Sex Differences in Plasma Lysophosphatidic Acid Species in Patients with Alcohol and Cocaine Use Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Recruitment
2.2. Eligibility Criteria
2.3. Ethics Statements
2.4. Clinical Evaluations
2.5. Collection and Processing of Plasma Samples
2.6. Analysis of LPA Species
2.7. Statistical Analysis
3. Results
3.1. Sociodemographic Characteristics and LPA Concentrations Based on Sample Group
3.2. Correlation Analyses between LPA Concentrations and Age and BMI
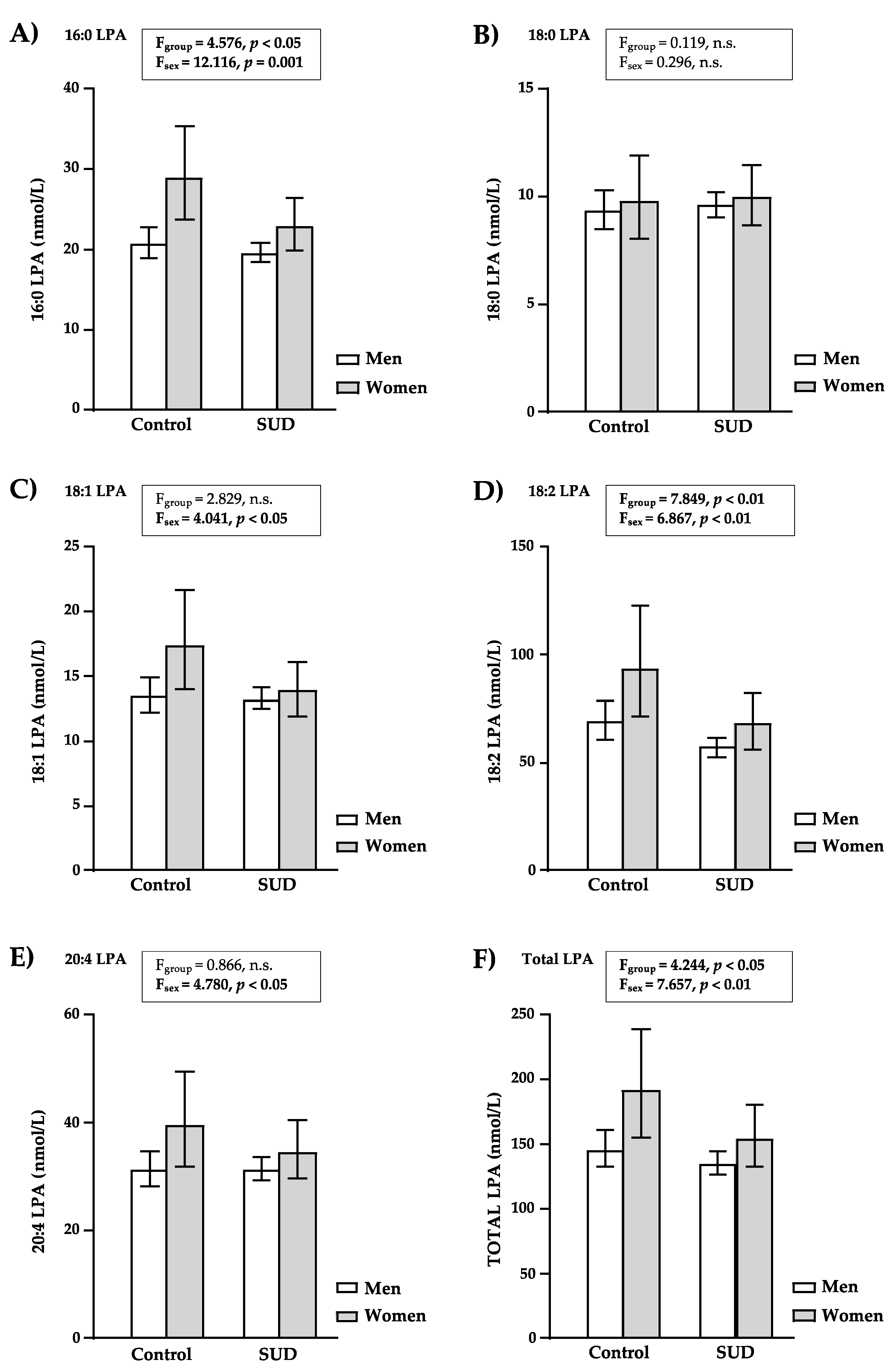
3.3. LPA Concentrations Based on Sample Group and Sex
3.3.1. LPA Concentrations in Controls and Patients with SUD
3.3.2. LPA Concentrations in Men and Women
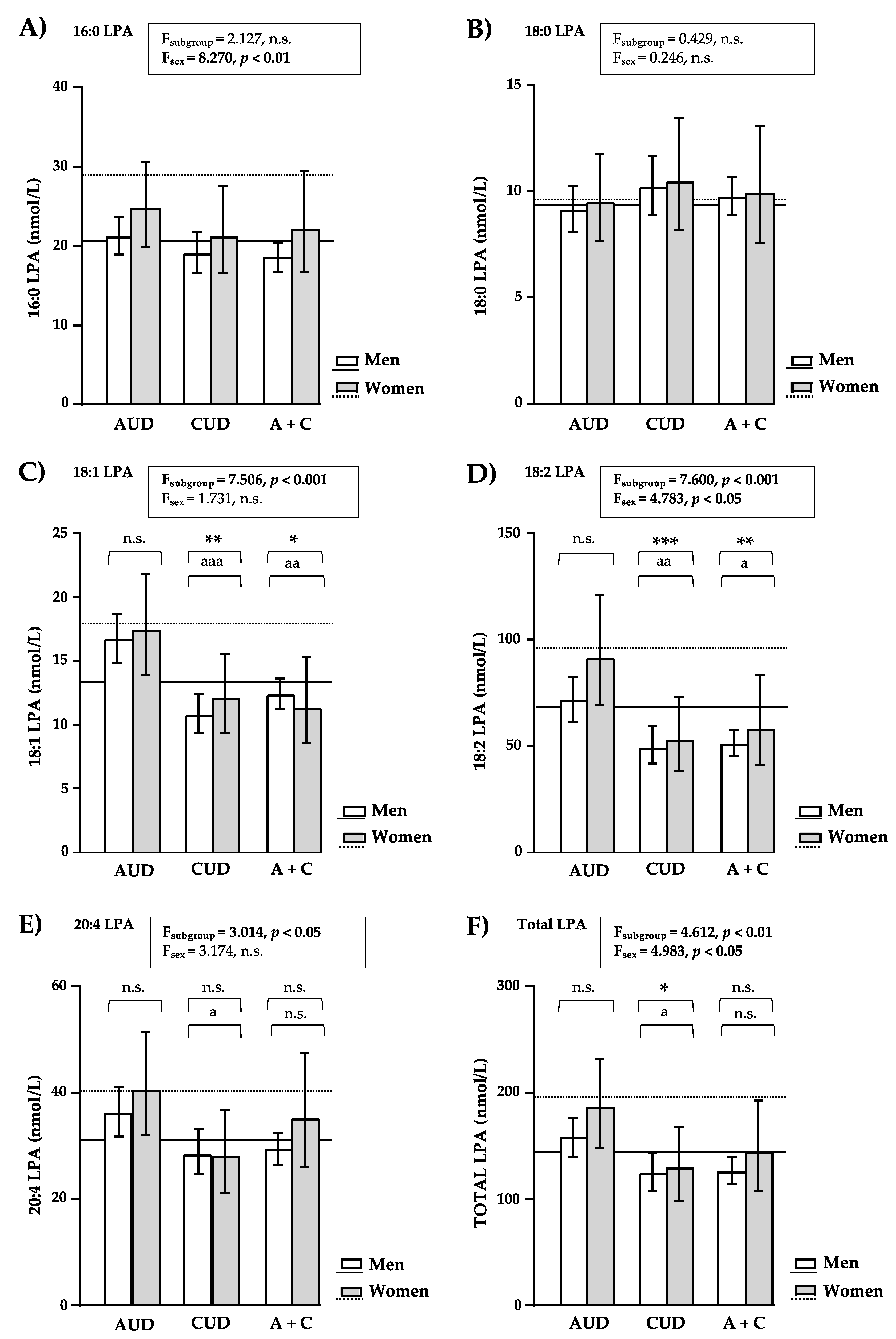
3.4. LPA Concentrations Based on Sample Subgroup and Sex
3.4.1. LPA Concentrations in Patients with AUD and/or CUD
3.4.2. LPA Concentrations in Men and Women of the Subgroups of Patients with SUD
3.5. Clinical and Psychiatric Variables of the Subgroups of SUD
3.5.1. Characterization Based on the Type of SUD
3.5.2. Characterization Based on Sex
4. Discussion
5. Limitations and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, J.W.; Herr, D.R.; Noguchi, K.; Yung, Y.C.; Lee, C.W.; Mutoh, T.; Lin, M.E.; Teo, S.T.; Park, K.E.; Mosley, A.N.; et al. LPA receptors: Subtypes and biological actions. Ann. Rev. Pharmacol. Toxicol. 2010, 50, 157–186. [Google Scholar] [CrossRef] [PubMed]
- Yung, Y.C.; Stoddard, N.C.; Mirendil, H.; Chun, J. Lysophosphatidic Acid signaling in the nervous system. Neuron 2015, 85, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Yung, Y.C.; Stoddard, N.C.; Chun, J. LPA receptor signaling: Pharmacology, physiology, and pathophysiology. J. Lipid Res. 2014, 55, 1192–1214. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Huang, Y.; Johansson, S. G-Protein-Coupled Lysophosphatidic Acid Receptors and Their Regulation of AKT Signaling. Int. J. Mol. Sci. 2016, 17, 215. [Google Scholar] [CrossRef]
- Aoki, J.; Taira, A.; Takanezawa, Y.; Kishi, Y.; Hama, K.; Kishimoto, T.; Mizuno, K.; Saku, K.; Taguchi, R.; Arai, H. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J. Biol. Chem. 2002, 277, 48737–48744. [Google Scholar] [CrossRef]
- Baker, D.L.; Umstot, E.S.; Desiderio, D.M.; Tigyi, G.J. Quantitative analysis of lysophosphatidic acid in human blood fractions. Ann. N. Y. Acad. Sci. 2000, 905, 267–269. [Google Scholar] [CrossRef]
