Transcranial Direct Current Stimulation Enhances Cognitive Function in Patients with Mild Cognitive Impairment and Early/Mid Alzheimer’s Disease: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Study Quality Assessment
2.4. Data Extraction and Analysis
3. Results
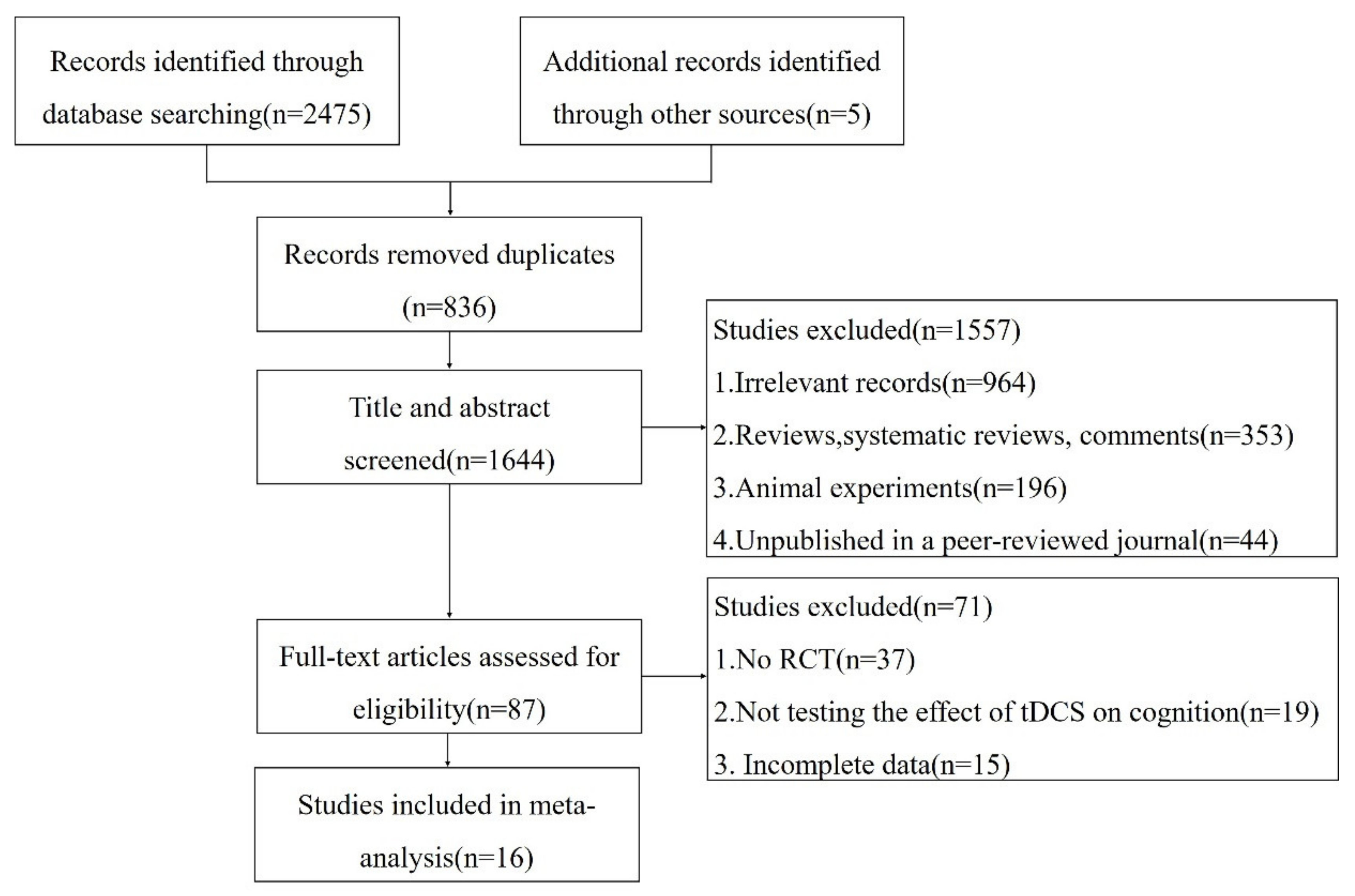
3.1. Study Selection and Characteristics
3.2. Quality Assessment
3.3. Primary Outcome
3.4. MMSE
3.5. ADAS-Cog
3.6. NPI
3.7. Word-Recognition Task
3.8. FDS
3.9. Clock Drawing Test
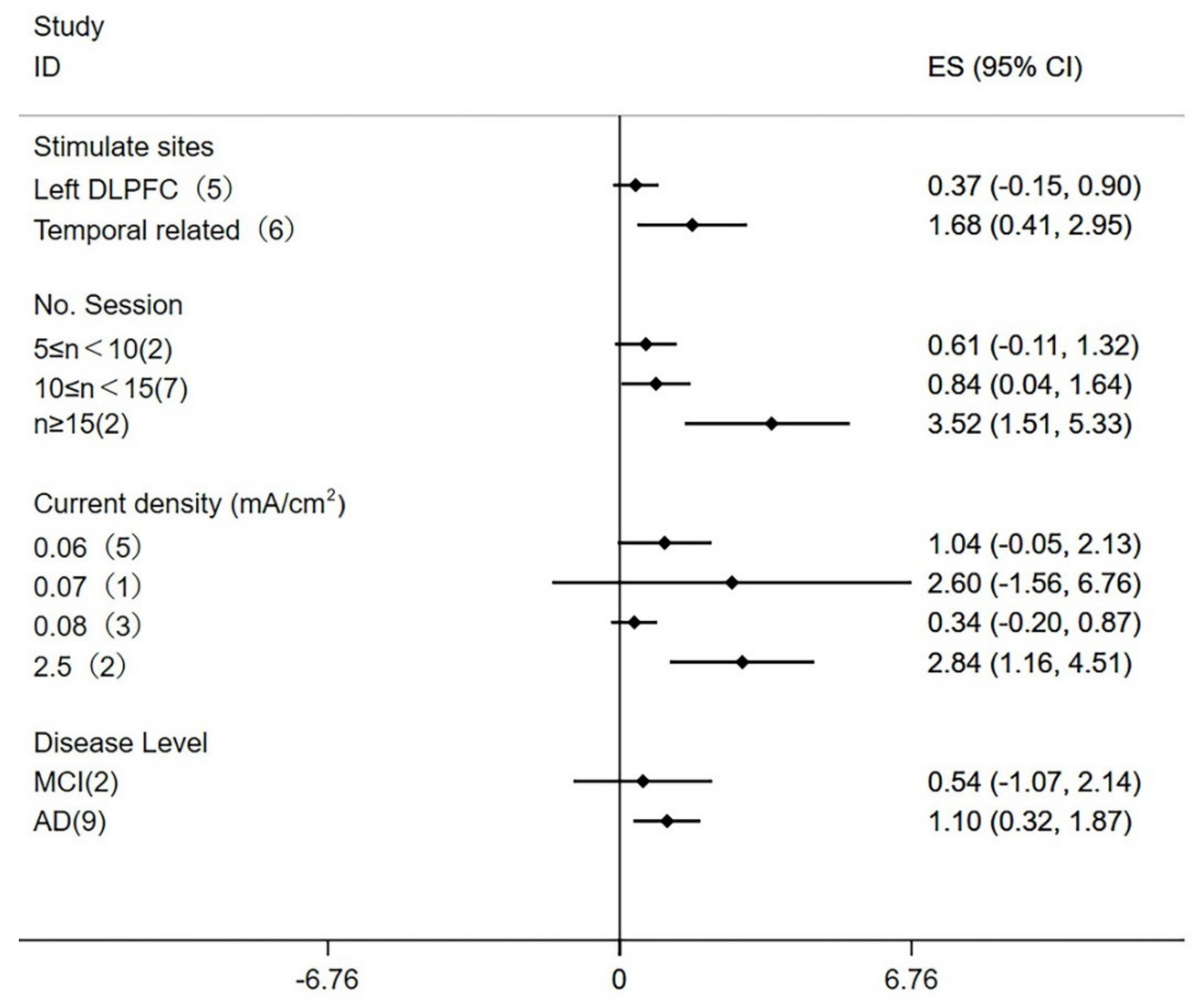
3.10. Subgroup Analysis and Meta-Regression
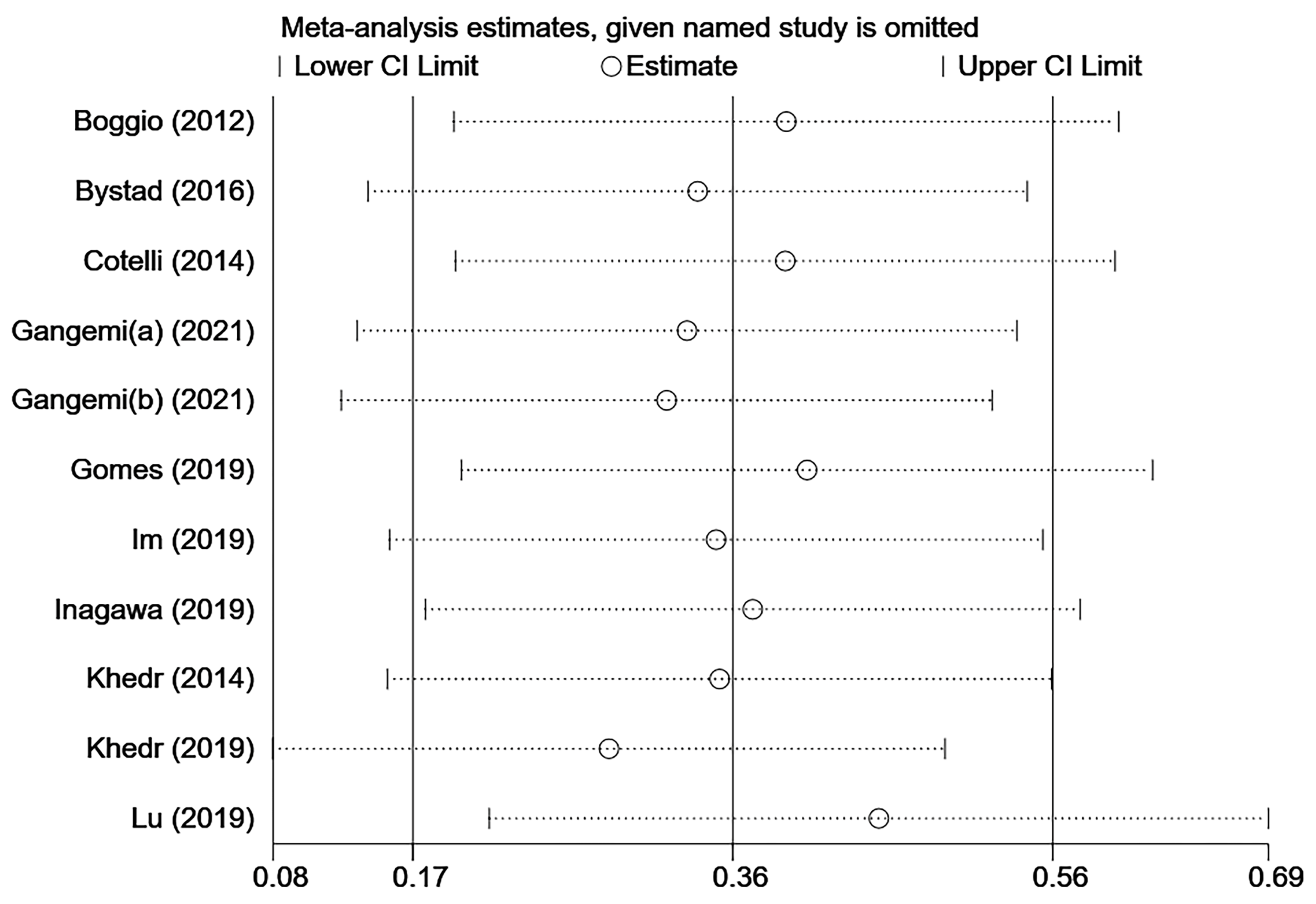
3.11. Sensitivity Analysis and Publication Bias
3.12. Secondary Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nance, C.; Ritter, A.; Miller, J.B.; Lapin, B.; Banks, S.J. The Pathology of Rapid Cognitive Decline in Clinically Diagnosed Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 70, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Lyketsos, C.G.; Carrillo, M.C.; Ryan, J.M.; Khachaturian, A.S. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Kirova, A.M.; Bays, R.B.; Lagalwar, S. Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. Biomed. Res. Int. 2015, 2015, 748212. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Beiser, A.; Machulda, M.M.; Fields, J.; Roberts, R.O.; Pankratz, V.S.; Aakre, J.; Cha, R.H.; Rocca, W.A.; Mielke, M.M.; et al. Spectrum of cognition short of dementia: Framingham Heart Study and Mayo Clinic Study of Aging. Neurology 2015, 85, 1712–1721. [Google Scholar] [CrossRef]
- Roberts, R.O.; Knopman, D.S.; Mielke, M.M.; Cha, R.H.; Pankratz, V.S.; Christianson, T.J.; Geda, Y.E.; Boeve, B.F.; Ivnik, R.J.; Tangalos, E.G.; et al. Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology 2014, 82, 317–325. [Google Scholar] [CrossRef]
- Birks, J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006, 1, CD00559. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and Management of Dementia: Review. JAMA 2019, 322, 1589–1599. [Google Scholar] [CrossRef]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef]
- Gonsalvez, I.; Baror, R.; Fried, P.; Santarnecchi, E.; Pascual-Leone, A. Therapeutic Noninvasive Brain Stimulation in Alzheimer’s Disease. Curr. Alzheimer Res. 2017, 14, 362–376. [Google Scholar] [CrossRef]
- Woods, A.J.; Antal, A.; Bikson, M.; Boggio, P.S.; Brunoni, A.R.; Celnik, P.; Cohen, L.G.; Fregni, F. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 2016, 127, 1031–1048. [Google Scholar] [CrossRef]
- Yokoi, Y.; Narita, Z.; Sumiyoshi, T. Transcranial Direct Current Stimulation in Depression and Psychosis: A Systematic Review. Clin. EEG Neurosci. 2018, 49, 93–102. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Schauenburg, A.; Lang, N.; Liebetanz, D.; Exner, C.; Paulus, W.; Tergau, F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J. Cogn. Neurosci. 2003, 15, 619–626. [Google Scholar] [CrossRef]
- Wagner, T.; Valero-Cabre, A.; Pascual-Leone, A. Noninvasive human brain stimulation. Annu. Rev. Biomed. Eng. 2007, 9, 527–565. [Google Scholar] [CrossRef]
- Philip, N.S.; Nelson, B.G.; Frohlich, F.; Lim, K.O.; Widge, A.S.; Carpenter, L.L. Low-Intensity Transcranial Current Stimulation in Psychiatry. Am. J. Psychiatry 2017, 174, 628–639. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527 Pt 3, 633–639. [Google Scholar] [CrossRef]
