A Pilot Randomized Controlled Trial of Goal Management Training in Canadian Military Members, Veterans, and Public Safety Personnel Experiencing Post-Traumatic Stress Symptoms
Abstract
:1. Introduction
Study Objectives and Hypotheses
2. Materials and Methods
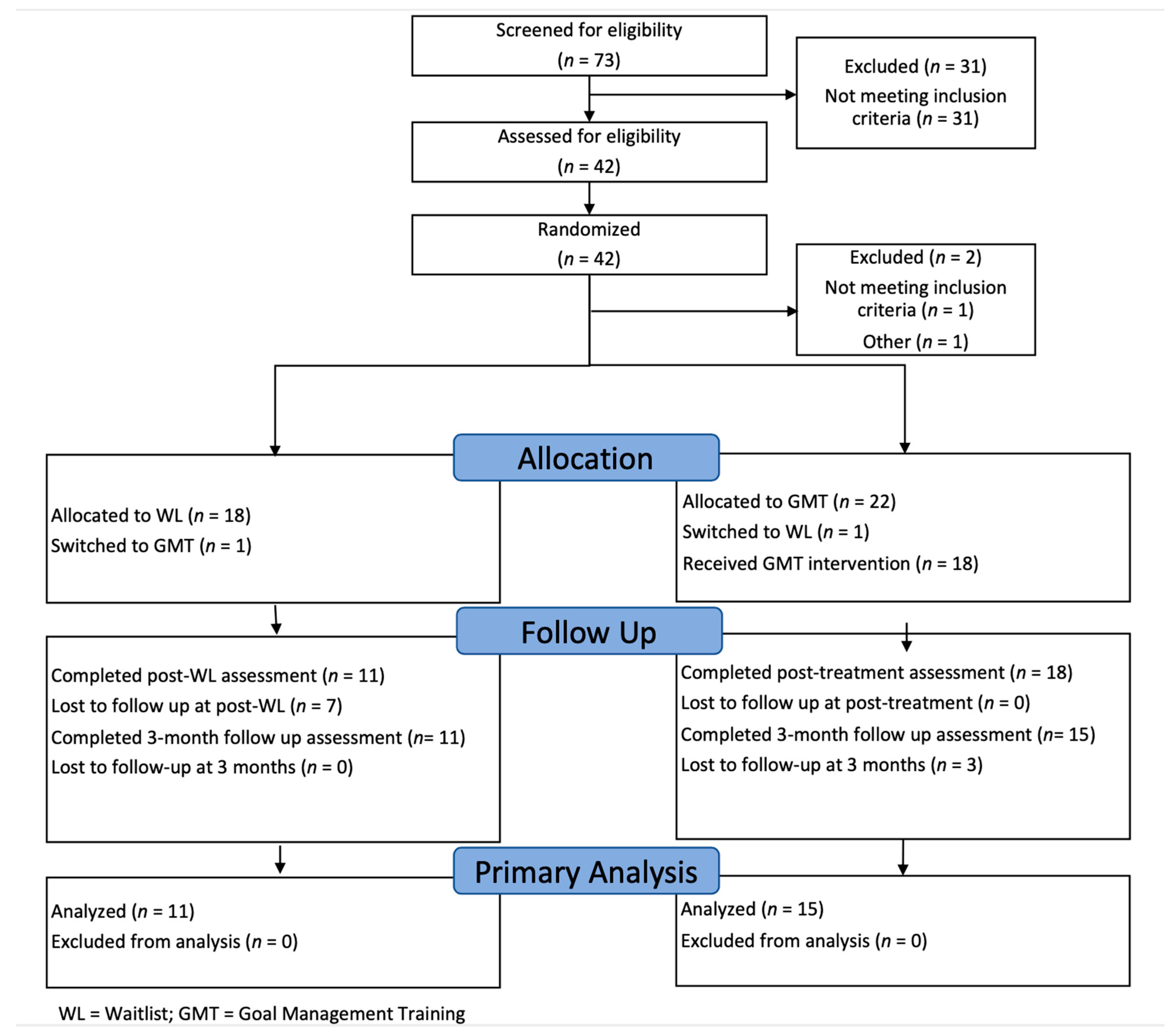
2.1. Participants
2.2. Experimental Design and Procedure
2.3. Study Conditions
2.4. Measures
2.4.1. Clinician-Administered Interviews
2.4.2. Neuropsychological Assessment
2.4.3. Subjective Cognition
2.4.4. Functional Outcomes
2.4.5. Self-Report Symptom Measures
2.5. Statistical Methods
3. Results
3.1. Pre- and Post-Analysis of Neuropsychological Assessment Performance
3.1.1. Tests of Executive Functioning, Processing Speed, and Attention
3.1.2. Declarative Memory
3.2. Pre and Post-Analysis of Subjective Cognition, Functioning, and Self-Report Symptom Measures
3.3. Trajectory of Change for Subjective Cognition and Self-Report Symptom Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Oliphant, R.C. Healthy Minds, Safe Communities: Supporting Our Public Safety Officers through a National Strategy for Operational Stress Injuries; Standing Committee on Public Safety and National Security, Ed.; Standing Committee on Public Safety and National Security: Ottawa, ON, Canada, 2016. [Google Scholar]
- Carleton, R.N.; Afifi, T.O.; Turner, S.; Taillieu, T.; Duranceau, S.; LeBouthillier, D.M.; Sareen, J.; Ricciardelli, R.; Macphee, R.S.; Groll, D.; et al. Mental Disorder Symptoms among Public Safety Personnel in Canada. Can. J. Psychiatry 2018, 63, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Boulos, D.; Zamorski, M.A. Deployment-related mental disorders among Canadian Forces personnel deployed in support of the mission in Afghanistan, 2001–2008. Can. Med. Assoc. J. 2013, 185, E545–E552. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.; VanTil, L.D.; Zamorski, M.A.; Garber, B.; Dursun, S.; Fikretoglu, D.; Ross, D.; Richardson, J.D.; Sareen, J.; Sudom, K.; et al. Mental health of Canadian Armed Forces Veterans: Review of population studies. J. Mil. Veter. Fam. Health 2016, 2, 70–86. [Google Scholar] [CrossRef]
- Van Ameringen, M.; Mancini, C.; Patterson, B.; Boyle, M.H. Post-Traumatic Stress Disorder in Canada. CNS Neurosci. Ther. 2008, 14, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Sareen, J.; Bolton, S.-L.; Mota, N.; Afifi, T.O.; Enns, M.W.; Taillieu, T.; Stewart-Tufescu, A.; El-Gabalawy, R.; Marrie, R.A.; Richardson, J.D.; et al. Lifetime Prevalence and Comorbidity of Mental Disorders in the Two-wave 2002–2018 Canadian Armed Forces Members and Veterans Mental Health Follow-up Survey (CAFVMHS): Prévalence et Comorbidité de Durée de vie Des Troubles Mentaux Dans l’Enquête de Suivi Sur la Santé Mentale Auprès des Membres des Forces Armées Canadiennes et Des ex-Militaires (ESSMFACM) en Deux Cycles de 2002 à 2018. Can. J. Psychiatry 2021, 66, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.; Armour, C.; Southwick, S.M.; Pietrzak, R.H. Dissociative subtype of DSM-5 posttraumatic stress disorder in U.S. veterans. J. Psychiatr. Res. 2015, 66–67, 67–74. [Google Scholar] [CrossRef]