- Bandoh, K.; Aoki, J.; Taira, A.; Tsujimoto, M.; Arai, H.; Inoue, K. Lysophosphatidic acid (LPA) receptors of the EDG family are differentially activated by LPA species. Structure-activity relationship of cloned LPA receptors. FEBS Lett. 2000, 478, 159–165. [Google Scholar] [CrossRef]
- Baker, D.L.; Desiderio, D.M.; Miller, D.D.; Tolley, B.; Tigyi, G.J. Direct quantitative analysis of lysophosphatidic acid molecular species by stable isotope dilution electrospray ionization liquid chromatography-mass spectrometry. Anal. Biochem. 2001, 292, 287–295. [Google Scholar] [CrossRef]
- Choi, J.W.; Chun, J. Lysophospholipids and their receptors in the central nervous system. Biochim. Biophys. Acta 2013, 1831, 20–32. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Yin, N.; Zhang, J. The Expression Regulation and Biological Function of Autotaxin. Cells 2021, 10, 939. [Google Scholar] [CrossRef]
- Kurano, M.; Suzuki, A.; Inoue, A.; Tokuhara, Y.; Kano, K.; Matsumoto, H.; Igarashi, K.; Ohkawa, R.; Nakamura, K.; Dohi, T.; et al. Possible involvement of minor lysophospholipids in the increase in plasma lysophosphatidic acid in acute coronary syndrome. Arter. Thromb. Vasc. Biol. 2015, 35, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Crack, P.J.; Zhang, M.; Morganti-Kossmann, M.C.; Morris, A.J.; Wojciak, J.M.; Fleming, J.K.; Karve, I.; Wright, D.; Sashindranath, M.; Goldshmit, Y.; et al. Anti-lysophosphatidic acid antibodies improve traumatic brain injury outcomes. J. Neuroinflamm. 2014, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Zhang, W.C.; Zhang, J.L.; Zheng, C.J.; Zhu, H.; Yu, H.M.; Fan, L.M. Plasma levels of lysophosphatidic acid in ovarian cancer versus controls: A meta-analysis. Lipids Health Dis. 2015, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Ikeda, H.; Nakamura, K.; Ohkawa, R.; Kume, Y.; Aoki, J.; Hama, K.; Okudaira, S.; Tanaka, M.; Tomiya, T.; et al. Both plasma lysophosphatidic acid and serum autotaxin levels are increased in chronic hepatitis C. J. Clin. Gastroenterol. 2007, 41, 616–623. [Google Scholar] [CrossRef]
- Herr, K.J.; Herr, D.R.; Lee, C.W.; Noguchi, K.; Chun, J. Stereotyped fetal brain disorganization is induced by hypoxia and requires lysophosphatidic acid receptor 1 (LPA1) signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 15444–15449. [Google Scholar] [CrossRef]
- Mirendil, H.; Thomas, E.A.; De Loera, C.; Okada, K.; Inomata, Y.; Chun, J. LPA signaling initiates schizophrenia-like brain and behavioral changes in a mouse model of prenatal brain hemorrhage. Transl. Psychiatry 2015, 5, e541. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Li, H.; Xu, W.; Guo, X. Evaluation of serum ATX and LPA as potential diagnostic biomarkers in patients with pancreatic cancer. BMC Gastroenterol. 2021, 21, 58. [Google Scholar] [CrossRef]
