Application of Real and Virtual Radial Arm Maze Task in Human
Abstract
1. Introduction
2. The Evolution of Virtual Reality Technology
3. Main Spatial Tasks Suitable for Virtual Environments
4. Method
5. Radial Arm Maze Task (RAM)
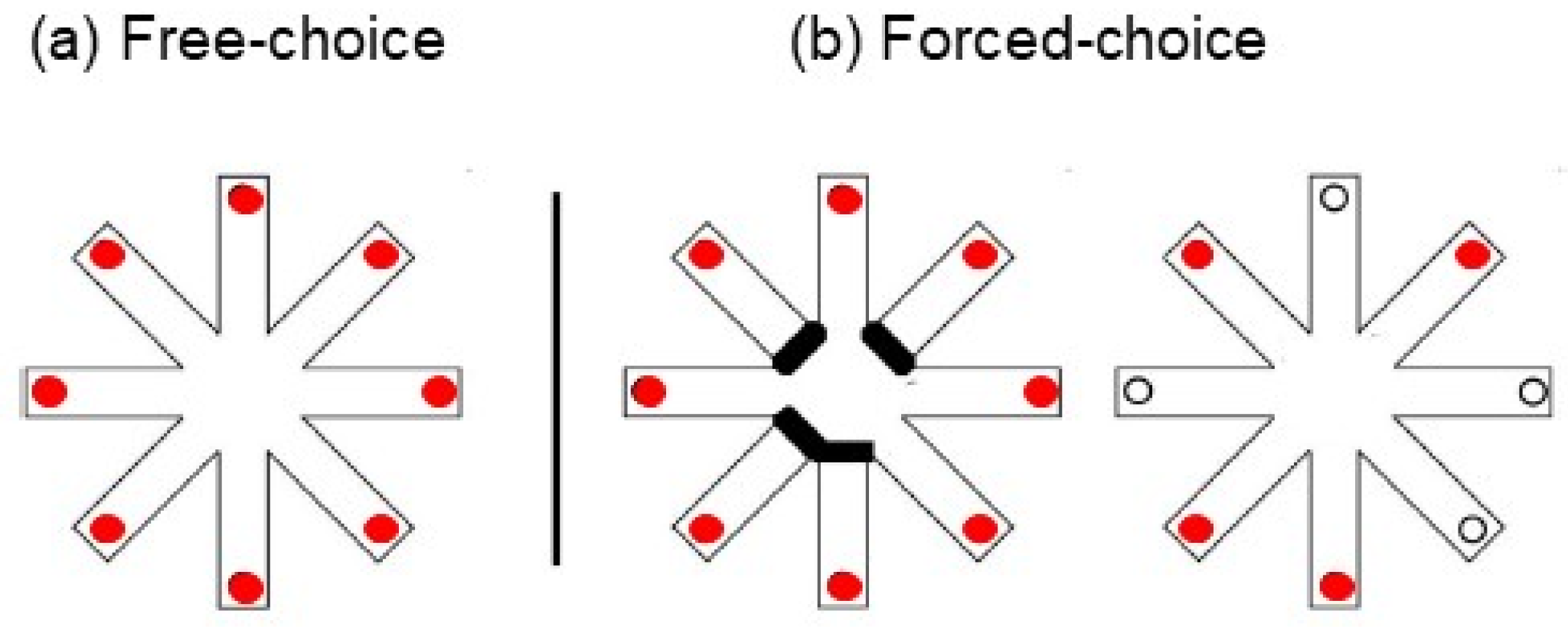
5.1. Free-Choice and Forced-Choice Version
5.2. Table RAM and Visuospatial Peripersonal Abilities
6. Applications of RAM Task in Real Environment
7. Potentiality and Applications of RAM Task in Virtual Environment
8. Conclusion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellis, S.R. What Are Virtual Environments? IEEE Comput. Graph. Appl. 1994, 14, 17–22. [Google Scholar] [CrossRef]
- Chirico, A.; Lucidi, F.; De Laurentiis, M.; Milanese, C.; Napoli, A.; Giordano, A. Virtual Reality in Health System: Beyond Entertainment. A Mini-Review on the Efficacy of VR during Cancer Treatment. J. Cell Physiol. 2016, 231, 275–287. [Google Scholar] [CrossRef]
- Chirico, A.; Maiorano, P.; Indovina, P.; Milanese, C.; Giordano, G.G.; Alivernini, F.; Iodice, G.; Gallo, L.; De Pietro, G.; Lucidi, F.; et al. Virtual reality and music therapy as distraction interventions to alleviate anxiety and improve mood states in breast cancer patients during chemotherapy. J. Cell Physiol. 2020, 235, 5353–5362. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.M. Route learning in a case of amnesia: A preliminary investigation into the efficacy of training in a virtual environment. Neuropsychol. Rehabil. 1999, 9, 63–76. [Google Scholar] [CrossRef]
- Hofmann, M.; Rösler, A.; Schwarz, W.; Muller-Spahn, F.; Krauchi, K.; Hock, C.; Seifiritz, E. Interactive computer-training as a therapeutic tool in Alzheimer’s disease. Compr. Psychiatry 2003, 44, 213–219. [Google Scholar] [CrossRef]
- Optale, G.; Urgesi, C.; Busato, V.; Marin, S.; Piron, L.; Priftis, K.; Gamberini, L.; Capodieci, S.; Bordin, A. Controlling memory impairment in elderly adults using virtual reality memory training: A randomized controlled pilot study. Neurorehabil. Neural Repair 2010, 24, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Jekel, K.; Damian, M.; Wattmo, C.; Hausner, L.; Bullock, R.; Connelly, P.; Dubois, B.; Eriksdotter, M.; Ewers, M.; Graessel, E.; et al. Mild cognitive impairment and deficits in instrumental activities of daily living: A systematic review. Alzheimer’s Res. Ther. 2015, 7, 17. [Google Scholar] [CrossRef]
- Claessen, M.H.G.; Visser-Meily, J.M.A.; De Rooij, N.K.; Postma, A.; Van Der Ham, I.J.M. A Direct Comparison of Real-World and Virtual Navigation Performance in Chronic Stroke Patients. J. Int. Neuropsychol. Soc. 2016, 22, 467–477. [Google Scholar] [CrossRef]
- Montana, J.I.; Tuena, C.; Serino, S.; Cipresso, P.; Riva, G. Neurorehabilitation of spatial memory using virtual environments: A systematic review. J. Clin. Med. 2019, 8, 1516. [Google Scholar] [CrossRef]
- Jonson, M.; Avramescu, S.; Chen, D.; Alam, F. The Role of Virtual Reality in Screening, Diagnosing, and Rehabilitating Spatial Memory Deficits. Front. Hum. Neurosci. 2021, 15, 628818. [Google Scholar] [CrossRef]
- Plancher, G.; Tirard, A.; Gyselinck, V.; Nicolas, S.; Piolino, P. Using virtual reality to characterize episodic memory profiles in amnestic mild cognitive impairment and Alzheimer’s disease: Influence of active and passive encoding. Neuropsychologia 2012, 50, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.A.; Nannery, R.; Spiers, H.J. Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain 2006, 129, 2894–2907. [Google Scholar] [CrossRef] [PubMed]
- Hort, J.; Laczó, J.; Vyhnálek, M.; Bojar, M.; Bureš, J.; Vlček, K. Spatial navigation deficit in amnestic mild cognitive impairment. Proc. Natl. Acad. Sci. USA 2007, 104, 4042–4047. [Google Scholar] [CrossRef] [PubMed]
- Cushman, L.A.; Stein, K.; Duffy, C.J. Detecting navigational deficits in cognitive aging and Alzheimer disease using virtual reality. Neurology 2008, 71, 888–895. [Google Scholar] [CrossRef] [PubMed]
- van der Ham, I.J.M.; Faber, A.M.E.; Venselaar, M.; van Kreveld, M.J.; Löffler, M. Ecological validity of virtual environments to assess human navigation ability. Front. Psychol. 2015, 6, 637. [Google Scholar] [CrossRef]
- Cogné, M.; Taillade, M.; N’Kaoua, B.; Tarruella, A.; Klinger, E.; Larrue, F.; Sauzéon, H.; Joseph, P.A.; Sorita, E. The contribution of virtual reality to the diagnosis of spatial navigation disorders and to the study of the role of navigational aids: A systematic literature review. Ann. Phys. Rehabil. Med. 2017, 60, 164–176. [Google Scholar] [CrossRef]
- Weniger, G.; Ruhleder, M.; Lange, C.; Wolf, S.; Irle, E. Egocentric and allocentric memory as assessed by virtual reality in individuals with amnestic mild cognitive impairment. Neuropsychologia 2011, 49, 518–527. [Google Scholar] [CrossRef]
