The Cost of Imagined Actions in a Reward-Valuation Task
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
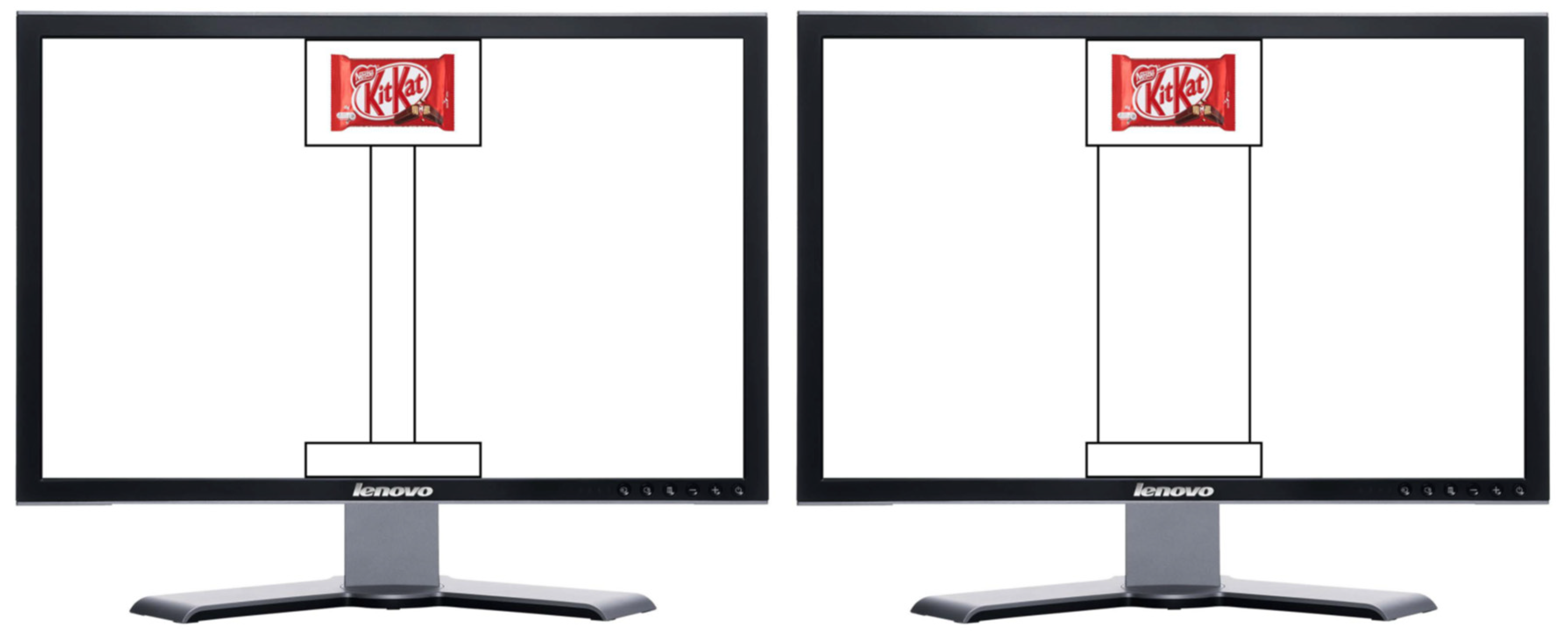
2.2. Apparatus and Stimuli
2.3. Tasks
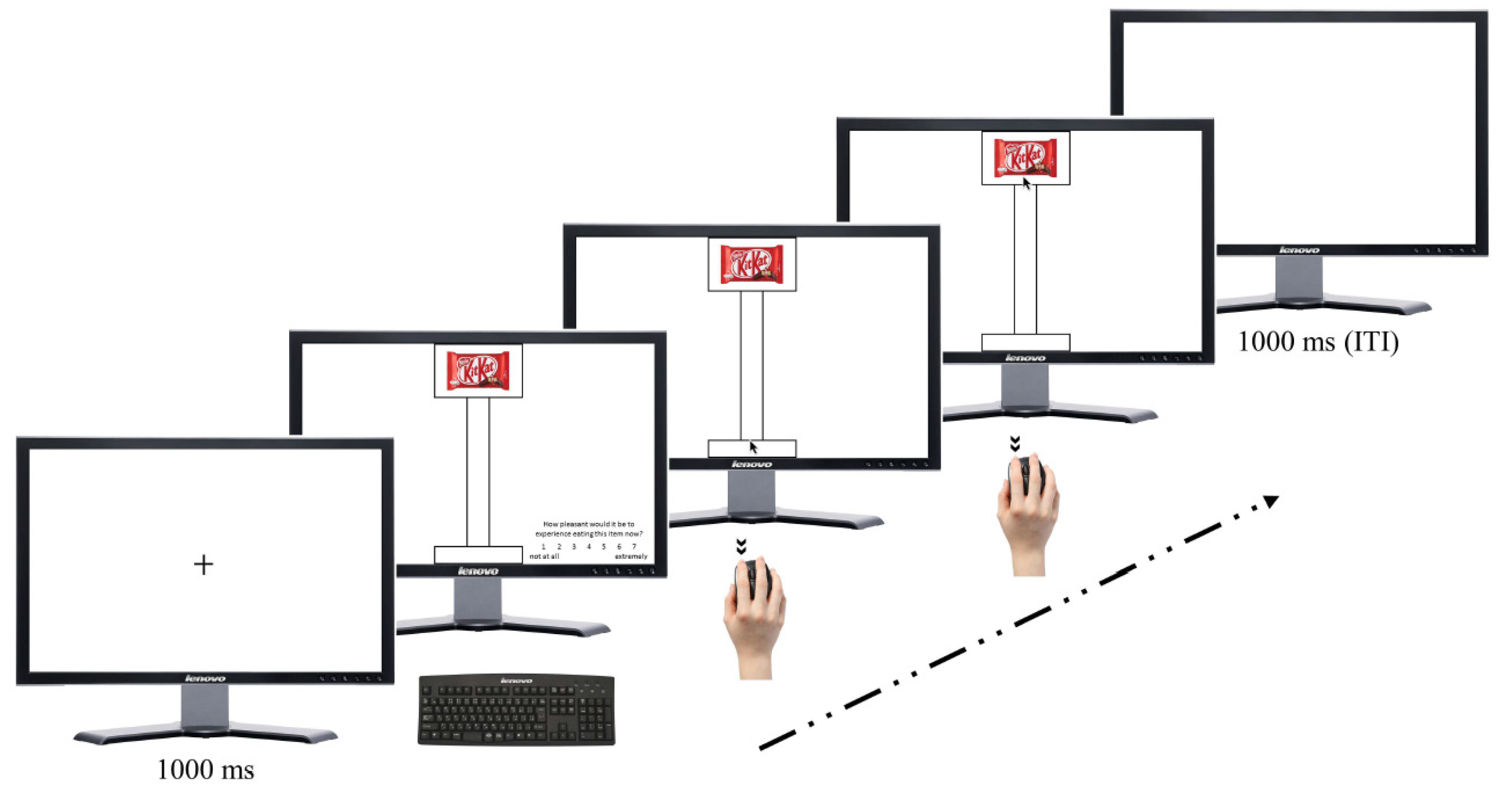
2.3.1. Experimental Valuation Task
2.3.2. Control Valuation Task
2.4. Experimental Procedures
2.5. Statistical Analyses
3. Results
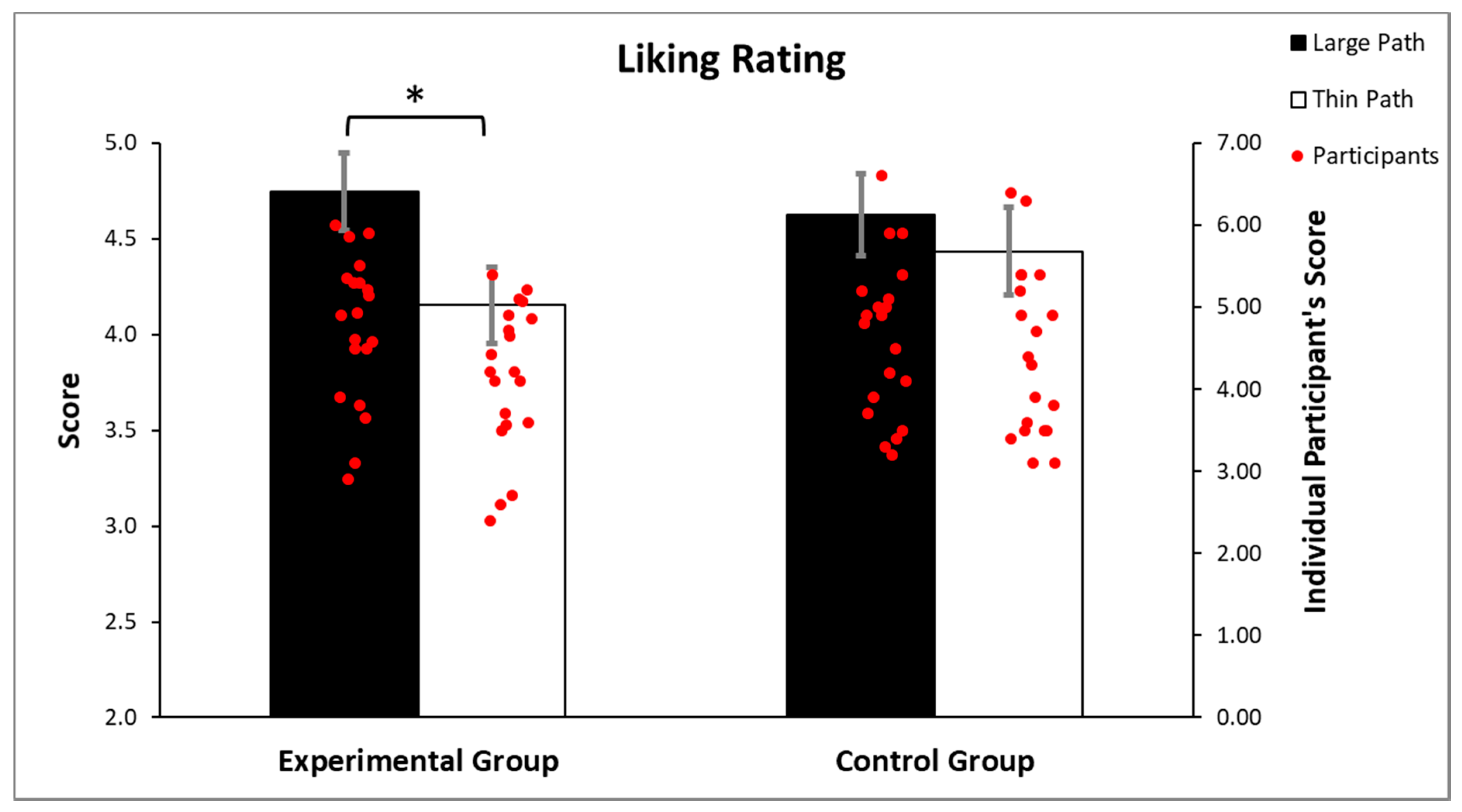
3.1. Liking
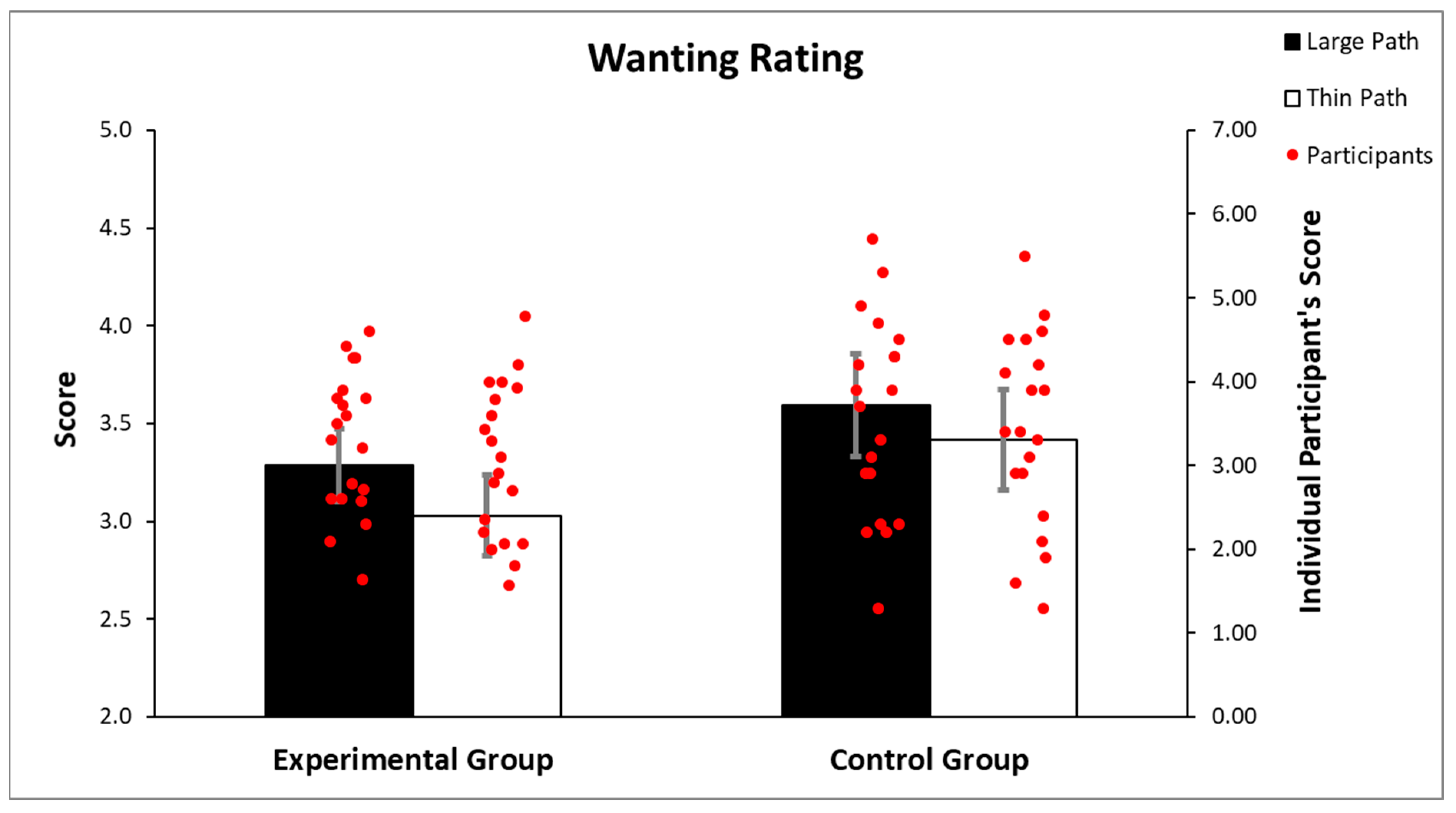
3.2. Wanting
3.3. Response Time
3.4. Questionnaires and Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walton, M.E.; Kennerley, S.W.; Bannerman, D.M.; Phillips, P.E.M.; Rushworth, M.F.S. Weighing up the Benefits of Work: Behavioral and Neural Analyses of Effort-Related Decision Making. Neural Netw. 2006, 19, 1302–1314. [Google Scholar] [CrossRef]
- Kolling, N.; Behrens, T.E.; Mars, R.B.; Rushworth, M.F. Neural Mechanisms of Foraging. Science 2012, 336, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Shenhav, A.; Straccia, M.A.; Cohen, J.D.; Botvinick, M.M. Anterior Cingulate Engagement in a Foraging Context Reflects Choice Difficulty, Not Foraging Value. Nat. Neurosci. 2014, 17, 1249–1254. [Google Scholar] [CrossRef]
- Seinstra, M.S.; Sellitto, M.; Kalenscher, T. Rate Maximization and Hyperbolic Discounting in Human Experiential Intertemporal Decision Making. Behav. Ecol. 2018, 29, 193–203. [Google Scholar] [CrossRef]
- Rangel, A.; Camerer, C.; Montague, P.R. A Framework for Studying the Neurobiology of Value-Based Decision Making. Nat. Rev. Neurosci. 2008, 9, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Botvinick, M.M.; Huffstetler, S.; McGuire, J.T. Effort Discounting in Human Nucleus Accumbens. Cogn. Affect. Behav. Neurosci. 2009, 9, 16–27. [Google Scholar] [CrossRef]
- Massar, S.A.A.; Lim, J.; Sasmita, K.; Chee, M.W.L. Rewards Boost Sustained Attention through Higher Effort: A Value-Based Decision Making Approach. Biol. Psychol. 2016, 120, 21–27. [Google Scholar] [CrossRef]
- Frömer, R.; Lin, H.; Wolf, C.K.D.; Inzlicht, M.; Shenhav, A. Expectations of Reward and Efficacy Guide Cognitive Control Allocation. Nat. Commun. 2021, 12, 1030. [Google Scholar] [CrossRef]
- Phillips, P.E.M.; Walton, M.E.; Jhou, T.C. Calculating Utility: Preclinical Evidence for Cost-Benefit Analysis by Mesolimbic Dopamine. Psychopharmacol 2007, 191, 483–495. [Google Scholar] [CrossRef]
- Rudebeck, P.H.; Walton, M.E.; Smyth, A.N.; Bannerman, D.M.; Rushworth, M.F.S. Separate Neural Pathways Process Different Decision Costs. Nat. Neurosci. 2006, 9, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Prévost, C.; Pessiglione, M.; Metereau, E.; Clery-Melin, M.-L.; Dreher, J.-C. Separate Valuation Subsystems for Delay and Effort Decision Costs. J. Neurosci. 2010, 30, 14080–14090. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, S.; Finotti, G.; Starita, F.; Bouchard, A.E.; Fecteau, S. Effort-Based Decision Making. The Sage Handbook of Cognitive and Systems Neuroscience II, 2022; in press. [Google Scholar]
- Apps, M.A.J.; Grima, L.L.; Manohar, S.; Husain, M. The Role of Cognitive Effort in Subjective Reward Devaluation and Risky Decision-Making. Sci. Rep. 2015, 5, 16880. [Google Scholar] [CrossRef]
- Vassena, E.; Deraeve, J.; Alexander, W.H. Predicting Motivation: Computational Models of PFC Can Explain Neural Coding of Motivation and Effort-Based Decision-Making in Health and Disease. J. Cogn. Neurosci. 2017, 29, 1633–1645. [Google Scholar] [CrossRef]