- Batsikadze, G.; Moliadze, V.; Paulus, W.; Kuo, M.F.; Nitsche, M.A. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J. Physiol. 2013, 591, 1987–2000. [Google Scholar] [CrossRef]
- Ferrucci, R.; Mameli, F.; Guidi, I.; Mrakic-Sposta, S.; Vergari, M.; Marceglia, S.; Cogiamanian, F.; Barbieri, S.; Scarpini, E.; Priori, A. Transcranial direct current stimulation improves recognition memory in Alzheimer disease. Neurology 2008, 71, 493–498. [Google Scholar] [CrossRef]
- Stagg, C.J.; Antal, A.; Nitsche, M.A. Physiology of Transcranial Direct Current Stimulation. J. ECT 2018, 34, 144–152. [Google Scholar] [CrossRef]
- Pellicciari, M.C.; Miniussi, C. Transcranial Direct Current Stimulation in Neurodegenerative Disorders. J. ECT 2018, 34, 193–202. [Google Scholar] [CrossRef]
- Beheshti, I.; Ko, J.H. Modulating brain networks associated with cognitive deficits in Parkinson’s disease. Mol. Med. 2021, 27, 24. [Google Scholar] [CrossRef]
- De Berker, A.O.; Bikson, M.; Bestmann, S. Predicting the behavioral impact of transcranial direct current stimulation: Issues and limitations. Front. Hum. Neurosci. 2013, 7, 613. [Google Scholar] [CrossRef]
- Gervits, F.; Ash, S.; Coslett, H.B.; Rascovsky, K.; Grossman, M.; Hamilton, R. Transcranial direct current stimulation for the treatment of primary progressive aphasia: An open-label pilot study. Brain Lang. 2016, 162, 35–41. [Google Scholar] [CrossRef]
- Dagan, M.; Herman, T.; Harrison, R.; Zhou, J.; Giladi, N.; Ruffini, G.; Manor, B.; Hausdorff, J.M. Multitarget transcranial direct current stimulation for freezing of gait in Parkinson’s disease. Mov. Disord. 2018, 33, 642–646. [Google Scholar] [CrossRef]
- Broeder, S.; Nackaerts, E.; Heremans, E.; Vervoort, G.; Meesen, R.; Verheyden, G.; Nieuwboer, A. Transcranial direct current stimulation in Parkinson’s disease: Neurophysiological mechanisms and behavioral effects. Neurosci. Biobehav. Rev. 2015, 57, 105–117. [Google Scholar] [CrossRef]
- Liu, C.S.; Herrmann, N.; Gallagher, D.; Rajji, T.K.; Kiss, A.; Vieira, D.; Lanctot, K.L. A Pilot Study Comparing Effects of Bifrontal versus Bitemporal Transcranial Direct Current Stimulation in Mild Cognitive Impairment and Mild Alzheimer Disease. J. ECT 2020, 36, 211–215. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, W.; Li, N.; Yang, X.; Zhu, B.; Wang, C.; Hou, W.; Wang, X.; Wen, H.; Tian, X. Anodal Transcranial Direct Current Stimulation Can Improve Spatial Learning and Memory and Attenuate Abeta42 Burden at the Early Stage of Alzheimer’s Disease in APP/PS1 Transgenic Mice. Front. Aging Neurosci. 2020, 12, 134. [Google Scholar] [CrossRef]
- Cocco, S.; Rinaudo, M.; Fusco, S.; Longo, V.; Gironi, K.; Renna, P.; Aceto, G.; Mastrodonato, A.; Li Puma, D.D.; Podda, M.V.; et al. Plasma BDNF Levels Following Transcranial Direct Current Stimulation Allow Prediction of Synaptic Plasticity and Memory Deficits in 3xTg-AD Mice. Front. Cell Dev. Biol. 2020, 8, 541. [Google Scholar] [CrossRef]
- Cai, M.; Guo, Z.; Xing, G.; Peng, H.; Zhou, L.; Chen, H.; McClure, M.A.; He, L.; Xiong, L.; He, B.; et al. Transcranial Direct Current Stimulation Improves Cognitive Function in Mild to Moderate Alzheimer Disease: A Meta-Analysis. Alzheimer Dis. Assoc. Disord. 2019, 33, 170–178. [Google Scholar] [CrossRef]