- Chirico, A.; Giovannetti, T.; Neroni, P.; Simone, S.; Gallo, L.; Galli, F.; Giancamilli, F.; Predazzi, M.; Lucidi, F.; De Pietro, G.; et al. Virtual Reality for the Assessment of Everyday Cognitive Functions in Older Adults: An Evaluation of the Virtual Reality Action Test and Two Interaction Devices in a 91-Year-Old Woman. Front. Psychol. 2020, 11, 123. [Google Scholar] [CrossRef]
- Davis, R.; Ohman, J.M.; Weisbeck, C. Salient cues and wayfinding in Alzheimer’s disease within a virtual senior residence. Environ. Behav. 2017, 49, 1038–1065. [Google Scholar] [CrossRef]
- Montenegro, J.M.F.; Argyriou, V. Cognitive evaluation for the diagnosis of Alzheimer’s disease based on turing test and virtual environments. Physiol. Behav. 2017, 173, 42–51. [Google Scholar] [CrossRef]
- Dovis, S.; Van der Oord, S.; Wiers, R.W.; Prins, P.J.M. Improving Executive Functioning in Children with ADHD: Training Multiple Executive Functions within the Context of a Computer Game. A Randomized Double- Blind Placebo Controlled Trial. PLoS ONE 2015, 10, e0121651. [Google Scholar] [CrossRef] [PubMed]
- Olton, D.S.; Samuelson, R.J. Remembrance of placed passed: Spatial memory in rats. J. Exp. Psychol. Anim. Behav. 1976, 2, 97–116. [Google Scholar] [CrossRef]
- Bailenson, J.N.; Yee, N.; Merget, D.; Schroeder, R. The effect of behavioral realism and form realism of real-time avatar faces on verbal disclosure, nonverbal disclosure, emotion recognition, and copresence in dyadic interaction. Presence Teleoperators Virtual Environ. 2006, 15, 359–372. [Google Scholar] [CrossRef]
- Andersen, S.M.; Thorpe, J.S. An IF-THEN theory of personality: Significant others and the relational self. J. Res. Pers. 2009, 43, 163–170. [Google Scholar] [CrossRef]
- Slater, M. Place illusion and plausibility can lead to realistic behaviour in immersive virtual environments. Phil. Trans. R. Soc. B 2009, 364, 3549–3557. [Google Scholar] [CrossRef]
- Marková, H.; Laczó, J.; Andel, R.; Hort, J.; Vlček, K. Perspective taking abilities in amnestic mild cognitive impairment and Alzheimer’s disease. Behav. Brain Res. 2015, 284, 229–238. [Google Scholar] [CrossRef]
- Cipresso, P.; Giglioli, I.A.C.; Raya, M.A.; Riva, G. The past, present, and future of virtual and augmented reality research: A network and cluster analysis of the literature. Front. Psychol. 2018, 9, 2086. [Google Scholar] [CrossRef]
- García-Betances, R.I.; Arredondo Waldmeyer, M.T.; Fico, G.; Cabrera-Umpiérrez, M.F. A succinct overview of virtual reality technology use in Alzheimer’s disease. Front. Aging Neurosci. 2015, 7, 80. [Google Scholar] [CrossRef]
- D’Cunha, N.M.; Nguyen, D.; Naumovski, N.; McKune, J.A.; Kellett, J.; Georgousopoulou, N.E.; Frost, J.; Isbel, S. A mini-review of virtual reality-based interventions to promote well-being for people living with dementia and mild cognitive impairment. Gerontology 2019, 65, 430–440. [Google Scholar] [CrossRef]
- Ware, C.; Arthur, K.; Booth, K.S. Fish tank virtual reality. In Proceedings of the INTERACT’93 and CHI’93 Conference on Human Factors in Computing Systems, (Amsterdam: ACM), New Orleans, LA, USA, 24–29 April 1993; pp. 37–42. [Google Scholar] [CrossRef]
- Minderer, M.; Harvey, C.D.; Donato, F.; Moser, E.I. Neuroscience: Virtual reality explored. Nature 2016, 533, 324–325. [Google Scholar] [CrossRef]
- Cornwell, B.R.; Johnson, L.L.; Holroyd, T.; Carver, F.W.; Grillon, C. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual Morris water maze. J. Neurosci. 2008, 28, 5983–5990. [Google Scholar] [CrossRef] [PubMed]
- Bohbot, V.D.; Iaria, G.; Petrides, M. Hippocampal function and spatial memory: Evidence from functional neuroimaging in healthy participants and performance of patients with medial temporal lobe resections. Neuropsychology 2004, 18, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Iaria, G.; Petrides, M.; Dagher, A.; Pike, B.; Bohbot, V.D. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: Variability and change with practice. J. Neurosci. 2003, 23, 5945–5952. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.A.; Frick, K.M.; Newhouse, P.A.; Astur, R.S. Estradiol effects on spatial memory in women. Behav. Brain Res. 2022, 417, 113592. [Google Scholar] [CrossRef] [PubMed]
- Leplow, B.; Lehnung, M.; Pohl, J.; Herzog, A.; Ferstl, R.; Mehdorn, M. Navigational place learning in children and young adults as assessed with a standardized locomotor search task. Br. J. Psychol. 2003, 94, 299–317. [Google Scholar] [CrossRef] [PubMed]
- Leon, I.; Tascòn, L.; Ortells-Pareja, J.J.; Cimadevilla, J.M. Virtual reality assessment of walking and non-walking space in men and women with virtual reality-based tasks. PLoS ONE 2018, 13, e0204995. [Google Scholar] [CrossRef]
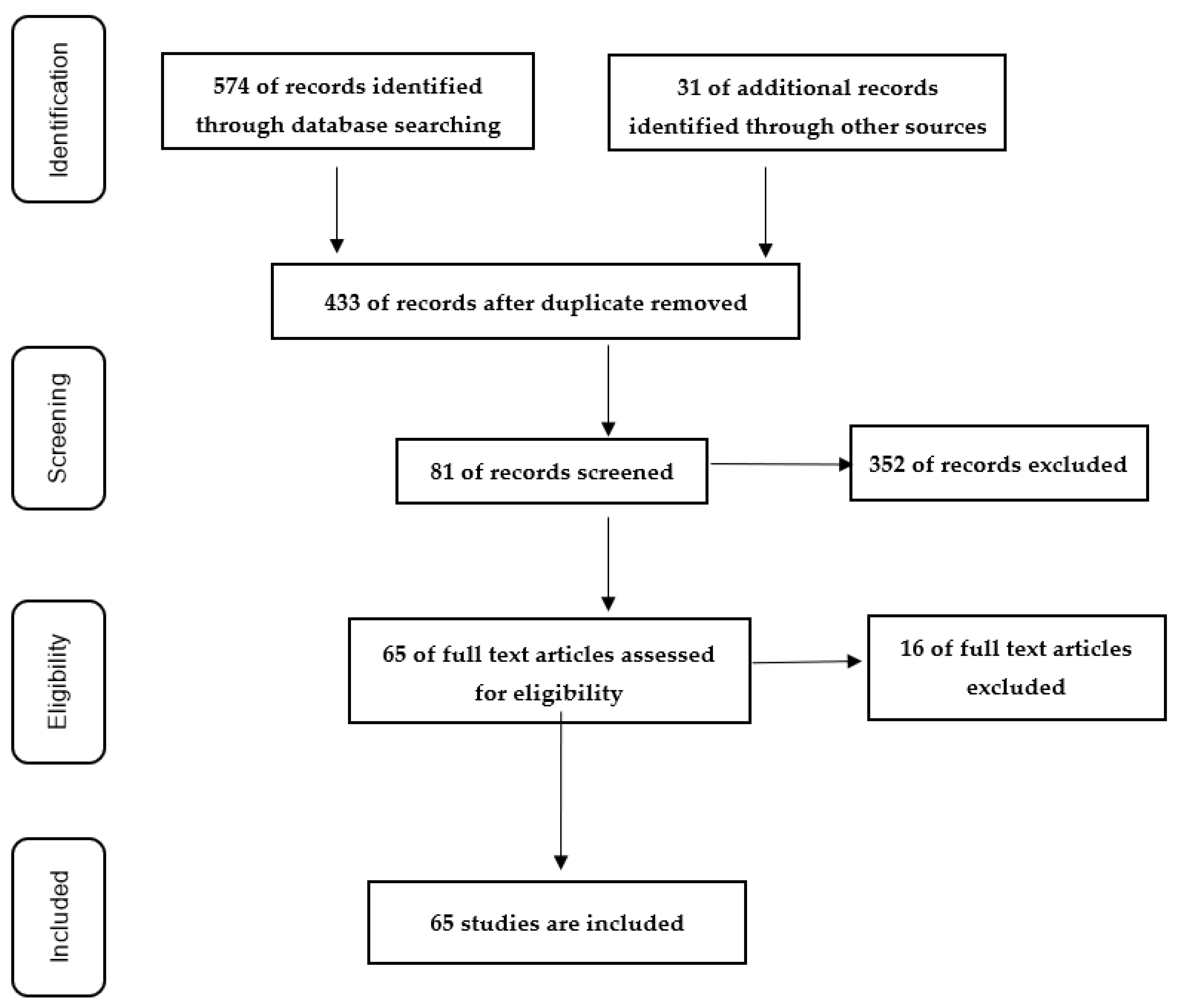