- Ainslie, G. Specious Reward: A Behavioral Theory of Impulsiveness and Impulse Control. Psychol. Bull. 1975, 82, 463–496. [Google Scholar] [CrossRef]
- Myerson, J.; Green, L. Discounting of Delayed Rewards: Models of Individual Choice. J. Exp. Anal. Behav. 1995, 64, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Frederick, S.; Loewenstein, G.; O’Donoghue, T. Time Discounting and Preference: A Critical Review. J. Econ. Lit. 2002, 40, 351–401. [Google Scholar] [CrossRef]
- Sellitto, M.; Ciaramelli, E.; Pellegrino, G. The Neurobiology of Intertemporal Choice: Insight from Imaging and Lesion Studies. Rev. Neurosci. 2011, 22, 565–574. [Google Scholar] [CrossRef]
- Guleken, Z.; Sutcubasi, B.; Metin, B. The Cognitive Dynamics of Small-Sooner over Large-Later Preferences during Temporal Discounting Task through Event-Related Oscillations (EROs). Neuropsychologia 2021, 162, 108046. [Google Scholar] [CrossRef]
- Shadmehr, R.; Orban de Xivry, J.J.; Xu-Wilson, M.; Shih, T.-Y. Temporal Discounting of Reward and the Cost of Time in Motor Control. J. Neurosci. 2010, 30, 10507–10516. [Google Scholar] [CrossRef]
- Berret, B.; Jean, F. Why Don’t We Move Slower? The Value of Time in the Neural Control of Action. J. Neurosci. 2016, 36, 1056–1070. [Google Scholar] [CrossRef]
- Shadmehr, R.; Huang, H.J.; Ahmed, A.A. A Representation of Effort in Decision-Making and Motor Control. Curr. Biol. 2016, 26, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Shadmehr, R. Control of Movements and Temporal Discounting of Reward. Curr. Opin. Neurobiol. 2010, 20, 726–730. [Google Scholar] [CrossRef]
- Choi, J.E.S.; Vaswani, P.A.; Shadmehr, R. Vigor of Movements and the Cost of Time in Decision Making. J. Neurosci. 2014, 34, 1212–1223. [Google Scholar] [CrossRef] [PubMed]
- Schultz, W. Dopamine Signals for Reward Value and Risk: Basic and Recent Data. Behav. Brain Funct. 2010, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Grogan, J.P.; Sandhu, T.R.; Hu, M.T.; Manohar, S.G. Dopamine Promotes Instrumental Motivation, but Reduces Reward-Related Vigour. Elife 2020, 9, e58321. [Google Scholar] [CrossRef]
- Sedaghat-Nejad, E.; Herzfeld, D.J.; Shadmehr, R. Reward Prediction Error Modulates Saccade Vigor. J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 5010–5017. [Google Scholar] [CrossRef]
- Shadmehr, R.; Reppert, T.R.; Summerside, E.M.; Yoon, T.; Ahmed, A.A. Movement Vigor as a Reflection of Subjective Economic Utility. Trends Neurosci. 2019, 42, 323–336. [Google Scholar] [CrossRef]
- Fitts, P.M. The Information Capacity of the Human Motor System in Controlling the Amplitude of Movement. J. Exp. Psychol. 1954, 47, 381–391. [Google Scholar] [CrossRef]
- Jeannerod, M. The Representing Brain: Neural Correlates of Motor Intention and Imagery. Behav. Brain Sci. 1994, 17, 187–202. [Google Scholar] [CrossRef]
- Paszkiel, S.; Dobrakowski, P. Brain—Computer Technology-Based Training System in the Field of Motor Imagery. IET Sci. Meas. Technol. 2021, 14, 1014–1018. [Google Scholar] [CrossRef]
