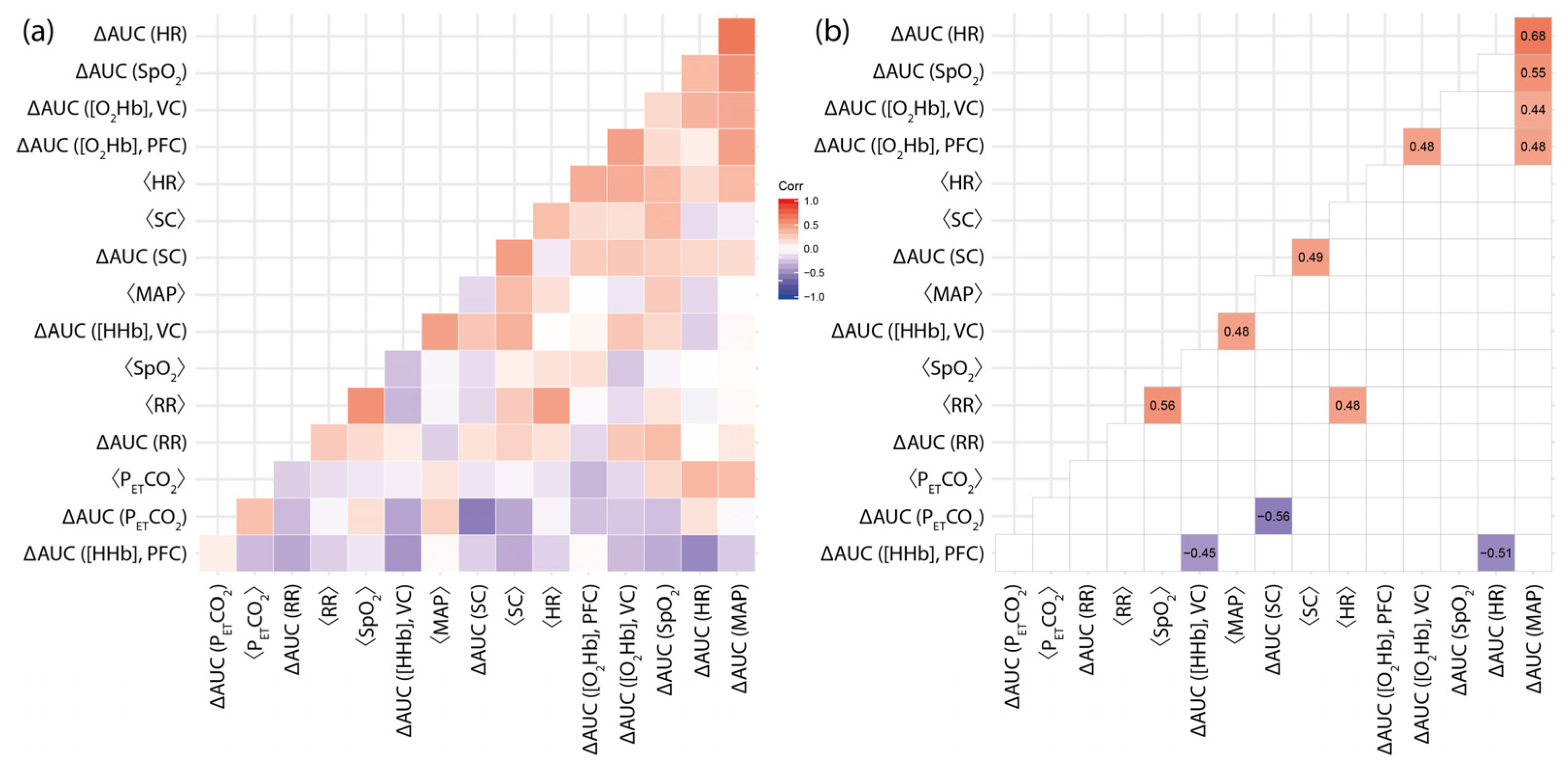
3.3. The Magnitude of Differential Changes in fNIRS Signals (Red vs. Blue Light Exposure) Are Correlated with Baseline Levels and Reactivity of Systemic Physiology
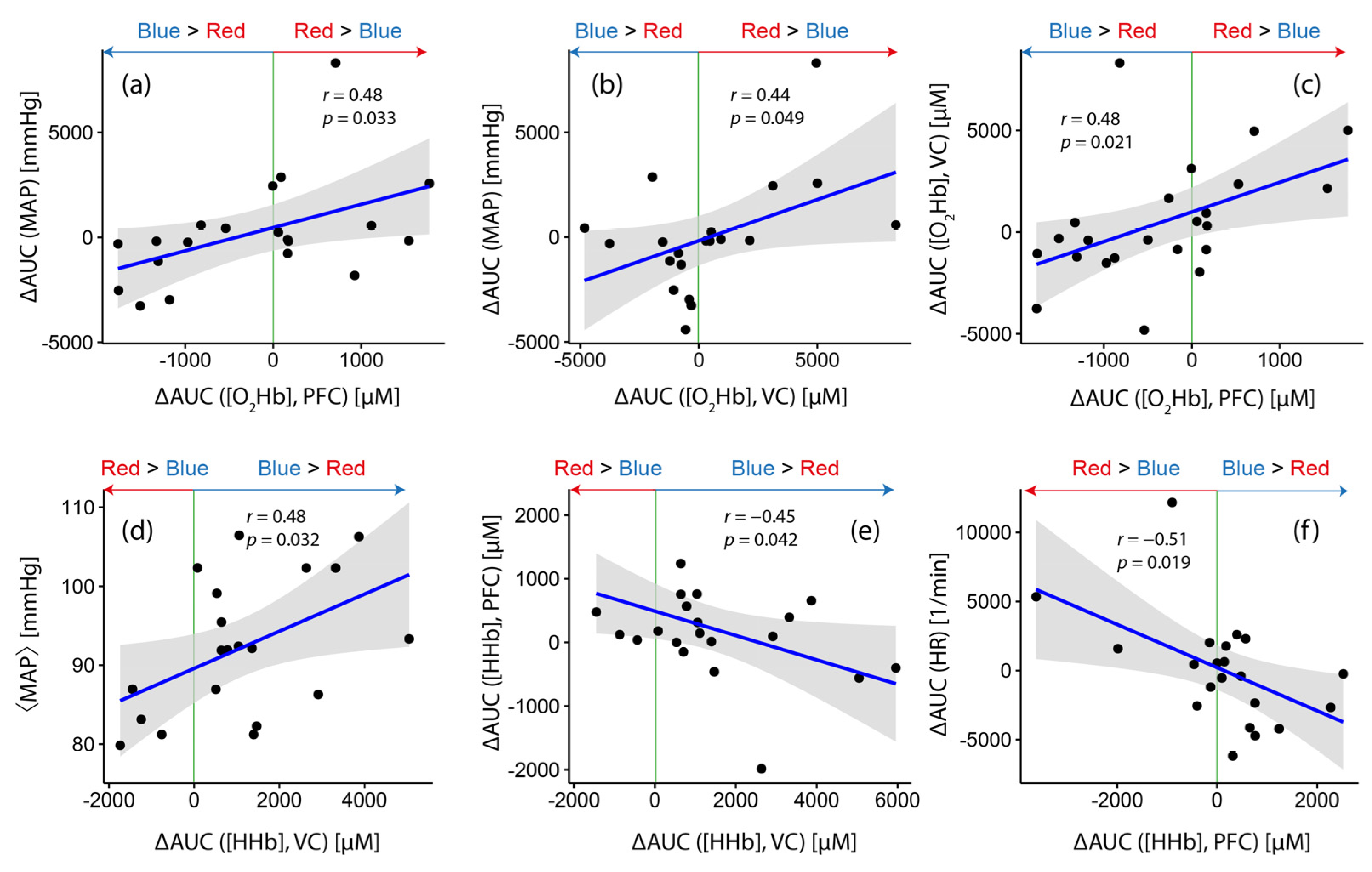
- While [HHb] at VC, on the group level, showed a stronger increase during blue light exposure (as already discussed in Section 3.1), the magnitude of the differences (∆AUC ([HHb]) was correlated with the baseline MAP level (⟨MAP⟩) (r = 0.48, p = 0.032; Figure 3d), i.e., the larger the baseline MAP of a subject, the higher the chance to have a stronger increase in [HHb] during blue light exposure compared to red light exposure (and vice versa). Furthermore, ∆AUC ([HHb]) at the VC was negatively correlated with ∆AUC ([HHb]) at PFC (r = −0.45, p = 0.042; Figure 3e).
- ∆AUC ([O2Hb]) at VC was positively correlated with ∆AUC ([MAP]) (r = 0.44, p = 0.049; Figure 3b), i.e., subjects with a larger increase in [O2Hb] during red light exposure compared to the blue one had a higher probability of also having a larger increase in [MAP] during red light exposure compared to the blue one.
- For PFC, three statistically significant correlations were found. ∆AUC ([HHb]) was negatively correlated with ∆AUC (HR) (r = −0.51, p = 0.019; Figure 3f), while ∆AUC ([O2Hb] showed positive correlations with ∆AUC (MAP) (r = 0.48, p = 0.033; Figure 3a) and ∆AUC ([O2Hb]) at VC (r = 0.48, p = 0.021; Figure 3c).