- Dedoncker, J.; Brunoni, A.R.; Baeken, C.; Vanderhasselt, M.A. A Systematic Review and Meta-Analysis of the Effects of Transcranial Direct Current Stimulation (tDCS) Over the Dorsolateral Prefrontal Cortex in Healthy and Neuropsychiatric Samples: Influence of Stimulation Parameters. Brain Stimul. 2016, 9, 501–517. [Google Scholar] [CrossRef]
- Cruz Gonzalez, P.; Fong, K.N.K.; Chung, R.C.K.; Ting, K.H.; Law, L.L.F.; Brown, T. Can Transcranial Direct-Current Stimulation Alone or Combined with Cognitive Training Be Used as a Clinical Intervention to Improve Cognitive Functioning in Persons with Mild Cognitive Impairment and Dementia? A Systematic Review and Meta-Analysis. Front. Hum. Neurosci. 2018, 12, 416. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; Rau, A.; Gallagher, D.; Rajji, T.K.; Lanctot, K.L.; Herrmann, N. Using transcranial direct current stimulation to treat symptoms in mild cognitive impairment and Alzheimer’s disease. Neurodegener. Dis. Manag. 2017, 7, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Inagawa, T.; Narita, Z.; Sugawara, N.; Maruo, K.; Stickley, A.; Yokoi, Y.; Sumiyoshi, T. A Meta-Analysis of the Effect of Multisession Transcranial Direct Current Stimulation on Cognition in Dementia and Mild Cognitive Impairment. Clin. EEG Neurosci. 2019, 50, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group PRISMA. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Chapter 10: Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2 (Updated February 2021); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2021; Available online: https://www.training.cochrane.org/handbook (accessed on 25 September 2021).
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Bystad, M.; Gronli, O.; Rasmussen, I.D.; Gundersen, N.; Nordvang, L.; Wang-Iversen, H.; Aslaksen, P.M. Transranial direct current stimulation as a memory enhancer in patients with Alzheimer’s disease: A randomized, placebo-controlled trial. Alzheimer’s Res. Ther. 2016, 8, 13. [Google Scholar] [CrossRef]
- Cotelli, M.; Manenti, R.; Brambilla, M.; Petesi, M.; Rosini, S.; Ferrari, C.; Zanetti, O.; Miniussi, C. Anodal tDCS during face-name associations memory training in Alzheimer’s patients. Front. Aging Neurosci. 2014, 6, 38. [Google Scholar] [CrossRef]
- Das, N.; Spence, J.S.; Aslan, S.; Vanneste, S.; Mudar, R.; Rackley, A.; Quiceno, M.; Chapman, S.B. Cognitive Training and Transcranial Direct Current Stimulation in Mild Cognitive Impairment: A Randomized Pilot Trial. Front. Neurosci. 2019, 13, 307. [Google Scholar] [CrossRef]
- Gangemi, A.; Colombo, B.; Fabio, R.A. Effects of short- and long-term neurostimulation (tDCS) on Alzheimer’s disease patients: Two randomized studies. Aging Clin. Exp. Res. 2021, 33, 383–390. [Google Scholar] [CrossRef]
- Gomes, M.A.; Akiba, H.T.; Gomes, J.S.; Trevizol, A.P.; de Lacerda, A.L.T.; Dias, A.M. Transcranial direct current stimulation (tDCS) in elderly with mild cognitive impairment: A pilot study. Dement. Neuropsychol. 2019, 13, 187–195. [Google Scholar] [CrossRef]
- Im, J.J.; Jeong, H.; Bikson, M.; Woods, A.J.; Unal, G.; Oh, J.K.; Na, S.; Park, J.S.; Knotkova, H.; Song, I.U.; et al. Effects of 6-month at-home transcranial direct current stimulation on cognition and cerebral glucose metabolism in Alzheimer’s disease. Brain Stimul. 2019, 12, 1222–1228. [Google Scholar] [CrossRef]
- Inagawa, T.; Yokoi, Y.; Narita, Z.; Maruo, K.; Okazaki, M.; Nakagome, K. Safety and Feasibility of Transcranial Direct Current Stimulation for Cognitive Rehabilitation in Patients with Mild or Major Neurocognitive Disorders: A Randomized Sham-Controlled Pilot Study. Front. Hum. Neurosci. 2019, 13, 273. [Google Scholar] [CrossRef]