- Hwang, H.-J.; Kwon, K.; Im, C.-H. Neurofeedback-Based Motor Imagery Training for Brain-Computer Interface (BCI). J. Neurosci. Methods 2009, 179, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Paszkiel, S.; Rojek, R.; Lei, N.; Castro, M. Review of Solutions for the Application of Example of Machine Learning Methods for Motor Imagery in Correlation with Brain-Computer Interfaces. Przegląd Elektrotechniczny 2021, 1, 113–118. [Google Scholar] [CrossRef]
- Mane, R.; Robinson, N.; Vinod, A.P.; Lee, S.-W.; Guan, C. A Multi-View CNN with Novel Variance Layer for Motor Imagery Brain Computer Interface. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 2950–2953. [Google Scholar] [CrossRef]
- Pawus, D.; Paszkiel, S. The Application of Integration of EEG Signals for Authorial Classification Algorithms in Implementation for a Mobile Robot Control Using Movement Imagery—Pilot Study. Appl. Sci. 2022, 12, 2161. [Google Scholar] [CrossRef]
- Decety, J.; Jeannerod, M.; Prablanc, C. The Timing of Mentally Represented Actions. Behav. Brain Res. 1989, 34, 35–42. [Google Scholar] [CrossRef]
- Porro, C.A.; Francescato, M.P.; Cettolo, V.; Diamond, M.E.; Baraldi, P.; Zuiani, C.; Bazzocchi, M.; Di Prampero, P.E. Primary Motor and Sensory Cortex Activation during Motor Performance and Motor Imagery: A Functional Magnetic Resonance Imaging Study. J. Neurosci. 1996, 16, 7688–7698. [Google Scholar] [CrossRef]
- Decety, J. The Neurophysiological Basis of Motor Imagery. Behav. Brain Res. 1996, 77, 45–52. [Google Scholar] [CrossRef]
- Batula, A.M.; Mark, J.A.; Kim, Y.E.; Ayaz, H. Comparison of Brain Activation during Motor Imagery and Motor Movement Using FNIRS. Comput. Intell. Neurosci. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Decety, J.; Jeannerod, M. Mentally Simulated Movements in Virtual Reality: Does Fitt’s Law Hold in Motor Imagery? Behav. Brain Res. 1995, 72, 127–134. [Google Scholar] [CrossRef]
- Sirigu, A.; Duhamel, J.; Cohen, L.; Pillon, B.; Dubois, B.; Agid, Y. The Mental Representation of Hand Movements after Parietal Cortex Damage. Science 1996, 273, 1564–1568. [Google Scholar] [CrossRef]
- Dahm, S.F.; Rieger, M. Cognitive Constraints on Motor Imagery. Psychol. Res. 2016, 80, 235–247. [Google Scholar] [CrossRef]
- Bertucco, M.; Cesari, P.; Latash, M.L. Fitts’ Law in Early Postural Adjustments. Neuroscience 2013, 231, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Cisek, P.; Kalaska, J.F. Neural Mechanisms for Interacting with a World Full of Action Choices. Annu. Rev. Neurosci. 2010, 33, 269–298. [Google Scholar] [CrossRef]
- Huda, R.; Goard, M.J.; Pho, G.N.; Sur, M. Neural Mechanisms of Sensorimotor Transformation and Action Selection. Eur. J. Neurosci. 2019, 49, 1055–1060. [Google Scholar] [CrossRef]
- Borgomaneri, S.; Serio, G.; Battaglia, S. Please, Don’t Do It! Fifteen Years of Progress of Non-Invasive Brain Stimulation in Action Inhibition. Cortex 2020, 132, 404–422. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Serio, G.; Scarpazza, C.; D’Ausilio, A.; Borgomaneri, S. Frozen in (e)Motion: How Reactive Motor Inhibition Is Influenced by the Emotional Content of Stimuli in Healthy and Psychiatric Populations. Behav. Res. Ther. 2021, 146, 103963. [Google Scholar] [CrossRef] [PubMed]
- O’Doherty, J.; Dayan, P.; Schultz, J.; Deichmann, R.; Friston, K.; Dolan, R.J. Dissociable Roles of Ventral and Dorsal Striatum in Instrumental Conditioning. Science 2004, 304, 452–454. [Google Scholar] [CrossRef]