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffman, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Serra, L.; Raimondi, S.; di Domenico, C.; Maffei, S.; Lardone, A.; Liparoti, M.; Sorrentino, P.; Caltagirone, C.; Petrosini, L.; Mandolesi, L. The beneficial effects of physical exercise on visuospatial working memory in preadolescent children. AIMS Neurosci. 2021, 8, 496–509. [Google Scholar] [CrossRef]
- Foti, F.; Martone, D.; Orrù, S.; Montuori, S.; Imperlini, E.; Buono, P.; Petrosini, L.; Mandolesi, L. Are young children able to learn exploratory strategies by observation? Psychol. Res. 2018, 82, 1212–1223. [Google Scholar] [CrossRef]
- Moraleda, E.; Broglio, C.; Rodríguez, F.; Gómez, A. Development of different spatial frames of reference for orientation in small-scale environments. Psicothema 2013, 25, 468–475. [Google Scholar] [CrossRef]
- Mandolesi, L.; Petrosini, L.; Menghini, D.; Addona, F.; Vicari, S. Children’s radial arm maze performance as a function of age and sex. Int. J. Dev. Neurosci. 2009, 27, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Foreman, N.; Gillett, R.; Jones, S. Choice autonomy and memory for spatial locations in six-year-old children. Br. J. Psychol. 1994, 85, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Foreman, N.; Warry, R.; Murray, P. Development of reference and working spatial memory in preschool children. J. Gen. Psychol. 1990, 117, 267–276. [Google Scholar] [PubMed]
- Foreman, N.P.; Arber, M.; Savage, J. Spatial memory in preschool infants. Dev. Psychobiol. 1984, 17, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Overman, W.H.; Pate, B.J.; Moore, K.; Peuster, A. Ontogeny of place learning in children as measured in the radial arm maze, Morris search task, and open field task. Behav. Neurosci. 1996, 110, 1205–1228. [Google Scholar] [CrossRef]
- Aadland, J.; Beatty, W.W.; Maki, R.H. Spatial memory of children and adults assessed in the radial maze. Dev. Psychobiol. 1985, 18, 163–172. [Google Scholar] [CrossRef]
- O’Connor, R.C.; Glassman, R.B. Human performance with a seventeen-arm radial maze analog. Brain Res. Bull. 1993, 30, 189–191. [Google Scholar] [CrossRef]
- Glassman, R.B.; Garvey, K.J.; Elkins, K.M.; Kasal, K.L.; Couillard, N.L. Spatial working memory score of humans in a large radial maze, similar to published score of rats, implies capacity close to the magical number 7 ± 22. Brain Res. Bull. 1994, 34, 151–159. [Google Scholar] [CrossRef]
- Glassman, R.B.; Leniek, K.M.; Haegerich, T.M. Human working memory capacity is 7 ± 2 in a radial maze with distracting interruption: Possible implication for neural mechanisms of declarative and implicit long-term memory. Brain Res. Bull. 1998, 47, 249–256. [Google Scholar] [CrossRef]
- Leitner, Y.; Heldman, D.; Harel, S.; Pick, C.G. Deficits in spatial orientation of children with intrauterine growth retardation. Brain Res. Bull. 2005, 67, 13–18. [Google Scholar] [CrossRef]
- Foti, F.; Menghini, D.; Petrosini, L.; Valerio, G.; Crinò, A.; Vicari, S.; Grimaldi, T.; Mandolesi, L. Spatial competences in Prader-Willi syndrome: A Radial Arm Maze study. Behav. Genet. 2011, 41, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Mandolesi, L.; Addona, F.; Foti, F.; Menghini, D.; Petrosini, L.; Vicari, S. Spatial competences in Williams syndrome: A radial arm maze study. Int. J. Dev. Neurosci. 2009, 27, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Foti, F.; Sorrentino, P.; Menghini, D.; Montuori, S.; Pesoli, M.; Turriziani, P.; Vicari, S.; Petrosini, L.; Mandolesi, L. Peripersonal Visuospatial Abilities in Williams Syndrome Analyzed by a Table Radial Arm Maze Task. Front. Hum. Neurosci. 2020, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Bertholet, L.; Escobar, M.T.; Depré, M.; Chavan, C.F.; Giuliani, F.; Gisquet-Verrier, P.; Preissmann, D.; Schenk, F. Spatial radial maze procedures and setups to dissociate local and distal relational spatial frameworks in humans. J. Neurosci. Methods 2015, 253, 126–141. [Google Scholar] [CrossRef]
- Palermo, L.; Foti, F.; Ferlazzo, F.; Guariglia, C.; Petrosini, L. I find my way in a maze but not in my own territory! Navigational processing in developmental topographical disorientation. Neuropsychology 2014, 28, 135–146. [Google Scholar] [CrossRef]
- Bohbot, V.D.; Jech, R.; Ruèicka, E.; Nadel, L.; Kanilna, M.; Stepankova, K.; Bures, J. Rat Spatial Memory Tasks Adapted for Humans: Characterization in Subjects with Intact Brain and Subjects with Medial Temporal Lobe Lesions. Physiol. Res. 2002, 51 (Suppl. S1), S49–S56. [Google Scholar]
- Kim, H.; Park, J.Y.; Kim, K.K. Spatial learning and memory using a radial arm maze with a head-mounted display. Psychiatry Investig. 2018, 15, 935–944. [Google Scholar] [CrossRef]
- Ben-Zeev, T.; Weiss, I.; Ashri, S.; Heled, Y.; Ketko, I.; Yanovich, R.; Okun, E. Mild Physical Activity Does Not Improve Spatial Learning in a Virtual Environment. Front. Behav. Neurosci. 2020, 14, 584052. [Google Scholar] [CrossRef]