3.4. The Magnitude of Differential Changes in Systemic Physiology (Red vs. Blue Light Exposure): Interrelationships and Correlations with Baseline Values
- ∆AUC ([MAP]) was positively correlated with (∆AUC ([HR]) (r = 0.68, p = 0.00036; Figure 4a) and (∆AUC ([SpO2]) (r = 0.55, p = 0.0048; Figure 4b). In the case of a stronger MAP response due to red light exposure compared to blue light exposure, the probability that a stronger response would also be observed in HR and SpO2 increased.
4. Discussion
4.1. The Magnitude of Changes in [HHb] at the Visual Cortex Depends on the Type of Color Exposure and Is Related to the Individual Baseline Blood Pressure Level
4.2. The Magnitude of Color-Dependent Changes in [O2Hb] at the Visual and Prefrontal Cortex Is Related to the Individual Magnitude of Color-Dependent Changes in MAP
4.3. A Color-Dependent Fronto-Occipital Coupling
4.4. The Magnitude of Color-Dependent Changes in [HHb] at the Prefrontal Cortex Is Related to the Individual Magnitude of Color-Dependent Changes in HR
4.5. Color-Dependent Changes in Systemic Physiology
4.6. Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrari, M.; Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 2012, 63, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Quaresima, V.; Ferrari, M. A Mini-Review on Functional Near-Infrared Spectroscopy (fNIRS): Where Do We Stand, and Where Should We Go? Photonics 2019, 6, 87. [Google Scholar] [CrossRef] [Green Version]
- Scholkmann, F.; Kleiser, S.; Metz, A.J.; Zimmermann, R.; Mata Pavia, J.; Wolf, U.; Wolf, M. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage 2014, 85, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Zohdi, H.; Egli, R.; Guthruf, D.; Scholkmann, F.; Wolf, U. Color-dependent changes in humans during a verbal fluency task under colored light exposure assessed by SPA-fNIRS. Sci. Rep. 2021, 11, 9654. [Google Scholar] [CrossRef] [PubMed]
- Zohdi, H.; Scholkmann, F.; Wolf, U. Individual Differences in Hemodynamic Responses Measured on the Head due to a Long-Term Stimulation Involving Colored Light Exposure and a Cognitive Task: A SPA-fNIRS Study. Brain Sci. 2021, 11, 54. [Google Scholar] [CrossRef]
- Zohdi, H.; Scholkmann, F.; Wolf, U. Long-Term Blue Light Exposure Changes Frontal and Occipital Cerebral Hemodynamics: Not All Subjects React the Same. Adv. Exp. Med. Biol. 2021, 1269, 217–222. [Google Scholar] [CrossRef]
- Metz, A.J.; Klein, S.D.; Scholkmann, F.; Wolf, U. Continuous coloured light altered human brain haemodynamics and oxygenation assessed by systemic physiology augmented functional near-infrared spectroscopy. Sci. Rep. 2017, 7, 10027. [Google Scholar] [CrossRef] [Green Version]
- Metz, A.J.; Klein, S.D.; Scholkmann, F.; Wolf, U. Physiological Effects of Continuous Colored Light Exposure on Mayer Wave Activity in Cerebral Hemodynamics: A Functional Near-Infrared Spectroscopy (fNIRS) Study. In Oxygen Transport to Tissue XXXIX; Springer: Berlin/Heidelberg, Germany, 2017; pp. 277–283. [Google Scholar] [CrossRef]
- Scholkmann, F.; Hafner, T.; Metz, A.J.; Wolf, M.; Wolf, U. Effect of short-term colored-light exposure on cerebral hemodynamics and oxygenation, and systemic physiological activity. Neurophotonics 2017, 4, 045005. [Google Scholar] [CrossRef] [Green Version]
- Hueber, D.M.; Franceschini, M.A.; Ma, H.Y.; Zhang, Q.; Ballesteros, J.R.; Fantini, S.; Wallace, D.; Ntziachristos, V.; Chance, B. Non-invasive and quantitative near-infrared haemoglobin spectrometry in the piglet brain during hypoxic stress, using a frequency-domain multidistance instrument. Phys. Med. Biol. 2001, 46, 41–62. [Google Scholar] [CrossRef] [Green Version]
- Jaspers, H.H. The ten-twenty electrode system of the international federation. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 370–375. [Google Scholar]
- Yucel, M.A.; Luhmann, A.V.; Scholkmann, F.; Gervain, J.; Dan, I.; Ayaz, H.; Boas, D.; Cooper, R.J.; Culver, J.; Elwell, C.E.; et al. Best practices for fNIRS publications. Neurophotonics 2021, 8, 012101. [Google Scholar] [CrossRef]
- Coulson, J.M.; Murphy, K.; Harris, A.D.; Fjodorova, M.; Cockcroft, J.R.; Wise, R.G. Correlation between baseline blood pressure and the brainstem FMRI response to isometric forearm contraction in human volunteers: A pilot study. J. Hum. Hypertens. 2014, 29, 449–455. [Google Scholar] [CrossRef]