- Abdul Rahman, M.; Mohamad Haron, D.E.; Hollows, R.J.; Abdul Ghani, Z.D.F.; Ali Mohd, M.; Chai, W.L.; Ng, C.C.; Lye, M.S.; Karsani, S.A.; Yap, L.F.; et al. Profiling lysophosphatidic acid levels in plasma from head and neck cancer patients. PeerJ 2020, 8, e9304. [Google Scholar] [CrossRef]
- Castilla-Ortega, E.; Pavon, F.J.; Sanchez-Marin, L.; Estivill-Torrus, G.; Pedraza, C.; Blanco, E.; Suarez, J.; Santin, L.; Rodriguez de Fonseca, F.; Serrano, A. Both genetic deletion and pharmacological blockade of lysophosphatidic acid LPA1 receptor results in increased alcohol consumption. Neuropharmacology 2016, 103, 92–103. [Google Scholar] [CrossRef]
- Sanchez-Marin, L.; Ladron de Guevara-Miranda, D.; Manas-Padilla, M.C.; Alen, F.; Moreno-Fernandez, R.D.; Diaz-Navarro, C.; Perez-Del Palacio, J.; Garcia-Fernandez, M.; Pedraza, C.; Pavon, F.J.; et al. Systemic blockade of LPA1/3 lysophosphatidic acid receptors by ki16425 modulates the effects of ethanol on the brain and behavior. Neuropharmacology 2018, 133, 189–201. [Google Scholar] [CrossRef]
- Ladron de Guevara-Miranda, D.; Moreno-Fernandez, R.D.; Gil-Rodriguez, S.; Rosell-Valle, C.; Estivill-Torrus, G.; Serrano, A.; Pavon, F.J.; Rodriguez de Fonseca, F.; Santin, L.J.; Castilla-Ortega, E. Lysophosphatidic acid-induced increase in adult hippocampal neurogenesis facilitates the forgetting of cocaine-contextual memory. Addict. Biol. 2019, 24, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Bilbao, A.; Luque-Rojas, M.J.; Palomino, A.; Bermudez-Silva, F.J.; Suarez, J.; Santin, L.J.; Estivill-Torrus, G.; Gutierrez, A.; Campos-Sandoval, J.A.; et al. Attenuation of cocaine-induced conditioned locomotion is associated with altered expression of hippocampal glutamate receptors in mice lacking LPA1 receptors. Psychopharmacology 2012, 220, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Flores-Lopez, M.; Garcia-Marchena, N.; Pavon, F.J.; Lara, E.; Porras-Perales, O.; Araos, P.; Requena-Ocana, N.; Torres-Galvan, S.; Manas-Padilla, M.C.; Rubio, G.; et al. Plasma Concentrations of Lysophosphatidic Acid and Autotaxin in Abstinent Patients with Alcohol Use Disorder and Comorbid Liver Disease. Biomedicines 2021, 9, 1207. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Marchena, N.; Pizarro, N.; Pavon, F.J.; Martinez-Huelamo, M.; Flores-Lopez, M.; Requena-Ocana, N.; Araos, P.; Silva-Pena, D.; Suarez, J.; Santin, L.J.; et al. Potential association of plasma lysophosphatidic acid (LPA) species with cognitive impairment in abstinent alcohol use disorders outpatients. Sci. Rep. 2020, 10, 17163. [Google Scholar] [CrossRef]