- Somma, F.; Bartolomeo, P.; Vallone, F.; Argiuolo, A.; Cerrato, A.; Miglino, O.; Mandolesi, L.; Zurlo, M.C.; Gigliotta, O. Further to the left: Stress-induced increase of spatial pseudoneglect during the covid-19 lockdown. Front. Psychol. 2021, 12, 573846. [Google Scholar] [CrossRef]
- Taheri Gorji, H.; Leocadi, M.; Grassi, F.; Galati, G. The art gallery maze: A novel tool to assess human navigational abilities. Cogn. Process. 2021, 22, 501–514. [Google Scholar] [CrossRef]
- Rechtman, E.; Curtin, P.; Papazaharias, D.M.; Renzetti, S.; Cagna, G.; Peli, M.; Levin-Schwartz, Y.; Placidi, D.; Smith, D.R.; Lucchini, R.G.; et al. Sex-specific associations between co-exposure to multiple metals and visuospatial learning in early adolescence. Transl. Psychiatry 2020, 10, 358. [Google Scholar] [CrossRef] [PubMed]
- Sodums, D.J.; Bohbot, V.D. Negative correlation between grey matter in the hippocampus and caudate nucleus in healthy aging. Hippocampus 2020, 30, 892–908. [Google Scholar] [CrossRef] [PubMed]
- Dahmani, L.; Courcot, B.; Near, J.; Patel, R.; Amaral, R.S.C.; Chakravarty, M.M.; Bohbot, V.D. Fimbria-Fornix Volume Is Associated With Spatial Memory and Olfactory Identification in Humans. Front. Syst. Neurosci. 2020, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Goodman, J.; McClay, M.; Dunsmoor, J.E. Threat-induced modulation of hippocampal and striatal memory systems during navigation of a virtual environment. Neurobiol. Learn. Mem. 2020, 168, 107160. [Google Scholar] [CrossRef]
- Yang, Y.; Merrill, E.C.; Wang, Q. Children’s response, landmark, and metric strategies in spatial navigation. J. Exp. Child Psychol. 2019, 181, 75–101. [Google Scholar] [CrossRef]
- Caplan, J.B.; Legge, E.L.G.; Cheng, B.; Madan, C.R. Effectiveness of the method of loci is only minimally related to factors that should influence imagined navigation. Q. J. Exp. Psychol. 2019, 72, 2541–2553. [Google Scholar] [CrossRef]
- Aumont, É.; Arguin, M.; Bohbot, V.; West, G.L. Increased flanker task and forward digit span performance in caudate-nucleus-dependent response strategies. Brain Cogn. 2019, 135, 103576. [Google Scholar] [CrossRef]
- Aumont, É.; Blanchette, C.A.; Bohbot, V.D.; West, G.L. Caudate nucleus-dependent navigation strategies are associated with increased risk-taking and set-shifting behavior. Learn. Mem. 2019, 26, 101–108. [Google Scholar] [CrossRef]
- Raiesdana, S. Modeling the interaction of navigational systems in a reward-based virtual navigation task. J. Integr. Neurosci. 2018, 17, 45–67. [Google Scholar] [CrossRef]
- Dahmani, L.; Patel, R.M.; Yang, Y.; Chakravarty, M.M.; Fellows, L.K.; Bohbot, V.D. An intrinsic association between olfactory identification and spatial memory in humans. Nat. Commun. 2018, 9, 4162. [Google Scholar] [CrossRef]
- Aumont, É.; Bohbot, V.D.; West, G.L. Spatial learners display enhanced oculomotor performance. J. Cogn. Psychol. 2018, 30, 872–879. [Google Scholar] [CrossRef]
- Konishi, K.; Joober, R.; Poirier, J.; MacDonald, K.; Chakravarty, M.; Patel, R.; Breitner, J.; Bohbot, V.D. Healthy versus entorhinal cortical atrophy identification in asymptomatic APOE4 carriers at risk for Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 61, 1493–1507. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.A.; Claus Henn, B.; Austin, C.; Zonic, S.; Fedrighi, C.; Cagna, G.; Placidi, D.; White, R.F.; Yang, Q.; Coulle, B.A.; et al. Manganese in teeth and neurobehavior: Sex-specific windows of susceptibility. Environ. Int. 2017, 108, 299–308. [Google Scholar] [CrossRef]
- Wilkins, L.K.; Girard, T.A.; Herdman, K.A.; Antonovaa, E.; Dawson, G.R.; Dourish, C.T.; Craig, K.J.; Simmons, A.; Wilcock, G.K.; McCulloch, E.; et al. Hippocampal activation and memory in schizophrenia depend on spatial strategy use in a virtual maze. Psychiatry Res.-Neuroimaging 2017, 268, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cyr, M.; Wang, Z.; Tau, G.Z.; Zhao, G.; Friedl, E.; Stefan, M.; Terranova, K.; Marsh, R. Reward-Based Spatial Learning in Teens With Bulimia Nervosa. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 962–971. [Google Scholar] [CrossRef]
- Migo, E.M.; O’Daly, O.; Mitterschiffthaler, M.; Antonova, E.; Dawson, G.R.; Dourish, C.T.; Craig, K.J.; Simmons, A.; Wilcock, G.K.; McCulloch, E.; et al. Investigating virtual reality navigation in amnestic mild cognitive impairment using fMRI. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2016, 23, 196–217. [Google Scholar] [CrossRef]
- Robaey, P.; McKenzie, S.; Schachar, R.; Boivin, M.; Bohbot, V.D. Stop and look! Evidence for a bias towards virtual navigation response strategies in children with ADHD symptoms. Behav. Brain Res. 2016, 298, 48–54. [Google Scholar] [CrossRef]