- Khedr, E.M.; Gamal, N.F.; El-Fetoh, N.A.; Khalifa, H.; Ahmed, E.M.; Ali, A.M.; Noaman, M.; El-Baki, A.A.; Karim, A.A. A double-blind randomized clinical trial on the efficacy of cortical direct current stimulation for the treatment of Alzheimer’s disease. Front. Aging Neurosci. 2014, 6, 275. [Google Scholar] [CrossRef]
- Khedr, E.M.; Salama, R.H.; Abdel Hameed, M.; Abo Elfetoh, N.; Seif, P. Therapeutic Role of Transcranial Direct Current Stimulation in Alzheimer Disease Patients: Double-Blind, Placebo-Controlled Clinical Trial. Neurorehabil. Neural Repair 2019, 33, 384–394. [Google Scholar] [CrossRef]
- Lu, H.; Chan, S.S.M.; Chan, W.C.; Lin, C.; Cheng, C.P.W.; Linda Chiu Wa, L. Randomized controlled trial of TDCS on cognition in 201 seniors with mild neurocognitive disorder. Ann. Clin. Transl. Neurol. 2019, 6, 1938–1948. [Google Scholar] [CrossRef]
- Martin, D.M.; Mohan, A.; Alonzo, A.; Gates, N.; Gbadeyan, O.; Meinzer, M. A Pilot Double-Blind Randomized Controlled Trial of Cognitive Training Combined with Transcranial Direct Current Stimulation for Amnestic Mild Cognitive Impairment. J. Alzheimer’s Dis. 2019, 71, 503–512. [Google Scholar] [CrossRef]
- Stonsaovapak, C.; Hemrungroj, S.; Terachinda, P.; Piravej, K. Effect of Anodal Transcranial Direct Current Stimulation at the Right Dorsolateral Prefrontal Cortex on the Cognitive Function in Patients with Mild Cognitive Impairment: A Randomized Double-Blind Controlled Trial. Arch. Phys. Med. Rehabil. 2020, 101, 1279–1287. [Google Scholar] [CrossRef]
- Suemoto, C.K.; Apolinario, D.; Nakamura-Palacios, E.M.; Lopes, L.; Leite, R.E.; Sales, M.C.; Nitrini, R.; Brucki, S.M.; Morillo, L.S.; Magaldi, R.M.; et al. Effects of a non-focal plasticity protocol on apathy in moderate Alzheimer’s disease: A randomized, double-blind, sham-controlled trial. Brain Stimul. 2014, 7, 308–313. [Google Scholar] [CrossRef]
- Yun, K.; Song, I.U.; Chung, Y.A. Changes in cerebral glucose metabolism after 3 weeks of noninvasive electrical stimulation of mild cognitive impairment patients. Alzheimer’s Res. Ther. 2016, 8, 49. [Google Scholar] [CrossRef]
- Boggio, P.S.; Ferrucci, R.; Mameli, F.; Martins, D.; Martins, O.; Vergari, M.; Tadini, L.; Scarpini, E.; Fregni, F.; Priori, A. Prolonged visual memory enhancement after direct current stimulation in Alzheimer’s disease. Brain Stimul. 2012, 5, 223–230. [Google Scholar] [CrossRef]
- Arevalo-Rodriguez, I.; Smailagic, N.; Roque, I.F.M.; Ciapponi, A.; Sanchez-Perez, E.; Giannakou, A.; Pedraza, O.L.; Bonfill Cosp, X.; Cullum, S. Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst. Rev. 2015, 3, CD010783. [Google Scholar]
- Mueller, K.D.; Hermann, B.; Mecollari, J.; Turkstra, L.S. Connected speech and language in mild cognitive impairment and Alzheimer’s disease: A review of picture description tasks. J. Clin. Exp. Neuropsychol. 2018, 40, 917–939. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Ma, L.; Cao, Y.; Wang, F.; Li, Z.; Liu, N.; Liu, M.; Wei, Y.; Li, H. Traditional Chinese Medicine for Alzheimer’s Disease and Other Cognitive Impairment: A Review. Am. J. Chin. Med. 2020, 48, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, A.; Calhoun, H.; Rimikis, S.; Lowe, M.S.; Wellner, R.; Edwards, D.J. Using tDCS to facilitate motor learning in speech production: The role of timing. Cortex 2019, 111, 274–285. [Google Scholar] [CrossRef]