- Michalczyk, A.; Budkowska, M.; Dolegowska, B.; Chlubek, D.; Safranow, K. Lysophosphatidic acid plasma concentrations in healthy subjects: Circadian rhythm and associations with demographic, anthropometric and biochemical parameters. Lipids Health Dis. 2017, 16, 140. [Google Scholar] [CrossRef]
- Hosogaya, S.; Yatomi, Y.; Nakamura, K.; Ohkawa, R.; Okubo, S.; Yokota, H.; Ohta, M.; Yamazaki, H.; Koike, T.; Ozaki, Y. Measurement of plasma lysophosphatidic acid concentration in healthy subjects: Strong correlation with lysophospholipase D activity. Ann. Clin. Biochem. 2008, 45, 364–368. [Google Scholar] [CrossRef]
- Quigley, J.A.; Logsdon, M.K.; Turner, C.A.; Gonzalez, I.L.; Leonardo, N.B.; Becker, J.B. Sex differences in vulnerability to addiction. Neuropharmacology 2021, 187, 108491. [Google Scholar] [CrossRef]
- Flores-Bonilla, A.; Richardson, H.N. Sex Differences in the Neurobiology of Alcohol Use Disorder. Alcohol Res. 2020, 40, 4. [Google Scholar] [CrossRef]
- Harp, S.J.; Martini, M.; Lynch, W.J.; Rissman, E.F. Sexual Differentiation and Substance Use: A Mini-Review. Endocrinology 2020, 161, bqaa129. [Google Scholar] [CrossRef]
- Torrens, M.; Serrano, D.; Astals, M.; Perez-Dominguez, G.; Martin-Santos, R. Diagnosing comorbid psychiatric disorders in substance abusers: Validity of the Spanish versions of the Psychiatric Research Interview for Substance and Mental Disorders and the Structured Clinical Interview for DSM-IV. Am. J. Psychiatry 2004, 161, 1231–1237. [Google Scholar] [CrossRef]
- Hasin, D.; Samet, S.; Nunes, E.; Meydan, J.; Matseoane, K.; Waxman, R. Diagnosis of comorbid psychiatric disorders in substance users assessed with the Psychiatric Research Interview for Substance and Mental Disorders for DSM-IV. Am. J. Psychiatry 2006, 163, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Robins, L.N.; Wing, J.; Wittchen, H.U.; Helzer, J.E.; Babor, T.F.; Burke, J.; Farmer, A.; Jablenski, A.; Pickens, R.; Regier, D.A.; et al. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch. Gen. Psychiatry 1988, 45, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Galvan, S.T.; Flores-Lopez, M.; Romero-Sanchiz, P.; Requena-Ocana, N.; Porras-Perales, O.; Nogueira-Arjona, R.; Mayoral, F.; Araos, P.; Serrano, A.; Muga, R.; et al. Plasma concentrations of granulocyte colony-stimulating factor (G-CSF) in patients with substance use disorders and comorbid major depressive disorder. Sci. Rep. 2021, 11, 13629. [Google Scholar] [CrossRef] [PubMed]