- Marsh, R.; Tau, G.Z.; Wang, Z.; Huo, Y.; Liu, G.; Hao, X.; Packard, M.J.; Peterson, B.S.; Simpson, B. Reward-based spatial learning in unmedicated adults with obsessive-compulsive disorder. Am. J. Psychiatry 2015, 172, 383–392. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kho, S.; Yoo, H.B.; Park, S.; Choi, J.S.; Kwon, J.S.; Cha, K.R.; Jung, H.Y. Spatial memory impairments in amnestic mild cognitive impairment in a virtual radial arm maze. Neuropsychiatr. Dis. Treat. 2014, 10, 653–660. [Google Scholar] [CrossRef]
- Pirogovsky, E.; Holden, H.M.; Jenkins, C.; Peavy, G.M.; Salmon, D.P.; Galasko, D.R.; Gilbert, P.E. Temporal sequence learning in healthy aging and amnestic mild cognitive impairment. Exp. Aging Res. 2013, 39, 371–381. [Google Scholar] [CrossRef][Green Version]
- Konishi, K.; Bohbot, V.D. Spatial navigational strategies correlate with gray matter in the hippocampus of healthy older adults tested in a virtual maze. Front. Aging Neurosci. 2013, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, L.K.; Girard, T.A.; Konishi, K.; King, M.; Herdman, K.A.; King, J.; Christensen, B.; Bohbot, V.D. Selective deficit in spatial memory strategies contrast to intact response strategies in patients with schizophrenia spectrum disorders tested in a virtual navigation task. Hippocampus 2013, 23, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Konishi, K.; Etchamendy, N.; Roy, S.; Marighetto, A.; Rajah, N.; Bohbot, V.D. Decreased functional magnetic resonance imaging activity in the hippocampus in favor of the caudate nucleus in older adults tested in a virtual navigation task. Hippocampus 2013, 23, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Andersen, N.E.; Dahmani, L.; Konishi, K.; Bohbot, V.D. Eye tracking, strategies, and sex differences in virtual navigation. Neurobiol. Learn. Mem. 2012, 97, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Lucchini, R.; Bellinger, D.C.; Hoffman, E.; Nazzaro, M.; Smith, D.R.; Wright, R.D. Predictors of virtual radial arm maze performance in adolescent Italian children. Neurotoxicology 2012, 33, 1203–1211. [Google Scholar] [CrossRef]
- Bohbot, V.D.; McKenzie, S.; Konishi, K.; Fouquet, C.; Kurdi, V.; Schachar, R.; Boivin, M.; Robaey, P. Virtual navigation strategies from childhood to senescence: Evidence for changes across the life span. Front. Aging Neurosci. 2012, 4, 28. [Google Scholar] [CrossRef]
- Spieker, E.A.; Astur, R.S.; West, J.T.; Griego, J.A.; Rowland, L.M. Spatial memory deficits in a virtual reality eight-arm radial maze in schizophrenia. Schizophr. Res. 2012, 135, 84–89. [Google Scholar] [CrossRef]
- Schwabe, L.; Bohbot, V.D.; Wolf, O.T. Prenatal stress changes learning strategies in adulthood. Hippocampus 2012, 22, 2136–2143. [Google Scholar] [CrossRef]
- Etchamendy, N.; Konishi, K.; Pike, G.B.; Marighetto, A.; Bohbot, V.D. Evidence for a virtual human analog of a rodent relational memory task: A study of aging and fMRI in young adults. Hippocampus 2012, 22, 869–880. [Google Scholar] [CrossRef]
- Bohbot, V.D.; Gupta, M.; Banner, H.; Dahmani, L. Caudate nucleus-dependent response strategies in a virtual navigation task are associated with lower basal cortisol and impaired episodic memory. Neurobiol. Learn. Mem. 2011, 96, 173–180. [Google Scholar] [CrossRef]
- Banner, H.; Bhat, V.; Etchamendy, N.; Joober, R.; Bohbot, V.D. The brain-derived neurotrophic factor Val66Met polymorphism is associated with reduced functional magnetic resonance imaging activity in the hippocampus and increased use of caudate nucleus-dependent strategies in a human virtual navigation task. Eur. J. Neurosci. 2011, 33, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Marsh, R.; Hao, X.; Xu, D.; Wang, Z.; Duan, Y.; Liu, J.; Kangarlu, A.; Martinez, D.; Garcia, F.; Tau, G.; et al. A virtual reality-based FMRI study of reward-based spatial learning. Neuropsychologia 2010, 48, 2912–2921. [Google Scholar] [CrossRef] [PubMed]
- Goodrich-Hunsaker, N.J.; Hopkins, R.O. Spatial memory deficits in a virtual radial arm maze in amnesic participants with hippocampal damage. Behav. Neurosci. 2010, 124, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Pirogowsky, E.; Goldstein, J.; Peavy, G.; Jacobson, M.W.; Corey-Bloom, J.; Gilbert, P.E. Temporal order memory deficits prior to clinical diagnosis in Huntington’s disease. J. Int. Neuropsychol. Soc. 2009, 15, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Q.; Koerting, J. Sexual orientation-related differences in allocentric spatial memory tasks. Hippocampus 2008, 18, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Bohbot, V.D.; Lerch, J.; Thorndycraft, B.; Iaria, G.; Zijdenbos, A.P. Gray matter differences correlate with spontaneous strategies in a human virtual navigation task. J. Neurosci. 2007, 27, 10078–10083. [Google Scholar] [CrossRef] [PubMed]