- Hameed, M.Q.; Dhamne, S.C.; Gersner, R.; Kaye, H.L.; Oberman, L.M.; Pascual-Leone, A.; Rotenberg, A. Transcranial Magnetic and Direct Current Stimulation in Children. Curr. Neurol. Neurosci. Rep. 2017, 17, 11. [Google Scholar] [CrossRef]
- Dubreuil-Vall, L.; Chau, P.; Ruffini, G.; Widge, A.S.; Camprodon, J.A. tDCS to the left DLPFC modulates cognitive and physiological correlates of executive function in a state-dependent manner. Brain Stimul. 2019, 12, 1456–1463. [Google Scholar] [CrossRef]
- Jeneson, A.; Squire, L.R. Working memory, long-term memory, and medial temporal lobe function. Learn. Mem. 2012, 19, 15–25. [Google Scholar] [CrossRef]
- Lara, A.H.; Wallis, J.D. The Role of Prefrontal Cortex in Working Memory: A Mini Review. Front. Syst. Neurosci. 2015, 9, 173. [Google Scholar] [CrossRef]
- Liang, P.; Wang, Z.; Yang, Y.; Jia, X.; Li, K. Functional disconnection and compensation in mild cognitive impairment: Evidence from DLPFC connectivity using resting-state fMRI. PLoS ONE 2011, 6, e22153. [Google Scholar]
- Yang, Y.; Liang, P.; Lu, S.; Li, K.; Zhong, N. The role of the DLPFC in inductive reasoning of MCI patients and normal agings: An fMRI study. Sci. China C Life Sci. 2009, 52, 789–795. [Google Scholar] [CrossRef]
- Meisenhelter, S.; Jobst, B.C. Neurostimulation for Memory Enhancement in Epilepsy. Curr. Neurol. Neurosci. Rep. 2018, 18, 30. [Google Scholar] [CrossRef]
- Liao, X.; Li, G.; Wang, A.; Liu, T.; Feng, S.; Guo, Z.; Tang, Q.; Jin, Y.; Xing, G.; McClure, M.A.; et al. Repetitive Transcranial Magnetic Stimulation as an Alternative Therapy for Cognitive Impairment in Alzheimer’s Disease: A Meta-Analysis. J. Alzheimer’s Dis. 2015, 48, 463–472. [Google Scholar] [CrossRef]
- Shariatirad, S.; Vaziri, A.; Hassani-Abharian, P.; Sharifi Fardshad, M.; Molavi, N.; Fitzgerald, P.B. Cumulative and booster effects of tdcs sessions on drug cravings, lapse, and cognitive impairment in methamphetamine use disorder: A case study report. Am. J. Addict. 2016, 25, 264–266. [Google Scholar] [CrossRef]
- Christova, M.; Rafolt, D.; Gallasch, E. Cumulative effects of anodal and priming cathodal tDCS on pegboard test performance and motor cortical excitability. Behav. Brain Res. 2015, 287, 27–33. [Google Scholar] [CrossRef]
- Besson, P.; Perrey, S.; Teo, W.P.; Muthalib, M. Commentary: Cumulative effects of anodal and priming cathodal tDCS on pegboard test performance and motor cortical excitability. Front. Hum. Neurosci. 2016, 10, 70. [Google Scholar] [CrossRef][Green Version]
- Boggio, P.S.; Nunes, A.; Rigonatti, S.P.; Nitsche, M.A.; Pascual-Leone, A.; Fregni, F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor. Neurol. Neurosci. 2007, 25, 123–129. [Google Scholar]
- Alonzo, A.; Brassil, J.; Taylor, J.L.; Martin, D.; Loo, C.K. Daily transcranial direct current stimulation (tDCS) leads to greater increases in cortical excitability than second daily transcranial direct current stimulation. Brain Stimul. 2012, 5, 208–213. [Google Scholar] [CrossRef]
- Silvanto, J.; Muggleton, N.; Walsh, V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn. Sci. 2008, 12, 447–454. [Google Scholar] [CrossRef]
- Bastani, A.; Jaberzadeh, S. Differential modulation of corticospinal excitability by different current densities of anodal transcranial direct current stimulation. PLoS ONE 2013, 8, e72254. [Google Scholar] [CrossRef]