- Kano, K.; Matsumoto, H.; Kono, N.; Kurano, M.; Yatomi, Y.; Aoki, J. Suppressing postcollection lysophosphatidic acid metabolism improves the precision of plasma LPA quantification. J. Lipid Res. 2021, 62, 100029. [Google Scholar] [CrossRef]
- McHugh, R.K.; Votaw, V.R.; Sugarman, D.E.; Greenfield, S.F. Sex and gender differences in substance use disorders. Clin. Psychol. Rev. 2018, 66, 12–23. [Google Scholar] [CrossRef]
- Ait-Daoud, N.; Blevins, D.; Khanna, S.; Sharma, S.; Holstege, C.P.; Amin, P. Women and Addiction: An Update. Med. Clin. North Am. 2019, 103, 699–711. [Google Scholar] [CrossRef]
- Grella, C.E. From generic to gender-responsive treatment: Changes in social policies, treatment services, and outcomes of women in substance abuse treatment. J. Psychoact. Drugs 2008, 40 (Suppl. S5), 327–343. [Google Scholar] [CrossRef]
- Pedraz, M.; Araos, P.; Garcia-Marchena, N.; Serrano, A.; Romero-Sanchiz, P.; Suarez, J.; Castilla-Ortega, E.; Mayoral-Cleries, F.; Ruiz, J.J.; Pastor, A.; et al. Sex differences in psychiatric comorbidity and plasma biomarkers for cocaine addiction in abstinent cocaine-addicted subjects in outpatient settings. Front. Psychiatry 2015, 6, 17. [Google Scholar] [CrossRef]
- Yao, C.S.; Yan, S.G.; Gao, L.S.; Sun, Z.R.; Liu, F.; Jiang, B.; Wang, H.B.; Wu, Q.Z. Patients with risk factors have higher plasma levels of lysophosphatidic acid: A promising surrogate marker for blood platelet activation. Blood Coagul. Fibrinolysis 2014, 25, 322–325. [Google Scholar] [CrossRef]
- Castilla-Ortega, E.; Escuredo, L.; Bilbao, A.; Pedraza, C.; Orio, L.; Estivill-Torrus, G.; Santin, L.J.; de Fonseca, F.R.; Pavon, F.J. 1-Oleoyl lysophosphatidic acid: A new mediator of emotional behavior in rats. PLoS ONE 2014, 9, e85348. [Google Scholar] [CrossRef]
- Moreno-Fernandez, R.D.; Perez-Martin, M.; Castilla-Ortega, E.; Rosell Del Valle, C.; Garcia-Fernandez, M.I.; Chun, J.; Estivill-Torrus, G.; Rodriguez de Fonseca, F.; Santin, L.J.; Pedraza, C. maLPA1-null mice as an endophenotype of anxious depression. Transl. Psychiatry 2017, 7, e1077. [Google Scholar] [CrossRef] [PubMed]