- Kessler, R.C.; Sonnega, A.; Bromet, E.; Hughes, M.; Nelson, C.B. Posttraumatic Stress Disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 1995, 52, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Gratz, K.L.; Roemer, L. Multidimensional Assessment of Emotion Regulation and Dysregulation: Development, Factor Structure, and Initial Validation of the Difficulties in Emotion Regulation Scale. J. Psychopathol. Behav. Assess. 2004, 26, 41–54. [Google Scholar] [CrossRef]
- Bardeen, J.R.; Kumpula, M.J.; Orcutt, H.K. Emotion regulation difficulties as a prospective predictor of posttraumatic stress symptoms following a mass shooting. J. Anxiety Disord. 2013, 27, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Coates, A.A.; Messman-Moore, T.L. A structural model of mechanisms predicting depressive symptoms in women following childhood psychological maltreatment. Child Abus. Negl. 2014, 38, 103–113. [Google Scholar] [CrossRef]
- Ehring, T.; Quack, D. Emotion Regulation Difficulties in Trauma Survivors: The Role of Trauma Type and PTSD Symptom Severity. Behav. Ther. 2010, 41, 587–598. [Google Scholar] [CrossRef]
- Klemanski, D.H.; Mennin, D.S.; Borelli, J.L.; Morrissey, P.M.; Aikins, D.E. Emotion-related regulatory difficulties contribute to negative psychological outcomes in active-duty Iraq war soldiers with and without posttraumatic stress disorder. Depress. Anxiety 2012, 29, 621–628. [Google Scholar] [CrossRef]
- Lanius, R.A.; Vermetten, E.; Loewenstein, R.J.; Brand, B.; Schmahl, C.; Bremner, J.D.; Spiegel, D. Emotion Modulation in PTSD: Clinical and Neurobiological Evidence for a Dissociative Subtype. Am. J. Psychiatry 2010, 167, 640–647. [Google Scholar] [CrossRef] [Green Version]
- Seligowski, A.V.; Lee, D.; Bardeen, J.R.; Orcutt, H.K. Emotion Regulation and Posttraumatic Stress Symptoms: A Meta-Analysis. Cogn. Behav. Ther. 2014, 44, 87–102. [Google Scholar] [CrossRef]
- Suveg, C.; Morelen, D.; Brewer, G.A.; Thomassin, K. The Emotion Dysregulation Model of Anxiety: A preliminary path analytic examination. J. Anxiety Disord. 2010, 24, 924–930. [Google Scholar] [CrossRef]
- Tull, M.T.; Barrett, H.M.; McMillan, E.S.; Roemer, L. A Preliminary Investigation of the Relationship between Emotion Regulation Difficulties and Posttraumatic Stress Symptoms. Behav. Ther. 2007, 38, 303–313. [Google Scholar] [CrossRef]
- Tull, M.T.; Berghoff, C.R.; Wheeless, L.E.; Cohen, R.T.; Gratz, K.L. PTSD Symptom Severity and Emotion Regulation Strategy Use during Trauma Cue Exposure among Patients with Substance Use Disorders: Associations With Negative Affect, Craving, and Cortisol Reactivity. Behav. Ther. 2018, 49, 57–70. [Google Scholar] [CrossRef]
- Tull, M.T.; Vidaña, A.G.; Betts, J.E. Emotion regulation difficulties in PTSD. In Emotion in Posttraumatic Stress Disorder: Etiology, Assessment, Neurobiology, and Treatment; Tull, M.T., Kimbrel, N.A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 295–310. [Google Scholar]
- Resick, P.A.; Monson, C.M.; Chard, K.M. Cognitive Processing Therapy for PTSD: A Comprehensive Manual; The Guilford Press: New York, NY, USA, 2016. [Google Scholar]