- Mahdavi, S.; Towhidkhah, F. Computational human head models of tDCS: Influence of brain atrophy on current density distribution. Brain Stimul. 2018, 11, 104–107. [Google Scholar] [CrossRef]
- Foerster, Á.S.; Rezaee, Z.; Paulus, W.; Nitsche, M.A.; Dutta, A. Effects of Cathode Location and the Size of Anode on Anodal Transcranial Direct Current Stimulation Over the Leg Motor Area in Healthy Humans. Front. Neurosci. 2018, 12, 443. [Google Scholar] [CrossRef]
- Chu, C.S.; Li, C.T.; Brunoni, A.R.; Yang, F.C.; Tseng, P.T.; Tu, Y.K.; Stubbs, B.; Carvalho, A.F.; Thompson, T.; Rajji, T.K.; et al. Cognitive effects and acceptability of non-invasive brain stimulation on Alzheimer’s disease and mild cognitive impairment: A component network meta-analysis. J. Neurol. Neurosurg. Psychiatry 2021, 92, 195–203. [Google Scholar] [CrossRef]
- Teselink, J.; Bawa, K.K.; Koo, G.K.; Sankhe, K.; Liu, C.S.; Rapoport, M.; Oh, P.; Marzolini, S.; Gallagher, D.; Swardfager, W.; et al. Efficacy of non-invasive brain stimulation on global cognition and neuropsychiatric symptoms in Alzheimer’s disease and mild cognitive impairment: A meta-analysis and systematic review. Ageing Res. Rev. 2021, 72, 101499. [Google Scholar] [CrossRef]
- Spencer, R.J.; Wendell, C.R.; Giggey, P.P.; Katzel, L.I.; Lefkowitz, D.M.; Siegel, E.L.; Waldstein, S.R. Psychometric limitations of the mini-mental state examination among nondemented older adults: An evaluation of neurocognitive and magnetic resonance imaging correlates. Exp. Aging Res. 2013, 39, 382–397. [Google Scholar] [CrossRef]



| Study (Time) | Sample Size | Design | Diagnosis | Gender (M/F) | Age (y) | Education (y) | Duration of Disease(y) | Outcomes for Cognition Function |
|---|---|---|---|---|---|---|---|---|
| Boggio et al. (2012) [51] | NE:15 NC:15 | Crossover | AD | 8/7 | 78.95 ± 8.07 | 14.42 ± 3.65 | 4.39 ± 1.88 | MMSE, VAT, ADAS-Cog, Word recall, Word recognition, Instruction remembering, VRT |
| Bystad et al. (2016) [37] | NE:12 NC:13 | Parallel | AD | 7/5 7/6 | 70.0 ± 8.0 75.0 ± 8.7 | NR | NR | CVLT-II, MMSE, Clock-drawing test, TMT-A, TMT-B |
| Cotelli et al. (2014) [38] | NE:12 NC:12 | Parallel | AD | 2/10 3/9 | 76.6 ± 4.6 74.7 ± 6.1 | 5.5 ± 2.4 8.9 ± 5.1 | NR | FNAT, MMSE, Tinetti balance scale, Tinetti gait scale, NPI, Picture naming task, BADA, Rivermead behavioral memory test, Rey auditory verbal learning test, TMT-A, TMT-B |
| Das et al. (2019) [39] | NE:12 NC:10 | Parallel | MCI | 8/4 8/2 | 62.58 ± 8.43 63.30 ± 7.38 | 17.92 ± 3.94 16.20 ± 1.75 | NR | TOSL, DKEFS, CVLT, MMQ |
| Ferrucci et al. (2008) [17] | NEa:10 NEb:10 NC:10 | Crossover | AD | 3/7 | 75.2 ± 7.3 | 10.9 ± 4.8 | NR | Word recognition task, VAT |
| Gangemi(a) et al. (2021) [40] | NE:13 NC:13 | Parallel | AD | NR | 67.5 ± 2.8 69.01 ± 3.1 | 6.5 ± 2.0 6.1 ± 2.1 | NR | MMSE, MODA |
| Gangemi(b) et al. (2021) [40] | NE:9 NC:9 | Parallel | AD | NR | 68.5 ± 2.8 68.7 ± 3.1 | 6.7 ± 2.0 6.2 ± 2.7 | NR | MMSE, MODA |
| Gomes et al. (2019) [41] | NE:29 NC:29 | Parallel | MCI | 9/20 7/22 | 73.0 ± 9.2 71.6 ± 7.9 | NR | NR | CAMCOG, MMSE, TMT-A, TMT-B, SVF, BNT, Clock-drawing test, WLMT, WAIS, N-back, FDS, BDS |
| Im et al. (2019) [42] | NE:11 NC:7 | Parallel | MCI | 1/10 2/5 | 71.9 ± 9.2 74.9 ± 5.0 | 6.3 ± 3.8 5.4 ± 5.9 | NR | MMSE, FDS, BDS, BNT, SVLT, COWAT, RCFT, Contrasting Program, Go-no go Test, Stroop Test, Clock-drawing test |