- Rich, E.L.; Wallis, J.D. Decoding Subjective Decisions from Orbitofrontal Cortex. Nat. Neurosci. 2016, 19, 973–980. [Google Scholar] [CrossRef]
- Ballesta, S.; Shi, W.; Conen, K.E.; Padoa-Schioppa, C. Values Encoded in Orbitofrontal Cortex Are Causally Related to Economic Choices. Nature 2020, 588, 450–453. [Google Scholar] [CrossRef]
- Gluth, S.; Rieskamp, J.; Büchel, C. Classic EEG Motor Potentials Track the Emergence of Value-Based Decisions. Neuroimage 2013, 79, 394–403. [Google Scholar] [CrossRef]
- Gold, J.I.; Shadlen, M.N. The Neural Basis of Decision Making. Annu. Rev. Neurosci. 2007, 30, 535–574. [Google Scholar] [CrossRef]
- Stuphorn, V.; Taylor, T.L.; Schall, J.D. Performance Monitoring by the Supplementary Eye Field. Nature 2000, 408, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Hikosaka, O. Reward-Dependent Gain and Bias of Visual Responses in Primate Superior Colliculus. Neuron 2003, 39, 693–700. [Google Scholar] [CrossRef]
- Roesch, M.R.; Olson, C.R. Impact of Expected Reward on Neuronal Activity in Prefrontal Cortex, Frontal and Supplementary Eye Fields and Premotor Cortex. J. Neurophysiol. 2003, 90, 1766–1789. [Google Scholar] [CrossRef] [PubMed]
- Galaro, J.K.; Celnik, P.; Chib, V.S. Motor Cortex Excitability Reflects the Subjective Value of Reward and Mediates Its Effects on Incentive-Motivated Performance. J. Neurosci. 2019, 39, 1236–1248. [Google Scholar] [CrossRef]
- Donner, T.H.; Siegel, M.; Fries, P.; Engel, A.K. Buildup of Choice-Predictive Activity in Human Motor Cortex during Perceptual Decision Making. Curr. Biol. 2009, 19, 1581–1585. [Google Scholar] [CrossRef]
- Aitken, F.; Turner, G.; Kok, P. Prior Expectations of Motion Direction Modulate Early Sensory Processing. J. Neurosci. 2020, 40, 6389–6397. [Google Scholar] [CrossRef]
- Pastor-Bernier, A.; Cisek, P. Neural Correlates of Biased Competition in Premotor Cortex. J. Neurosci. 2011, 31, 7083–7088. [Google Scholar] [CrossRef]
- Gupta, N.; Aron, A.R. Urges for Food and Money Spill over into Motor System Excitability before Action Is Taken. Eur. J. Neurosci. 2011, 33, 183–188. [Google Scholar] [CrossRef][Green Version]
- Borgomaneri, S.; Vitale, F.; Battaglia, S.; Avenanti, A. Early Right Motor Cortex Response to Happy and Fearful Facial Expressions: A TMS Motor-Evoked Potential Study. Brain Sci. 2021, 11, 1203. [Google Scholar] [CrossRef]
- Domínguez-Zamora, F.J.; Marigold, D.S. Motor Cost Affects the Decision of When to Shift Gaze for Guiding Movement. J. Neurophysiol. 2019, 122, 378–388. [Google Scholar] [CrossRef]
- Vourvopoulos, A.; Bermudez, S.; Liarokapis, F. EEG Correlates of Video Game Experience and User Profile in Motor-Imagery-Based Brain—Computer Interaction. Vis. Comput. 2017, 33, 533–546. [Google Scholar] [CrossRef]
- Klement, J.; Kubera, B.; Eggeling, J.; Rädel, C.; Wagner, C.; Park, S.Q.; Peters, A. Effects of Blood Glucose on Delay Discounting, Food Intake and Counterregulation in Lean and Obese Men. Psychoneuroendocrinology 2018, 89, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Massar, S.A.A.; Pu, Z.; Chen, C.; Chee, M.W.L. Losses Motivate Cognitive Effort More Than Gains in Effort-Based Decision Making and Performance. Front. Hum. Neurosci. 2020, 14, 287. [Google Scholar] [CrossRef]
- Bowyer, C.; Brush, C.J.; Threadgill, H.; Harmon-Jones, E.; Treadway, M.; Patrick, C.J.; Hajcak, G. The Effort-Doors Task: Examining the Temporal Dynamics of Effort-Based Reward Processing Using ERPs. Neuroimage 2021, 228, 117656. [Google Scholar] [CrossRef]