- Resick, P.A.; Suvak, M.K.; Johnides, B.D.; Mitchell, K.S.; Iverson, K.M. The impact of dissociation on PTSD treatment with cognitive processing therapy. Depress. Anxiety 2012, 29, 718–730. [Google Scholar] [CrossRef]
- Foa, E.B.; Rothbaum, B.O. Treatment Manuals for Practitioners. Treating the Trauma of Rape: Cognitive-Behavioral Therapy for PTSD; Guilford Press: New York, NY, USA, 1998. [Google Scholar]
- Millan, M.J.; Agid, Y.; Brüne, M.; Bullmore, E.; Carter, C.S.; Clayton, N.; Connor, R.; Davis, S.; Deakin, J.; DeRubeis, R.; et al. Cognitive dysfunction in psychiatric disorders: Characteristics, causes and the quest for improved therapy. Nat. Rev. Drug Discov. 2012, 11, 141–168. [Google Scholar] [CrossRef]
- Brewin, C.R.; Kleiner, J.S.; Vasterling, J.J.; Field, A.P. Memory for emotionally neutral information in posttraumatic stress disorder: A meta-analytic investigation. J. Abnorm. Psychol. 2007, 116, 448–463. [Google Scholar] [CrossRef] [Green Version]
- Jak, A.J.; Crocker, L.D.; Aupperle, R.L.; Clausen, A.; Bomyea, J. Neurocognition in PTSD: Treatment insights and implications. In Behavioral Neurobiology of PTSD; Vermetten, E., Baker, D.G., Risbrough, V.B., Eds.; Springer: Cham, Switzerland, 2016; pp. 93–116. [Google Scholar]
- Kw, S. Post-traumatic stress disorder and declarative memory functioning: A review. Dialogues Clin. Neurosci. 2011, 13, 346–351. [Google Scholar] [CrossRef]
- Scott, J.C.; Matt, G.; Wrocklage, K.M.; Crnich, C.; Jordan, J.; Southwick, S.M.; Krystal, J.H.; Schweinsburg, B.C. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol. Bull. 2015, 141, 105–140. [Google Scholar] [CrossRef]
- Allard, C.; Aupperle, R.L.; Grimes, E.M.; Simmons, A.N.; Flagan, T.; Behrooznia, M.; Cissell, S.H.; Twamley, E.W.; Thorp, S.R.; Norman, S.B.; et al. Dorsolateral Prefrontal Cortex Activation During Emotional Anticipation and Neuropsychological Performance in Posttraumatic Stress Disorder. Arch. Gen. Psychiatry 2012, 69, 360–371. [Google Scholar] [CrossRef] [Green Version]
- Aupperle, R.L.; Melrose, A.J.; Stein, M.B.; Paulus, M.P. Executive function and PTSD: Disengaging from trauma. Neuropharmacology 2012, 62, 686–694. [Google Scholar] [CrossRef] [Green Version]
- Polak, A.R.; Witteveen, A.B.; Reitsma, J.B.; Olff, M. The role of executive function in posttraumatic stress disorder: A systematic review. J. Affect. Disord. 2012, 141, 11–21. [Google Scholar] [CrossRef]
- Woon, F.L.; Farrer, T.J.; Braman, C.R.; Mabey, J.K.; Hedges, D.W. A meta-analysis of the relationship between symptom severity of Posttraumatic Stress Disorder and executive function. Cogn. Neuropsychiatry 2016, 22, 1–16. [Google Scholar] [CrossRef]
- McKinnon, M.C.; Boyd, J.E.; Frewen, P.A.; Lanius, U.F.; Jetly, R.; Richardson, J.D.; Lanius, R.A. A review of the relation between dissociation, memory, executive functioning and social cognition in military members and civilians with neuropsychiatric conditions. Neuropsychologia 2016, 90, 210–234. [Google Scholar] [CrossRef] [Green Version]
- Rivera-Vélez, G.M.; González-Viruet, M.; Martínez-Taboas, A.; Pérez-Mojica, D. Post-Traumatic Stress Disorder, Dissociation, and Neuropsychological Performance in Latina Victims of Childhood Sexual Abuse. J. Child Sex. Abus. 2014, 23, 55–73. [Google Scholar] [CrossRef]