| Inagawa et al. (2019) [43] | NE:7 NC:13 | Parallel | AD | 3/4 7/6 | 76.6 ± 5.7 76.2 ± 7.7 | NR | 0.9 ± 1.2 1.2 ± 1.5 | ADAS-Cog, MMSE, FAB |
| Khedr et al. (2014) [44] | NEa:11 NEb:12 NC:11 | Parallel | AD | 6/5 8/4 5/6 | 68.5 ± 7.2 70.7 ± 5.4 67.3 ± 5.9 | NR | 3.0 ± 2.6 2.9 ± 1.9 3.5 ± 1.7 | MMSE, WAIS |
| Khedr et al. (2019) [45] | NE:23 NC:21 | Parallel | AD | 13/10 13/8 | 64.22 ± 3.64 65.23 ± 4.52 | 1.17 ± 0.48 1.17 ± 0.39 | 4.04 ± 2.83 3.52 ± 1.96 | MMSE, Clock-drawing test, MoCA |
| Lu et al. (2019) [46] | NE:69 NC:64 | Parallel | AD | 21/42 17/36 | 74.2 ± 6.7 74.5 ± 6.6 | 7.3 ± 4.8 6.5 ± 4.3 | NR | ADAS-Cog, MMSE, NPI, CVFT, FDS, BDS, TMT-A, TMT-B |
| Martin et al. (2019) [47] | NE:33 NC:35 | Parallel | MCI | 13/20 10/25 | 71.8 ± 6.39 71.6 ± 6.35 | 14.5 ± 3.51 14.9 ± 3.23 | NR | CVLT-II, CANTAB, SDMT, CFQ |
| Stonsaovapak et al. (2020) [48] | NE:23 NC:22 | Parallel | MCI | 2/21 2/20 | 68.39 ± 8.37 69.68 ± 7.60 | NR | NR | CANTAB |
| Suemoto et al. (2014) [49] | NE:20 NC:20 | Parallel | AD | 5/15 7/13 | 79.4 ± 7.1 81.6 ± 8.0 | 5 ± 4.2 4.5 ± 3.9 | NR | NPI, ADAS-Cog, Digit cancellation task, Word list learning task, Word recognition task |
| Yun et al. (2016) [50] | NE:8 NC:8 | Parallel | MCI | 3/5 2/6 | 74.75 ± 7.47 73.12 ± 4.25 | 8.06 ± 4.93 5.56 ± 2.41 | NR | MMQ |
| Study (Time) | Type of Stimulation | Number of Sessions | Duration (min) | Stimulation Site | Current (mA) | Montage Size (cm2) | Stimulation Model | Adverse Effects |
|---|---|---|---|---|---|---|---|---|
| Boggio et al. (2012) [51] | Anode Sham | per day for 5 consecutive days | 30 | Temporal cortex bilaterally | 2 | 35 | Offline | No adverse effects were recorded after five daily tDCS sessions |
| Bystad et al. (2016) [37] | Anode Sham | 6 sessions for 10 days | 30 | Left temporal lobe | 2 | 35 | Offline | No adverse effects were reported |
| Cotelli et al. (2014) [38] | Anode+ICMT Sham+ICMT | 5 sessions per week for 2 weeks | 25 | Left DLPFC | 2 | 25 | Online | NR |
| Das et al. (2019) [39] | Anode+SMART Sham+SMART | 8 sessions for 4 weeks | 20 | Left IFG | 2 | 15 | Offline | NR |
| Ferrucci et al. (2008) [17] | Anodal Cathodal Sham | 1 session | 15 | Temporoparietal areas bilaterally | 1.5 | 25 | Offline | NR |
| Gangemi(a) et al. (2021) [40] | Anode Sham | Daily, for 10 days | 20 | Left frontotemporal cortex | 2 | 0.8 | Offline | NR |
| Gangemi(b) et al. (2021) [40] | Anode Sham | 10 sessions each month for 8 months | 20 | Left frontotemporal cortex | 2 | 0.8 | Offline | NR |
| Gomes et al. (2019) [41] | Anode Sham | Twice per week for 5 weeks | 30 | Left DLPFC | 2 | 25 | Offline | NR |
| Im et al. (2019) [42] | Anode Sham | Daily, for 6 months | 30 | Left DLPFC | 2 | 28 | Offline | NR |
| Inagawa et al. (2019) [43] | Anode+CT| Sham+CT | 2 sessions per day for 5 consecutive days | 20 | Left DLPFC | 2 | 35 | Online | Neither severe adverse events nor the need for medications caused by adverse events |
| Khedr et al. (2014) - [44] | Anodal Cathodal Sham | Daily, for 10 days | 25 | Left DLPFC | 2 | 24 | Offline | Two patients under active stimulation recorded itching, headache, and dizziness that were disappear after few hours |
| Khedr et al. (2019) [45] | Anode Sham | 5 sessions per week for 2 consecutive weeks | 20 (each side) | Left TP lobe and right TP lobe | 2 | 35 | Offline | All the patients tolerated tDCS well without major adverse effects |