- Smalley, K.; Knerr, N.; Kendrick, V.; Colliver, A.; Owen, O. Reassessment of Body Mass Indices. Am. J. Clin. Nutr. 1990, 52, 405–408. [Google Scholar] [CrossRef]
- Bakker, M.; De Lange, F.P.; Stevens, J.A.; Toni, I.; Bloem, B.R. Motor Imagery of Gait: A Quantitative Approach. Exp. Brain Res. 2007, 179, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, G.; King, N.; Blundell, J. The Role of Implicit Wanting in Relation to Explicit Liking and Wanting for Food: Implications for Appetite Control. Appetite 2008, 50, 120–127. [Google Scholar] [CrossRef]
- Born, J.M.; Lemmens, S.G.T.; Martens, M.J.I.; Goebel, R. Differences between Liking and Wanting Signals in the Human Brain and Relations with Cognitive Dietary Restraint and Body Mass Index. Am. J. Clin. Nutr. 2011, 94, 393–403. [Google Scholar] [CrossRef][Green Version]
- Marks, D.F. Visual Imagery Differences in the Recall of Pictures. Br. J. Psychol. 1973, 64, 17–24. [Google Scholar] [CrossRef]
- Isaac, A.; Marks, D.F.; Russell, D.G. An Instrument for Assessing Imagery of Movement: The Vividness of Movement Imagery Questionnaire (VMIQ). J. Ment. Imag. 1986, 10, 23–30. [Google Scholar]
- Glimcher, P.W. Decisions, Decisions, Decisions: Choosing a Biological Science of Choice. Neuron 2002, 36, 323–332. [Google Scholar] [CrossRef]
- Kennerley, S.W.; Dahmubed, A.F.; Lara, A.H.; Wallis, J.D. Neurons in the Frontal Lobe Encode the Value of Multiple Decision Variables. J. Cogn. Neurosci. 2009, 21, 1162–1178. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, S.; Battaglia, S.; Di Pellegrino, G. Individual Differences in Working Memory Capacity and Cue-Guided Behavior in Humans. Sci. Rep. 2019, 9, 7327. [Google Scholar] [CrossRef]
- Garofalo, S.; Battaglia, S.; Starita, F.; di Pellegrino, G. Modulation of Cue-Guided Choices by Transcranial Direct Current Stimulation. Cortex 2021, 137, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Kahneman, D.; Tversky, A.; Kahneman, B.Y.D.; Tversky, A. Prospect Theory: An Analysis of Decision under Risk Linked. Econometrica 1979, 47, 263–292. [Google Scholar] [CrossRef]
- Rigoli, F.; Martinelli, C.; Shergill, S.S. The Role of Expecting Feedback during Decision-Making under Risk. Neuroimage 2019, 202, 116079. [Google Scholar] [CrossRef] [PubMed]
- Mühlhoff, N.; Stevens, J.R.; Reader, S.M. Spatial Discounting of Food and Social Rewards in Guppies (Poecilia Reticulata). Front. Psychol. 2011, 2, 68. [Google Scholar] [CrossRef]
- Stevens, J.R.; Rosati, A.G.; Ross, K.R.; Hauser, M.D. Will Travel for Food: Spatial Discounting in Two New World Monkeys. Curr. Biol. 2005, 15, 1855–1860. [Google Scholar] [CrossRef]
- Dezfouli, A.; Balleine, B.W. Learning the Structure of the World: The Adaptive Nature of State-Space and Action Representations in Multi-Stage Decision-Making. PLoS Comput. Biol. 2019, 15, e1007334. [Google Scholar] [CrossRef]
- Salamone, J.D.; Correa, M.; Ferrigno, S.; Yang, J.-H.; Rotolo, R.A.; Presby, R.E. The Psychopharmacology of Effort-Related Decision Making: Dopamine, Adenosine, and Insights into the Neurochemistry of Motivation. Pharmacol. Rev. 2018, 70, 747–762. [Google Scholar] [CrossRef]