- Roca, V.; Hart, J.; Kimbrell, T.; Freeman, T. Cognitive function and dissociative disorder status among veteran subjects with chronic posttraumatic stress disorder: A preliminary study. J. Neuropsychiatry Clin. Neurosci. 2006, 18, 226–230. [Google Scholar] [CrossRef]
- Samuelson, K.W.; Engle, K.; Bartel, A.; Jordan, J.T.; Powers, T.; Abadjian, L.; Benight, C.C. The power of appraisals in predicting PTSD symptom improvement following cognitive rehabilitation: A randomized clinical trial. J. Affect. Disord. 2021, 282, 561–573. [Google Scholar] [CrossRef]
- O’Neil, M.E.; Laman-Maharg, B.; Schnurr, P.P.; Carlson, K.F.; Twamley, E.W.; Peterson, C.; Storzbach, D.; Helfand, M.; Sayer, N.A. Objective cognitive impairment and subjective cognitive problems in veterans initiating psychotherapy for posttraumatic stress disorder: An exploratory study. Appl. Neuropsychol. Adult 2019, 26, 247–254. [Google Scholar] [CrossRef]
- Samuelson, K.W.; Abadjian, L.; Jordan, J.T.; Bartel, A.; Vasterling, J.; Seal, K. The Association Between PTSD and Functional Outcome Is Mediated by Perception of Cognitive Problems Rather Than Objective Neuropsychological Test Performance. J. Trauma. Stress 2017, 30, 521–530. [Google Scholar] [CrossRef]
- Samuelson, K.W.; Bartel, A.; Valadez, R.; Jordan, J.T. PTSD symptoms and perception of cognitive problems: The roles of posttraumatic cognitions and trauma coping self-efficacy. Psychol. Trauma Theory Res. Pract. Policy 2017, 9, 537–544. [Google Scholar] [CrossRef]
- Wrocklage, K.M.; Schweinsburg, B.C.; Krystal, J.H.; Trejo, M.; Roy, A.; Weisser, V.; Moore, T.M.; Southwick, S.M.; Scott, J.C. Neuropsychological Functioning in Veterans with Posttraumatic Stress Disorder: Associations with Performance Validity, Comorbidities, and Functional Outcomes. J. Int. Neuropsychol. Soc. 2016, 22, 399–411. [Google Scholar] [CrossRef]
- Geuze, E.; Vermetten, E.; de Kloet, C.S.; Hijman, R.; Westenberg, H.G. Neuropsychological performance is related to current social and occupational functioning in veterans with posttraumatic stress disorder. Depress. Anxiety 2009, 26, 7–15. [Google Scholar] [CrossRef]
- Crocker, L.D.; Jurick, S.M.; Thomas, K.; Keller, A.; Sanderson-Cimino, M.; Boyd, B.; Rodgers, C.; Twamley, E.W.; Jak, A.J. Worse baseline executive functioning is associated with dropout and poorer response to trauma-focused treatment for veterans with PTSD and comorbid traumatic brain injury. Behav. Res. Ther. 2018, 108, 68–77. [Google Scholar] [CrossRef]
- Haaland, K.Y.; Sadek, J.R.; Keller, J.E.; Castillo, D.T. Neurocognitive Correlates of Successful Treatment of PTSD in Female Veterans. J. Int. Neuropsychol. Soc. 2016, 22, 643–651. [Google Scholar] [CrossRef]
- Nijdam, M.J.; Gersons, B.P.; Olff, M. Response to psychotherapy for posttraumatic stress disorder: The role of pretreatment verbal memory performance. J. Clin. Psychiatry 2015, 76, e1023-8. [Google Scholar] [CrossRef]
- Wild, J.; Gur, R.C. Verbal memory and treatment response in post-traumatic stress disorder. Br. J. Psychiatry 2008, 193, 254–255. [Google Scholar] [CrossRef]