- Levy, L.J.; Astur, R.S.; Frick, K.M. Men and women differ in object memory but not performance of a virtual radial maze. Behav. Neurosci. 2005, 119, 853–862. [Google Scholar] [CrossRef]
- Astur, R.S.; Tropp, J.; Sava, S.; Constable, R.T.; Markus, E.J. Sex differences and correlations in a virtual Morris water task, a virtual radial arm maze, and mental rotation. Behav. Brain Res. 2004, 151, 103–115. [Google Scholar] [CrossRef]
- Astur, R.S.; Taylor, L.B.; Mamelak, A.N.; Philpott, L.; Sutherland, R.J. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav. Brain Res. 2002, 132, 77–84. [Google Scholar] [CrossRef]
- O’Keefe, J.; Nadel, L. The Hippocampus as a Cognitive Map; Clarendon Press: Oxford, UK, 1978. [Google Scholar]
- Mandolesi, L.; Leggio, M.G.; Graziano, A.; Neri, P.; Petrosini, L. Cerebellar contribution to spatial event processing: Involvement in procedural and working memory components. Eur. J. Neurosci. 2001, 14, 2020–2022. [Google Scholar] [CrossRef]
- Mandolesi, L.; Leggio, M.G.; Spirito, F.; Petrosini, L. Cerebellar contribution to spatial event processing: Do spatial procedures contribute to formation of spatial declarative knowledge? Eur. J. Neurosci. 2003, 18, 2618–2626. [Google Scholar] [CrossRef] [PubMed]
- Jarrard, L.E. On the role of the hippocampus in learning and memory in the rat. Behav. Neural Biol. 1993, 60, 9–26. [Google Scholar] [CrossRef]
- Farran, E.K.; Purser, H.R.M.; Courbois, Y.; Ballé, M.; Sockeel, P.; Mellier, D.; Blades, M. Route knowledge and configural knowledge in typical and atypical development: A comparison of sparse and rich environments. J. Neurodev. Disord. 2015, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- McLaren-Gradinaru, M.; Burles, F.; Dhillon, I.; Retsinas, A.; Umiltà, A.; Hannah, J.; Dolhan, K.; Iaria, G. A Novel Training Program to Improve Human Spatial Orientation: Preliminary Findings. Front. Hum. Neurosci. 2020, 14, 5. [Google Scholar] [CrossRef]
- Serino, A. Peripersonal space (PPS) as a multisensory interface between the individual and the environment, defining the space of the self. Neurosci. Biobehav. Rev. 2019, 99, 138–159. [Google Scholar] [CrossRef]
- Krokos, E.; Plaisant, C.; Varshney, A. Virtual memory palaces: Immersion aids recall. Virtual Real. 2019, 23, 1–15. [Google Scholar] [CrossRef]
- Bohbot, V.D.; Copara, M.S.; Gotman, J.; Ekstrom, A.D. Low-frequency theta oscillations in the human hippocampus during real-world and virtual navigation. Nat. Commun. 2017, 8, 14415. [Google Scholar] [CrossRef]
- Astur, R.S.; St. Germain, S.A.; Baker, E.K.; Calhoun, V.; Pearlson, G.D.; Constable, R.T. fMRI hippocampal activity during a virtual radial arm maze. Appl. Psychophysiol. Biofeedback 2005, 30, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Thornberry, C.; Cimadevilla, J.M.; Commins, S. Virtual Morris water maze: Opportunities and challenges. Rev. Neurosci. 2021, 32, 887–903. [Google Scholar] [CrossRef]
| Authors | Sample Characteristics | RAM Paradigm | Arms |
|---|---|---|---|
| Serra et al., 2021 [39] | Children (healthy) | Forced-choice/ Table RAM | 8 |
| N = 28; Age: m = 8.1, SD = 1.1 | |||
| Foti et al., 2018 [40] | Children (healthy) | Free-choice | 8 |
| N = 36; Age: m = 5.3, SD = 0.2 | |||
| Moraleda et al., 2013 [41] | Children (healthy) | Free-choice/ Table RAM | 4 |
| N = 48; Reported age range from 6 to 10 years | |||
| Mandolesi et al., 2009 [42] | Children (healthy) | Free-choice Forced-choice | 8 |
| N = 90; Reported age range from 3 to 8 years | |||
| Foreman et al., 1994 [43] | Children (healthy) | Free-choice/ Forced-choice | 12 |
| N = 28; Reported age: m = 6, SD = 4.5 | |||
| Foreman et al., 1990 [44] | Children (healthy) | Forced-choice | 10 |
| N = 18; Age: m = 3.3, SD: Not reported | |||
| Foreman et al., 1984 [45] | Children (healthy) | Free-choice | 8 |
| N = 10; Reported age range from 2 to 4 years | |||
| Overman et al., 1996 [46] | Children/young adults (healthy) | Free-choice/ Forced-choice | 8 |
| N = 43; Children reported age range from 20 to 151 months; Young adults age range from 17 to 21 years | |||
| Aadland et al., 1985 [47] | Children/young adults (healthy) | Free-choice/ Forced-choice | 8 |
| N = 146; Reported age range from 18 to 71 months | |||
| O’Connor & Glassman, 1993 [48] | Young adults (healthy) | Free-choice (drawn on paper) | 17 |
| N = 15; Age: Not reported | |||
| Glassman et al., 1994 [49] | Young adults (healthy) | Free-choice | 17/13 |
| N = 57; Age: Not reported | |||
| Glassman et al., 1998 [50] | Young adults/adults (healthy) | Free-choice | 8 |
| N = 21; Reported age range from 18 to 35 years | |||
| Leitner et al., 2005 [51] | Children (Intrauterine growth retardation) | Free-choice/ Forced-choice | 8 |
| N = 28; Reported age: 6 years, SD: Not reported | |||
| Foti et al., 2011 [52] | Children/adolescents (Prader-Willi syndrome and Williams syndrome) | Free-choice/ Forced-choice | 8 |