- Cos, I.; Bélanger, N.; Cisek, P. The Influence of Predicted Arm Biomechanics on Decision Making. J. Neurophysiol. 2011, 105, 3022–3033. [Google Scholar] [CrossRef] [PubMed]
- Cos, I.; Duque, J.; Cisek, P. Rapid Prediction of Biomechanical Costs during Action Decisions. J. Neurophysiol. 2014, 112, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Pierrieau, E.; Lepage, J.-F.; Bernier, P.-M. Action Costs Rapidly and Automatically Interfere with Reward-Based Decision-Making in a Reaching Task. Eneuro 2021, 8, ENEURO.0247-21.2021. [Google Scholar] [CrossRef] [PubMed]
- Pool, E.; Sennwald, V.; Delplanque, S.; Brosch, T.; Sander, D. Measuring Wanting and Liking from Animals to Humans: A Systematic Review. Neurosci. Biobehav. Rev. 2016, 63, 124–142. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, A.; Balleine, B. Motivational Control of Goal-Directed Action. Anim. Learn. Behav. 1994, 22, 1–18. [Google Scholar] [CrossRef]
- Kahneman, D. Choices, Values, and Frames. Am. Psychol. 1984, 39, 341–350. [Google Scholar] [CrossRef]
- Di Gregorio, F.; Ernst, B.; Steinhauser, M. Differential effects of instructed and objective feedback reliability on feedback-related brain activity. Psychophysiology 2019, 56, e13399. [Google Scholar] [CrossRef]
- Berridge, K.C.; O’Doherty, J.P. From Experienced Utility to Decision Utility. Neuroeconomics 2013, 335–354. [Google Scholar] [CrossRef]
- Berridge, K.C.; Robinson, T.E. Parsing Reward. Trends Neurosci. 2003, 26, 507–513. [Google Scholar] [CrossRef]
- Klein-Flügge, M.C.; Kennerley, S.W.; Friston, K.; Bestmann, S. Neural Signatures of Value Comparison in Human Cingulate Cortex during Decisions Requiring an Effort-Reward Trade-Off. J. Neurosci. 2016, 36, 10002–10015. [Google Scholar] [CrossRef]
- Finlayson, G.; King, N.; Blundell, J.E. Is It Possible to Dissociate ‘Liking’ and ‘Wanting’ for Foods in Humans? A Novel Experimental Procedure. Physiol. Behav. 2007, 90, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Telegdy, G.; Adamik, A.; Tanaka, M.; Schally, A.V. Effects of the LHRH Antagonist Cetrorelix on Affective and Cognitive Functions in Rats. Regul. Pept. 2010, 159, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Telegdy, G.; Tanaka, M.; Schally, A.V. Effects of the LHRH Antagonist Cetrorelix on the Brain Function in Mice. Neuropeptides 2009, 43, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Martos, D.; Tuka, B.; Tanaka, M.; Vécsei, L.; Telegdy, G. Memory Enhancement with Kynurenic Acid and Its Mechanisms in Neurotransmission. Biomedicines 2022, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Csabafi, K.; Telegdy, G. Neurotransmissions of Antidepressant-like Effects of Kisspeptin-13. Regul. Pept. 2013, 180, 1–4. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Monitoring the Kynurenine System: Concentrations, Ratios or What Else? Adv. Clin. Exp. Med. 2021, 30, 775–778. [Google Scholar] [CrossRef]
- Telegdy, G.; Tanaka, M.; Schally, A.V. Effects of the Growth Hormone-Releasing Hormone (GH-RH) Antagonist on Brain Functions in Mice. Behav. Brain Res. 2011, 224, 155–158. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Editorial of Special Issue “Crosstalk between Depression, Anxiety, and Dementia: Comorbidity in Behavioral Neurology and Neuropsychiatry”. Biomedicines 2021, 9, 517. [Google Scholar] [CrossRef]