- Orio, L.; Pavon, F.J.; Blanco, E.; Serrano, A.; Araos, P.; Pedraz, M.; Rivera, P.; Calado, M.; Suarez, J.; de Fonseca, F.R. Lipid transmitter signaling as a new target for treatment of cocaine addiction: New roles for acylethanolamides and lysophosphatidic acid. Curr. Pharm. Des. 2013, 19, 7036–7049. [Google Scholar] [CrossRef] [PubMed]
- Requena-Ocana, N.; Flores-Lopez, M.; Martin, A.S.; Garcia-Marchena, N.; Pedraz, M.; Ruiz, J.J.; Serrano, A.; Suarez, J.; Pavon, F.J.; de Fonseca, F.R.; et al. Influence of gender and education on cocaine users in an outpatient cohort in Spain. Sci. Rep. 2021, 11, 20928. [Google Scholar] [CrossRef] [PubMed]
| VARIABLE | GROUP | p-Value | ||
|---|---|---|---|---|
| CONTROL | SUD | |||
| n = 91 | n = 214 | |||
| Sex (n (%)) | Women Men | 17 (18.7) 74 (81.7) | 35 (16.4) 179 (83.6) | 0.621 a |
| Age (years) | Mean ± SD | 40.9 ± 7.0 | 41.1 ± 9.8 | 0.861 b |
| Median (IQR) | 38.0 (37.0–44.0) | 40.0 (33.0–49.3) | ||
| BMI (kg/m2) | Mean ± SD | 25.5 ± 3.6 | 25.6 ± 4.1 | 0.887 a |
| Median (IQR) | 24.7 (23.3–28.7) | 25.2 (22.8–28.0) | ||
| Marital status (n (%)) | Single Married/cohabiting Divorced/separated Widowed | 22 (24.2) 50 (54.9) 19 (20.9) 0 (0.0) | 82 (38.3) 68 (31.8) 62 (29.0) 2 (0.9) | 0.081 a |
| Education (n (%)) | ≤Primary Secondary Tertiary | 3 (3.3) 52 (57.1) 36 (39.6) | 52 (24.3) 127 (59.3) 35 (16.4) | <0.001 a |
| LPA | GROUP | p-Value | ||
|---|---|---|---|---|
| CONTROL | SUD | |||
| n = 91 | n = 214 | |||
| 16:0-LPA(1-palmitoyl-LPA) (nmol/L) | Median (IQR) | 20.72 (16.53–28.49) | 19.60 (15.98–25.80) | 0.128 a |
| 18:0-LPA (1-stearoyl-LPA) (nmol/L) | Median (IQR) | 9.20 (7.92–10.30) | 9.20 (7.86–10.13) | 0.867 a |
| 18:1-LPA(1-oleoyl-LPA) (nmol/L) | Median (IQR) | 13.70 (9.48–20.20) | 13.31 (9.27–17.63) | 0.198 a |
| 18:2-LPA(1-linoleoyl-LPA) (nmol/L) | Median (IQR) | 70.72 (50.96–104.84) | 58.27 (43.38–87.37) | 0.004 a |
| 20:4-LPA(1-arachidonoyl-LPA) (nmol/L) | Median (IQR) | 30.08 (22.17–47.70) | 32.05 (23.89–41.90) | 0.969 a |
| Total LPA (nmol/L) | Median (IQR) | 145.30 (112.80–211.70) | 137.52 (106.43–177.39) | 0.097 a |
| VARIABLES | Age (Years) | BMI (kg/m2) | |||||
|---|---|---|---|---|---|---|---|
| TOTAL | CONTROL | SUD | TOTAL | CONTROL | SUD | ||
| 16:0-LPA(1-palmitoyl-LPA) (nmol/L) | r | +0.177 | +0.262 | +0.158 | +0.045 | −0.108 | +0.086 |
| p-value | 0.002 | 0.012 | 0.021 | 0.435 | 0.310 | 0.210 | |
| 18:0-LPA (1-stearoyl-LPA) (nmol/L) | r | −0.003 | +0.160 | −0.033 | +0.105 | +0.062 | +0.117 |
| p-value | 0.955 | 0.130 | 0.626 | 0.066 | 0.559 | 0.088 | |
| 18:1-LPA(1-oleoyl-LPA) (nmol/L) | r | +0.414 | +0.412 | +0.418 | +0.027 | −0.060 | +0.054 |