| Lu et al. (2019) [46] | Anode+WMT Sham+WMT | 3 sessions per week for 4 weeks | 20 | Left LTC | 2 | 35 | Offline | three cases had skin lesions under the cathodal electrode during the repeated sessions of tDCS |
| Martin et al. (2019) [47] | Anode+CT Sham+CT | 3 sessions per week for 5 weeks | 30 | Left DLPFC | 2 | 35 | Online | No adverse effects were reported |
| Stonsaovapak et al. (2020) [48] | Anode Sham | 3 times per week for 4 weeks | 20 | Right DLPFC | 2 | 25 | Offline | Dizziness was found in one participant from the atDCS group. All side effects disappeared within 24 hours |
| Suemoto et al. (2014) [49] | Anode Sham | 3 sessions per week for 2 weeks | 20 | Left DLPFC | 2 | 35 | Offline | TDCS was well tolerated and not associated with significant adverse effects |
| Yun et al. (2016) [50] | Anode Sham | 3 sessions per week for 3 weeks | 30 | Left DLPFC | 2 | 25 | Offline | No patient reported adverse effects |
| Study | Sequence Generation | Allocation Concealment | Blinding of Participants | Personnel and Outcomes Assessors | Incomplete Outcome Data | Selective Outcomes Reporting | Baseline Characteristics |
|---|---|---|---|---|---|---|---|
| Boggio (2012) [51] | ? | ? | ? | + | + | ? | - |
| Bystad (2016) [37] | + | + | ? | ? | + | + | ? |
| Cotelli (2014) [38] | ? | ? | + | + | ? | + | + |
| Das (2019) [39] | + | + | + | + | ? | + | ? |
| Ferrucci (2008) [17] | + | + | + | + | + | ? | ? |
| Gangemi(a) (2021) [40] | ? | ? | + | + | ? | + | ? |
| Gangemi(b) (2021) [40] | ? | ? | + | + | ? | + | ? |
| Gomes (2019) [41] | ? | ? | ? | + | ? | + | - |
| Im (2019) [42] | + | + | ? | + | + | + | + |
| Inagawa (2019) [43] | + | + | ? | + | + | + | + |
| Khedr (2014) [44] | + | ? | + | + | + | + | ? |
| Khedr (2019) [45] | + | ? | + | + | + | + | ? |
| Lu (2019) [46] | + | ? | + | + | + | + | + |
| Martin (2019) [47] | + | + | ? | + | ? | + | + |
| Stonsaovapak (2020) [48] | + | + | + | + | + | + | + |
| Suemoto (2014) [49] | + | + | + | + | + | + | + |
| Yun (2016) [50] | + | + | + | + | + | + | ? |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Wang, Z.; Chen, Q.; Fu, Y.; Zheng, K. Transcranial Direct Current Stimulation Enhances Cognitive Function in Patients with Mild Cognitive Impairment and Early/Mid Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Brain Sci. 2022, 12, 562. https://doi.org/10.3390/brainsci12050562
Chen J, Wang Z, Chen Q, Fu Y, Zheng K. Transcranial Direct Current Stimulation Enhances Cognitive Function in Patients with Mild Cognitive Impairment and Early/Mid Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Brain Sciences. 2022; 12(5):562. https://doi.org/10.3390/brainsci12050562
Chicago/Turabian StyleChen, Jiajie, Zheng Wang, Qin Chen, Yu Fu, and Kai Zheng. 2022. "Transcranial Direct Current Stimulation Enhances Cognitive Function in Patients with Mild Cognitive Impairment and Early/Mid Alzheimer’s Disease: A Systematic Review and Meta-Analysis" Brain Sciences 12, no. 5: 562. https://doi.org/10.3390/brainsci12050562
APA StyleChen, J., Wang, Z., Chen, Q., Fu, Y., & Zheng, K. (2022). Transcranial Direct Current Stimulation Enhances Cognitive Function in Patients with Mild Cognitive Impairment and Early/Mid Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Brain Sciences, 12(5), 562. https://doi.org/10.3390/brainsci12050562