- Spekker, E.; Tanaka, M.; Szabó, Á.; Vécsei, L. Neurogenic Inflammation: The Participant in Migraine and Recent Advancements in Translational Research. Biomedicines 2021, 10, 76. [Google Scholar] [CrossRef]
- Tanaka, M.; Török, N.; Vécsei, L. Are 5-HT(1) Receptor Agonists Effective Anti-Migraine Drugs? Expert Opin. Pharmacother. 2021, 22, 1221–1225. [Google Scholar] [CrossRef]
- Tanaka, M.; Bohár, Z.; Vécsei, L. Are Kynurenines Accomplices or Principal Villains in Dementia? Maintenance of Kynurenine Metabolism. Molecules 2020, 25, 564. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L. The Measurement of Pleasure and Pain. Perspect. Psychol. Sci. 2014, 9, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Pichon, A.M.; Coppin, G.; Cayeux, I.; Porcherot, C.; Sander, D.; Delplanque, S. Sensitivity of Physiological Emotional Measures to Odors Depends on the Product and the Pleasantness Ranges Used. Front. Psychol. 2015, 6, 1821. [Google Scholar] [CrossRef]
- Kivetz, R. The Effects of Effort and Intrinsic Motivation on Risky Choice. Mark. Sci. 2003, 22, 477–502. [Google Scholar] [CrossRef]
- Bushong, B.; King, L.M.; Camerer, C.F.; Rangel, A. Pavlovian Processes in Consumer Choice: The Physical Presence of a Good Increases Willingness-to-Pay. Am. Econ. Rev. 2010, 100, 1556–1571. [Google Scholar] [CrossRef]
- Barsalou, L.W. Perceptual Symbol Systems. Behav. Brain Sci. 1999, 22, 577–660. [Google Scholar] [CrossRef]
- Barsalou, L.W. Simulation, Situated Conceptualization, and Prediction. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1281–1289. [Google Scholar] [CrossRef]
- Niedenthal, P.M.; Barsalou, L.W.; Winkielman, P.; Krauth-Gruber, S.; Ric, F. Embodiment in Attitudes, Social Perception, and Emotion. Personal. Soc. Psychol. Rev. 2005, 9, 184–211. [Google Scholar] [CrossRef]
- Padoa-Schioppa, C. Neurobiology of Economic Choice: A Good-Based Model. Annu. Rev. Neurosci. 2011, 34, 333–359. [Google Scholar] [CrossRef]
- Plassmann, H.; O’Doherty, J.; Rangel, A. Orbitofrontal Cortex Encodes Willingness to Pay in Everyday Economic Transactions. J. Neurosci. 2007, 27, 9984–9988. [Google Scholar] [CrossRef] [PubMed]
- Wallis, J.D. Orbitofrontal Cortex and Its Contribution to Decision-Making. Annu. Rev. Neurosci. 2007, 30, 31–56. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Garofalo, S.; di Pellegrino, G.; Starita, F. Revaluing the Role of VmPFC in the Acquisition of Pavlovian Threat Conditioning in Humans. J. Neurosci. 2020, 40, 8491–8500. [Google Scholar] [CrossRef]
- Battaglia, S.; Harrison, B.J.; Fullana, M.A. Does the Human Ventromedial Prefrontal Cortex Support Fear Learning, Fear Extinction or Both? A Commentary on Subregional Contributions. Mol. Psychiatry 2021, 1–3. [Google Scholar] [CrossRef]
- Battaglia, S. Neurobiological Advances of Learned Fear in Humans. Adv. Clin. Exp. Med. 2022, 31, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Maier, M.E.; Di Gregorio, F.; Muricchio, T.; Di Pellegrino, G. Impaired rapid error monitoring but intact error signaling following rostral anterior cingulate cortex lesions in humans. Front. Hum. Neurosci. 2015, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Thayer, J.F. Functional interplay between central and autonomic nervous systems in human fear conditioning. Trends Neurosci. 2022; in press. [Google Scholar]
- Platt, M.L.; Glimcher, P.W. Neural Correlates of Decision Variables in Parietal Cortex. Nature 1999, 400, 233–238. [Google Scholar] [CrossRef]