| p-value | <0.001 | <0.001 | <0.001 | 0.645 | 0.573 | 0.435 | |
| 18:2-LPA(1-linoleoyl-LPA) (nmol/L) | r | +0.245 | +0.267 | +0.240 | +0.004 | −0.191 | +0.055 |
| p-value | <0.001 | 0.010 | <0.001 | 0.940 | 0.070 | 0.425 | |
| 20:4-LPA(1-arachidonoyl-LPA) (nmol/L) | r | +0.278 | +0.345 | +0.265 | −0.004 | −0.014 | −0.001 |
| p-value | <0.001 | 0.001 | <0.001 | 0.946 | 0.894 | 0.991 | |
| Total LPA (nmol/L) | r | +0.266 | +0.313 | +0.255 | +0.018 | −0.125 | +0.060 |
| p-value | <0.001 | 0.003 | <0.001 | 0.753 | 0.236 | 0.214 | |
| VARIABLE | SUBGROUP | p-Value | |||
|---|---|---|---|---|---|
| AUD | CUD | AUD + CUD | |||
| n = 73 | n = 48 | n = 93 | |||
| Sex (n (%) | Men Women | 58 (79.5) 15 (20.5) | 37 (77.1) 11 (22.9) | 84 (90.3) 9 (9.7) | 0.065 a |
| Abstinence duration (day) (median (IQR)) | Alcohol | 90 (45–180) | - | 50 (8–150) | 0.053 b |
| Cocaine | - | 15 (4–60) | 21 (7–60) | 0.221 b | |
| DSM criteria for SUD (median (IQR)) | (0–11) AUD | 7.0 (6.0–9.0) | - | 8.0 (6.0–9.0) | 0.495 b |
| (0–11) CUD | - | 8.0 (6.3–10.0) | 8.0 (6.0–10.0) | 0.350 b | |
| Comorbid SUDs (n (%)) | No Yes * | 59 (80.8) 14 (19.2) | 38 (79.2) 10 (20.8) | 61 (65.6) 32 (34.4) | 0.054 a |
| Cannabis Sedatives Stimulants Opioids | 11 (15.1) 2 (2.7) 2 (2.7) 2 (2.7) | 6 (12.5) 3 (6.3) 1 (2.1) 1 (2.1) | 22 (23.7) 12 (12.9) 1 (1.1) 5 (5.4) | ||
| Comorbid psychiatric disorders (n (%)) | No Yes | 32 (43.8) 41 (56.2) | 24 (50.0) 24 (50.0) | 31 (33.3) 62 (66.7) | 0.128 a |
| Mood Psychotic Anxiety Eating Personality | 30 (41.1) 3 (4.1) 24 (32.9) 2 (2.7) 9 (12.3) | 12 (25.0) 2 (4.2) 12 (25.0) 1 (2.1) 14 (29.2) | 32 (34.4) 10 (10.8) 25 (26.9) 5 (5.4) 28 (30.1) | ||
| Psychotropic medication (last year) (n (%)) | No Yes | 22 (30.1) 51 (69.9) | 20 (41.7) 28 (58.3) | 26 (28.0) 67 (72.0) | 0.237 a |
| Antidepressant Anxiolytic Anticraving Antipsychotic | 30 (41.1) 26 (35.6) 20 (27.4) 3 (4.1) | 14 (29.2) 21 (43.8) 4 (8.3) 4 (8.3) | 39 (41.9) 39 (41.9) 18 (19.4) 11 (11.8) | ||
| Comorbid digestive diseases (n (%)) | No Yes | 49 (67.1) 24 (32.9) | 47 (97.9) 1 (2.1) | 87 (93.5) 6 (6.5) | <0.001 a |
| VARIABLE | SEX | p-Value | ||
|---|---|---|---|---|
| Men | Women | |||
| n = 179 | n = 35 | |||
| SUD (n (%)) | AUD CUD AUD + CUD | 58 (32.4) 37 (20.7) 84 (46.9) | 15 (42.9) 11 (31.4) 9 (25.7) | 0.065 a |
| Abstinence duration (day) (median (IQR)) | Alcohol | 60 (22–150) | 90 (30–225) | 0.401 b |
| Cocaine | 18 (4–60) | 38 (6–139) | 0.114 b | |