- Larsen, S.E.; Fleming, C.J.E.; Resick, P.A. Residual symptoms following empirically supported treatment for PTSD. Psychol. Trauma Theory Res. Pract. Policy 2019, 11, 207–215. [Google Scholar] [CrossRef]
- Liberzon, I.; Sripada, C.S. The functional neuroanatomy of PTSD: A critical review. Prog. Brain Res. 2007, 167, 151–169. [Google Scholar] [CrossRef]
- Hayes, J.P.; VanElzakker, M.; Shin, L.M. Emotion and cognition interactions in PTSD: A review of neurocognitive and neuroimaging studies. Front. Integr. Neurosci. 2012, 6, 89. [Google Scholar] [CrossRef] [Green Version]
- Brown, V.M.; Morey, R.A. Neural Systems for Cognitive and Emotional Processing in Posttraumatic Stress Disorder. Front. Psychol. 2012, 3, 449. [Google Scholar] [CrossRef] [Green Version]
- Lanius, R.A.; Brand, B.; Vermetten, E.; Frewen, P.A.; Spiegel, D. The dissociative subtype of posttraumatic stress disorder: Rationale, clinical and neurobiological evidence, and implications. Depress. Anxiety 2012, 29, 701–708. [Google Scholar] [CrossRef]
- Nicholson, A.A.; Rabellino, D.; Densmore, M.; Frewen, P.A.; Paret, C.; Kluetsch, R.; Schmahl, C.; Theberge, J.; Neufeld, R.W.; McKinnon, M.C.; et al. The neurobiology of emotion regulation in posttraumatic stress disorder: Amygdala downregulation via real-time fMRI neurofeedback. Hum. Brain Mapp. 2017, 38, 541–560. [Google Scholar] [CrossRef]
- Fitzgerald, J.M.; DiGangi, J.A.; Phan, K.L. Functional Neuroanatomy of Emotion and Its Regulation in PTSD. Harv. Rev. Psychiatry 2018, 26, 116–128. [Google Scholar] [CrossRef]
- Andrewes, D.G.; Jenkins, L.M. The Role of the Amygdala and the Ventromedial Prefrontal Cortex in Emotional Regulation: Implications for Post-traumatic Stress Disorder. Neuropsychol. Rev. 2019, 29, 220–243. [Google Scholar] [CrossRef]
- Breukelaar, I.A.; Bryant, R.A.; Korgaonkar, M.S. The functional connectome in posttraumatic stress disorder. Neurobiol. Stress 2021, 14, 100321. [Google Scholar] [CrossRef]
- Akiki, T.; Averill, C.L.; Abdallah, C.G. A Network-Based Neurobiological Model of PTSD: Evidence From Structural and Functional Neuroimaging Studies. Curr. Psychiatry Rep. 2017, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Lanius, R.A.; Frewen, P.A.; Tursich, M.; Jetly, R.; McKinnon, M.C. Restoring large-scale brain networks in PTSD and related disorders: A proposal for neuroscientifically-informed treatment interventions. Eur. J. Psychotraumatol. 2015, 6, 27313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, R.; Spreng, R.N.; Shin, L.M.; Girard, T. Neurocircuitry models of posttraumatic stress disorder and beyond: A meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2012, 36, 2130–2142. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.L.; Clark, C.R.; McFarlane, A.C.; Moores, K.A.; Morris, P.; Egan, G.F. Abnormal frontal and parietal activity during working memory updating in post-traumatic stress disorder. Psychiatry Res. Neuroimaging 2005, 140, 27–44. [Google Scholar] [CrossRef]
- Levine, B.; Schweizer, T.A.; O’Connor, C.; Turner, G.; Gillingham, S.; Stuss, D.T.; Manly, T.; Robertson, I.H. Rehabilitation of Executive Functioning in Patients with Frontal Lobe Brain Damage with Goal Management Training. Front. Hum. Neurosci. 2011, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef]