| N = 24; PWS Mental age: m = 6.0, SD = 0.5; WS Mental age: m = 6.0, SD = 0.3 | |||
| Mandolesi et al., 2009 [53] | Children/Adolescents (Williams syndrome) | Free-choice/ Forced-choice | 8 |
| N = 14; Mental age: m = 6.2, SD = 1.4 | |||
| Foti et al., 2020 [54] | Adolescents (Williams syndrome) | Free-choice/ Forced-choice | 8 |
| N = 15; Mental age: m = 18.1, SD = 5.2 | |||
| Bertholet et al., 2015 [55] | Young adults (Intellectual disability and healthy) | Free-choice/ Forced-choice | 8 |
| N = 107; Age: m = 22.8, SD = 0.7 | |||
| Palermo et al., 2014 [56] | Adults (Developmental topographical disorientation) | Free-choice/ Forced-choice | 8 |
| N = 2; patient 1: reported age 29 years; patient 2: reported age 38 years | |||
| Bohbot et al., 2002 [57] | Adults (Temporal lesions and healthy) | Free-choice | 8 |
| N = 52; Age: m = 38, SD = 1.3 |
| Authors | Sample Characteristics | Virtual Modality | RAM Paradigm | Arms |
|---|---|---|---|---|
| Kim et al., 2018 [58] | Young adults (healthy) | Immersive | Forced-choice | 8 |
| N = 80; Age: m = 23.2 SD = 2.4 | ||||
| Ben-Zeev et al., 2020 [59] | Young adults (healthy) | Immersive | Free-choice | 8 |
| N = 40; Age: m = 25.5, SD = 1.6, | ||||
| Patel et al., 2021 [35] | Young adults (healthy) | Non-immersive | Forced-choice | 8 |
| N = 86; Age: m = 19.0, SD = 1.0 | ||||
| Somma et al., 2021 [60] | Young adults (healthy) | Non-immersive | Free-choice | 8 |
| N = 47; Age: m = 20, SD = 1.0 | ||||
| Taheri Gorji et al., 2021 [61] | Young adults (healthy) | Non-immersive | Forced-choice | 6 |
| N = 42; Age: m = 24.4, SD = 2.4 | ||||
| Rechtman et al., 2020 [62] | Children (exposed to manganese) | Non-immersive | Forced-choice | 8 |
| N = 188; Age: m = 12.01, SD = 0.9 | ||||
| Sodums & Bohbot, 2020 [63] | Elderly people (healthy) | Non-immersive | Forced-choice | 12 |
| N = 39; Age: m = 64.6, SD = 4.1 | ||||
| Dahmani et al., 2020 [64] | Young adults (healthy) | Non-immersive | Forced-choice | 8 |
| N = 55; Age: m = 22.9, SD = 3.5 | ||||
| Goodman et al., 2020 [65] | Young adults (healthy) | Non-immersive | Forced-choice | 8 |
| N = 62; Age: m = 23, SD = 6.3 | ||||
| Yang et al., 2019 [66] | Children/young adults (healthy) | Non-immersive | Free-choice | 6 |
| N = 83; Children: reported age range from 6 to 10 years; Young adults: reported age range from 18 to 22 years | ||||
| Caplan et al., 2019 [67] | Young adults (healthy) | Non-immersive | Free-choice | 8 |
| N = 173; Age: m = 19.5, SD = 2.5 | ||||
| Aumont et al., 2019 [68] | Young adults (healthy) | Non-immersive | Forced-choice | 8 |
| N = 50; Age: m = 23.4, SD = 4.1 | ||||
| Aumont et al., 2019 [69] | Young adults (healthy) | Non-immersive | Forced-choice | 8 |
| N = 53; Age: m = 23.9, SD = 4.4 | ||||
| Raiesdana, 2018 [70] | Young adults (healthy) | Non-immersive | Free-choice | 8 |
| N = 8; Age: m = 22.1, SD = 2.3 | ||||
| Dahmani et al., 2018 [71] | Young adults/adult (healthy/focal lesion to the frontal lobe) | Non-immersive | Forced-choice | 8 |
| N = 78; Young adults: Age: m = 22.9, SD = 3.5; Patients: group 1: Age m = 56.6, SD = 16.2; group 2: Age m = 60, SD = 6.7 | ||||
| Aumont et al., 2018 [72] | Young adults (healthy) | Non-immersive | Forced-choice | 8 |
| N = 50; Age: m = 23.4, SD = 4.1 | ||||
| Konishi et al., 2018 [73] | Elderly people (healthy) | Non-immersive | Forced-choice | 12 |
| N = 66; Age: m = 66.1, SD = 4.5 | ||||
| Bauer et al., 2017 [74] | Children (exposed to manganese) | Non-immersive | Forced-choice | 8 |
| N = 142; Age: m = 12.4, SD = 0.9 | ||||
| Wilkins et al., 2017 [75] | Adults (Schizophrenia) | Non-immersive | Forced-choice | 8 |
| N = 16; Age: m = 44.4, SD = 6.1; | ||||
| Cyr et al., 2016 [76] | Adolescents (Bulimia nervosa) | Non-immersive | Free-choice | 8 |
| N = 27; Age: m = 16.6 SD = 1.5 | ||||
| Migo et al., 2016 [77] | Elderly people (Mild cognitive impairment) | Non-immersive | Free-choice | 8 |
| N = 8; Age: m = 69.6, SD = 5.8 | ||||
| Robaey et al., 2016 [78] | Children (ADHD) | Non-immersive | Forced-choice | 8 |
| N = 223; Age: m = 8.4, SD = 0.1 | ||||
| Marsh et al., 2015 [79] | Adults (Obsessive compulsive disorder) | Non-immersive | Free-choice | 8 |
| N = 33; Age: m = 29.4, SD = 8.1 | ||||
| Lee et al., 2014 [80] | Elderly people (Alzheimer’s Disease, Mild cognitive impairment) | Non-immersive | Forced-choice | 6 |
| N = 40; AD Age: m = 72.4, SD = 5.6; MCI: Age m = 70.7, SD = 5 | ||||
| Pirogovsky et al., 2013 [81] | Elderly people (Mild Cognitiv Impairment) | Non-immersive | Free-choice | 8 |
| N = 10; Age: m = 76.8, SD = 2.3 | ||||
| Konishi & Bohbot, 2013 [82] | Elderly people (healthy) | Non-immersive | Forced-choice | 8 |
| N = 45; Age: m = 64.3, SD = 4 | ||||
| Wilkins et al., 2013 [83] | Adults (Schizophrenia) | Non-immersive | Forced-choice | 8 |