| DSM criteria for SUD (median (IQR)) | (0–11) AUD | 7.0 (6.0–9.0) | 8.0 (7.0–10.0) | 0.100 b |
| (0–11) CUD | 8.0 (6.0–10.0) | 8.5 (7.0–10.0) | 0.327 b | |
| Comorbid SUDs (n (%)) | No Yes | 128 (71.5) 51 (28.5) | 30 (85.7) 5 (14.3) | 0.094 a |
| Cannabis Sedatives Stimulants Opioids | 38 (21.2) 15 (8.4) 3 (1.7) 8 (4.5) | 2 (5.7) 2 (5.7) 1 (2.9) 0 (0.0) | ||
| Comorbid psychiatric disorders (n (%)) | No Yes | 79 (44.1) 100 (55.9) | 8 (22.9) 27 (77.1) | 0.023 a |
| Mood Psychotic Anxiety Eating Personality | 61 (34.1) 12 (6.7) 42 (23.5) 1 (0.6) 39 (21.8) | 13 (37.1) 3 (8.6) 19 (54.3) 7 (20.0) 12 (34.3) | ||
| Psychotropic medication (last year) (n (%)) | No Yes | 65 (36.3) 114 (63.7) | 3 (8.6) 32 (91.4) | 0.012 a |
| Antidepressant Anxiolytic Anticraving Antipsychotic | 66 (36.9) 65 (36.3) 33 (18.4) 14 (7.8) | 19 (54.3) 21 (60.0) 11 (31.4) 4 (11.4) | ||
| Comorbid digestive diseases (n (%)) | No Yes | 150 (83.8) 29 (16.2) | 34 (97.1) 1 (2.9) | 0.035 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-López, M.; García-Marchena, N.; Araos, P.; Requena-Ocaña, N.; Porras-Perales, O.; Torres-Galván, S.; Suarez, J.; Pizarro, N.; de la Torre, R.; Rubio, G.; et al. Sex Differences in Plasma Lysophosphatidic Acid Species in Patients with Alcohol and Cocaine Use Disorders. Brain Sci. 2022, 12, 588. https://doi.org/10.3390/brainsci12050588
Flores-López M, García-Marchena N, Araos P, Requena-Ocaña N, Porras-Perales O, Torres-Galván S, Suarez J, Pizarro N, de la Torre R, Rubio G, et al. Sex Differences in Plasma Lysophosphatidic Acid Species in Patients with Alcohol and Cocaine Use Disorders. Brain Sciences. 2022; 12(5):588. https://doi.org/10.3390/brainsci12050588
Chicago/Turabian StyleFlores-López, María, Nuria García-Marchena, Pedro Araos, Nerea Requena-Ocaña, Oscar Porras-Perales, Sandra Torres-Galván, Juan Suarez, Nieves Pizarro, Rafael de la Torre, Gabriel Rubio, and et al. 2022. "Sex Differences in Plasma Lysophosphatidic Acid Species in Patients with Alcohol and Cocaine Use Disorders" Brain Sciences 12, no. 5: 588. https://doi.org/10.3390/brainsci12050588
APA StyleFlores-López, M., García-Marchena, N., Araos, P., Requena-Ocaña, N., Porras-Perales, O., Torres-Galván, S., Suarez, J., Pizarro, N., de la Torre, R., Rubio, G., Ruiz-Ruiz, J. J., Rodríguez de Fonseca, F., Serrano, A., & Pavón-Morón, F. J. (2022). Sex Differences in Plasma Lysophosphatidic Acid Species in Patients with Alcohol and Cocaine Use Disorders. Brain Sciences, 12(5), 588. https://doi.org/10.3390/brainsci12050588