- Bari, B.A.; Cohen, J.Y. Dynamic Decision Making and Value Computations in Medial Frontal Cortex. In What Does Medial Frontal Cortex Signal During Behavior? Insights from Behavioral Neurophysiology; Brockett, A.T., Amarante, L.M., Laubach, M., Roesch, M.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 158, pp. 83–113. [Google Scholar] [CrossRef]
- Glimcher, P.W.; Dorris, M.C.; Bayer, H.M. Physiological Utility Theory and the Neuroeconomics of Choice. Games Econ. Behav. 2005, 52, 213–256. [Google Scholar] [CrossRef]
- Denk, F.; Walton, M.E.; Jennings, K.A.; Sharp, T.; Rushworth, M.F.S.; Bannerman, D.M. Differential Involvement of Serotonin and Dopamine Systems in Cost-Benefit Decisions about Delay or Effort. Psychopharmacology 2005, 179, 587–596. [Google Scholar] [CrossRef]
- Floresco, S.B.; Ghods-Sharifi, S. Amygdala-Prefrontal Cortical Circuitry Regulates Effort-Based Decision Making. Cereb. Cortex 2007, 17, 251–260. [Google Scholar] [CrossRef]
- Salamone, J.D.; Correa, M.; Mingote, S.; Weber, S.M. Nucleus Accumbens Dopamine and the Regulation of Effort in Food-Seeking Behavior: Implications for Studies of Natural Motivation, Psychiatry, and Drug Abuse. J. Pharmacol. Exp. Ther. 2003, 305, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.E.; Devlin, J.T.; Rushworth, M.F.S. Interactions between Decision Making and Performance Monitoring within Prefrontal Cortex. Nat. Neurosci. 2004, 7, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Trajkovic, J.; Di Gregorio, F.; Ferri, F.; Marzi, C.; Diciotti, S.; Romei, V. Resting State Alpha Oscillatory Activity Is a Valid and Reliable Marker of Schizotypy. Sci. Rep. 2021, 11, 10379. [Google Scholar] [CrossRef]
- Marciniak, M.A.; Shanahan, L.; Binder, H.; Kalisch, R.; Kleim, B. Positive Prospective Mental Imagery Characteristics in Young Adults and Their Associations with Depressive Symptoms. PsyArXiv, 2022; pre-print. [Google Scholar] [CrossRef]

| Group | n | Age | Education | Hunger | Fasting | BMI |
|---|---|---|---|---|---|---|
| Experimental | 20 | 23.35 (2.16) | 16.8 (1.85) | 2.25 (1.45) | 2.08 (1.34) | 21.49 (2.49) |
| Control | 20 | 23.85 (3.36) | 16.95 (2.46) | 2.75 (1.16) | 2.18 (1.32) | 21.52 (3.16) |
| VVIQ | VMIQ | |||
|---|---|---|---|---|
| Group | Experimental | Control | Experimental | Control |
| Mean | 58.4 | 63.9 | 84.7 | 89.3 |
| SD | 7.93 | 8.41 | 15.75 | 14.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sellitto, M.; Terenzi, D.; Starita, F.; di Pellegrino, G.; Battaglia, S. The Cost of Imagined Actions in a Reward-Valuation Task. Brain Sci. 2022, 12, 582. https://doi.org/10.3390/brainsci12050582
Sellitto M, Terenzi D, Starita F, di Pellegrino G, Battaglia S. The Cost of Imagined Actions in a Reward-Valuation Task. Brain Sciences. 2022; 12(5):582. https://doi.org/10.3390/brainsci12050582
Chicago/Turabian StyleSellitto, Manuela, Damiano Terenzi, Francesca Starita, Giuseppe di Pellegrino, and Simone Battaglia. 2022. "The Cost of Imagined Actions in a Reward-Valuation Task" Brain Sciences 12, no. 5: 582. https://doi.org/10.3390/brainsci12050582
APA StyleSellitto, M., Terenzi, D., Starita, F., di Pellegrino, G., & Battaglia, S. (2022). The Cost of Imagined Actions in a Reward-Valuation Task. Brain Sciences, 12(5), 582. https://doi.org/10.3390/brainsci12050582