| N = 17; Age: m = 44.9, SD = 4.8; Age: m = 39.6, SD = 9.8 | ||||
| Konishi et al., 2013 [84] | Young adults/elderly people (healthy) | Non-immersive | Forced-choice | 12 |
| N = 52; Young adults: Age: m = 23.8, SD = 3.8; Elderly people: Age: m = 64.2, SD = 4.7 | ||||
| Andersen et al., 2012 [85] | Young adults (healthy) | Non-immersive | Forced-choice | 8 |
| N = 7; Age: m = 28.1, SD = 5.6 | ||||
| Braun et al., 2012 [86] | Children (exposed to manganese) | Non-immersive | Forced-choice | 8 |
| N = 255; Age: m = 13, SD = 0.9 | ||||
| Bohbot et al., 2012 [87] | Children/young adults/elderly people (healthy) | Non-immersive | Forced-choice | 8 |
| N = 599; Children: Age: m = 8.4, SD = 0.1; Young adults: Age: m = 25.6, SD = 4.6; Elderly people: Age: m = 65.6, SD = 5.6 | ||||
| Spieker et al., 2012 [88] | Adults (Schizophrenia) | Non-immersive | Forced-choice | 8 |
| N = 33; Age: m = 40.0, SD = 11.9 | ||||
| Schwabe et al., 2012 [89] | Young adults (healthy) | Non-immersive | Forced-choice | 8 |
| N = 60; Age: m = 24.4, SD = 0.4 | ||||
| Etchamendy et al., 2012 [90] | Young adults/elderly people (healthy) | Non-immersive | Forced-choice | 12 |
| N = 55; Young adults: Age: m = 25.1, SD = 4.1; Elderly people: Age: m = 66.9, SD = 7.9 | ||||
| Bohbot et al., 2011 [91] | Young adults (healthy) | Non-immersive | Forced-choice | 8 |
| N = 66; Age: m = 21.6, SD = 0.81; | ||||
| Banner et al., 2011 [92] | Young adults (healthy) | Non-immersive | Forced-choice | 8 |
| N = 106; Age: m = 23.4, SD = 1.1 | ||||
| Marsh et al., 2010 [93] | Adults (healthy) | Non-immersive | Free-choice | 8 |
| N = 25; Age: m = 32.5, SD = 7.6 | ||||
| Goodrich-Hunsaker & Hopkins, 2010 [94] | Adults (Hippocampal damage) | Non-immersive | Forced-choice | 8 |
| N = 5; Age: m = 45.6, SD = 9.4 | ||||
| Pirogovsky et al., 2009 [95] | Adults (Huntington’s disease) | Non-immersive | Free-choice | 8 |
| N = 18; Age: m = 46.4, SD = 2.1 | ||||
| Rahman & Koerting, 2008 [96] | Young adults/adults (healthy) | Non-immersive | Forced-choice | 8 |
| N = 140; Reported age range from 19 to 45 years | ||||
| Bohbot et al., 2007 [97] | Young adults (healthy) | Non-immersive | Forced-choice | 8 |
| N = 30; Age: m = 27.9, SD = 4.1 | ||||
| Levy et al., 2005 [98] | Young adults (healthy) | Non-immersive | Forced-choice | 12 |
| N = 55; Reported age range from 18 to 30 years | ||||
| Astur et al., 2004 [99] | Young adults (healthy) | Non-immersive | Forced-choice | 8 |
| N = 13; Reported age range from 18 to 30 years | ||||
| Astur et al., 2002 [100] | Young adults (healthy) | Non-immersive | Forced-choice | 8 |
| N = 61; Age: m = 19.4, SD = 4.8 | ||||
| Bohbot et al.,2004 [33] | Adults (medial temporal lobe resections) | Non-immersive | Forced-choice | 8 |
| N = 15; Age: m = 42.5, SD = 8.7 | ||||
| Iaria et al., 2003 [34] | Young adults (healthy) | Non-immersive | Forced-choice | 8 |
| N = 50; Age: m = 27.7, SD = 4.7 |
| Free-Choice RAM Version | Forced-Choice RAM Version (Referred to the Second Phase of the Task) |
|---|---|
| Total time to complete the entire task Time to reach each reward | Total time to complete the second phase of the task |
| Latency to select the first arm | Latency to select the first arm |
| Total entries (arms correct and incorrect visited) | Total entries (arms correct and incorrect visited) |
| Distance travelled | Distance travelled |
| Movement speed | Errors |
| Frequency of successes/Percentage of correct visits/Search efficiency | Across-phase errors |
| Errors/Error-free trials | Within-phase errors |
| The longest sequence of correctly visited arms | The longest sequence of correctly visited arms |
| Percentage of angles turned (45°, 90°, 135°, 180° or 360°)/Angle change/Strategy fixation | |
| Perseverations (consecutive entries into the same arm or the re-entries into a fixed sequence of arms) | |
| Declarative mastery |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palombi, T.; Mandolesi, L.; Alivernini, F.; Chirico, A.; Lucidi, F. Application of Real and Virtual Radial Arm Maze Task in Human. Brain Sci. 2022, 12, 468. https://doi.org/10.3390/brainsci12040468
Palombi T, Mandolesi L, Alivernini F, Chirico A, Lucidi F. Application of Real and Virtual Radial Arm Maze Task in Human. Brain Sciences. 2022; 12(4):468. https://doi.org/10.3390/brainsci12040468
Chicago/Turabian StylePalombi, Tommaso, Laura Mandolesi, Fabio Alivernini, Andrea Chirico, and Fabio Lucidi. 2022. "Application of Real and Virtual Radial Arm Maze Task in Human" Brain Sciences 12, no. 4: 468. https://doi.org/10.3390/brainsci12040468
APA StylePalombi, T., Mandolesi, L., Alivernini, F., Chirico, A., & Lucidi, F. (2022). Application of Real and Virtual Radial Arm Maze Task in Human. Brain Sciences, 12(4), 468. https://doi.org/10.3390/brainsci12040468