- Kobuch, S.; Macefield, V.G.; Henderson, L.A. Resting regional brain activity and connectivity vary with resting blood pressure but not muscle sympathetic nerve activity in normotensive humans: An exploratory study. J. Cereb. Blood Flow Metab. 2018, 39, 2433–2444. [Google Scholar] [CrossRef]
- Sklerov, M.; Dayan, E.; Browner, N. Functional neuroimaging of the central autonomic network: Recent developments and clinical implications. Clin. Auton. Res. 2019, 29, 555–566. [Google Scholar] [CrossRef] [Green Version]
- Valenza, G.; Passamonti, L.; Duggento, A.; Toschi, N.; Barbieri, R. Uncovering complex central autonomic networks at rest: A functional magnetic resonance imaging study on complex cardiovascular oscillations. J. R. Soc. Interface 2020, 17, 20190878. [Google Scholar] [CrossRef] [Green Version]
- Scholkmann, F.; Gerber, U.; Wolf, M.; Wolf, U. End-tidal CO2: An important parameter for a correct interpretation in functional brain studies using speech tasks. Neuroimage 2013, 66, 71–79. [Google Scholar] [CrossRef]
- Scholkmann, F.; Wolf, M.; Wolf, U. The effect of inner speech on arterial CO2 and cerebral hemodynamics and oxygenation: A functional NIRS study. Adv. Exp. Med. Biol. 2013, 789, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, M.; Scholkmann, F.; Wolf, U.; Wolf, M.; Elwell, C.E.; Tachtsidis, I. Modelling confounding effects from extracerebral contamination and systemic factors on functional near-infrared spectroscopy. NeuroImage 2016, 143, 91–105. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Wolf, M.; Toronov, V.; Wolf, U.; Polzonetti, C.; Hueber, D.M.; Safonova, L.; Gupta, R.; Michalos, A.; Mantulin, W.; et al. Noninvasive determination of the optical properties of adult brain: Near-infrared spectroscopy approach. J. Biomed. Opt. 2004, 9, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Franceschini, M.A.; Fantini, S.; Paunescu, L.A.; Maier, J.S.; Gratton, E. Influence of a superficial layer in the quantitative spectroscopic study of strongly scattering media. Appl. Opt. 1998, 37, 7447–7458. [Google Scholar] [CrossRef] [Green Version]
- Scholkmann, F.; Metz, A.J.; Wolf, M. Measuring tissue hemodynamics and oxygenation by continuous-wave functional near-infrared spectroscopy—how robust are the different calculation methods against movement artifacts? Physiol. Meas. 2014, 35, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Photobiomodulation or low-level laser therapy. J. Biophotonics 2016, 9, 1122–1124. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, F.; Mahmoudi, J.; Kamari, F.; Sadigh-Eteghad, S.; Rasta, S.H.; Hamblin, M.R. Brain Photobiomodulation Therapy: A Narrative Review. Mol. Neurobiol. 2018, 55, 6601–6636. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, M.; Hamblin, M.R. Photobiomodulation and the brain: A new paradigm. J. Opt. 2017, 19, 013003. [Google Scholar] [CrossRef] [Green Version]
- Hamblin, M.R. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin. 2016, 6, 113–124. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scholkmann, F.; Zohdi, H.; Wolf, U. The Role of Systemic Physiology in Individual Hemodynamic Responses Measured on the Head Due to Long-Term Stimulation Involving Colored Light Exposure and a Cognitive Task: An SPA-fNIRS Study. Brain Sci. 2022, 12, 597. https://doi.org/10.3390/brainsci12050597
Scholkmann F, Zohdi H, Wolf U. The Role of Systemic Physiology in Individual Hemodynamic Responses Measured on the Head Due to Long-Term Stimulation Involving Colored Light Exposure and a Cognitive Task: An SPA-fNIRS Study. Brain Sciences. 2022; 12(5):597. https://doi.org/10.3390/brainsci12050597
Chicago/Turabian StyleScholkmann, Felix, Hamoon Zohdi, and Ursula Wolf. 2022. "The Role of Systemic Physiology in Individual Hemodynamic Responses Measured on the Head Due to Long-Term Stimulation Involving Colored Light Exposure and a Cognitive Task: An SPA-fNIRS Study" Brain Sciences 12, no. 5: 597. https://doi.org/10.3390/brainsci12050597
APA StyleScholkmann, F., Zohdi, H., & Wolf, U. (2022). The Role of Systemic Physiology in Individual Hemodynamic Responses Measured on the Head Due to Long-Term Stimulation Involving Colored Light Exposure and a Cognitive Task: An SPA-fNIRS Study. Brain Sciences, 12(5), 597. https://doi.org/10.3390/brainsci12050597