- Menon, V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margulies, D.S.; Vincent, J.L.; Kelly, C.; Lohmann, G.; Uddin, L.Q.; Biswal, B.B.; Villringer, A.; Castellanos, F.X.; Milham, M.P.; Petrides, M. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc. Natl. Acad. Sci. USA 2009, 106, 20069–20074. [Google Scholar] [CrossRef] [Green Version]
- Lanius, R.A.; Bluhm, R.L.; Coupland, N.J.; Hegadoren, K.M.; Rowe, B.; Theberge, J.; Neufeld, R.W.J.; Williamson, P.C.; Brimson, M. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr. Scand. 2010, 121, 33–40. [Google Scholar] [CrossRef]
- Nicholson, A.A.; Densmore, M.; Frewen, P.A.; Théberge, J.; Neufeld, R.W.; McKinnon, M.C.; Lanius, R.A. The Dissociative Subtype of Posttraumatic Stress Disorder: Unique Resting-State Functional Connectivity of Basolateral and Centromedial Amygdala Complexes. Neuropsychopharmacology 2015, 40, 2317–2326. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, A.A.; Sapru, I.; Densmore, M.; Frewen, P.A.; Neufeld, R.W.; Theberge, J.; McKinnon, M.C.; Lanius, R.A. Unique insula subregion resting-state functional connectivity with amygdala complexes in posttraumatic stress disorder and its dissociative subtype. Psychiatry Res. Neuroimaging 2016, 250, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.A.; Friston, K.; Zeidman, P.; Harricharan, S.; McKinnon, M.C.; Densmore, M.; Neufeld, R.W.; Theberge, J.; Corrigan, F.; Jetly, R.; et al. Dynamic causal modeling in PTSD and its dissociative subtype: Bottom-up versus top-down processing within fear and emotion regulation circuitry. Hum. Brain Mapp. 2017, 38, 5551–5561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholson, A.A.; Densmore, M.; McKinnon, M.C.; Neufeld, R.W.; Frewen, P.A.; Théberge, J.; Jetly, R.; Richardson, J.D.; Lanius, R.A. Machine learning multivariate pattern analysis predicts classification of posttraumatic stress disorder and its dissociative subtype: A multimodal neuroimaging approach. Psychol. Med. 2019, 49, 2049–2059. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.A.; Harricharan, S.; Densmore, M.; Neufeld, R.W.; Ros, T.; McKinnon, M.C.; Frewen, P.A.; Théberge, J.; Jetly, R.; Pedlar, D.; et al. Classifying heterogeneous presentations of PTSD via the default mode, central executive, and salience networks with machine learning. NeuroImage Clin. 2020, 27, 102262. [Google Scholar] [CrossRef] [PubMed]
- Olivé, I.; Densmore, M.; Harricharan, S.; Théberge, J.; McKinnon, M.C.; Lanius, R. Superior colliculus resting state networks in post-traumatic stress disorder and its dissociative subtype. Hum. Brain Mapp. 2018, 39, 563–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabellino, D.; Tursich, M.; Frewen, P.A.; Daniels, J.K.; Densmore, M.; Theberge, J.; Lanius, R.A. Intrinsic Connectivity Networks in post-traumatic stress disorder during sub- and supraliminal processing of threat-related stimuli. Acta Psychiatr. Scand. 2015, 132, 365–378. [Google Scholar] [CrossRef]
- Tursich, M.; Ros, T.; Frewen, P.A.; Kluetsch, R.C.; Calhoun, V.D.; Lanius, R.A. Distinct intrinsic network connectivity patterns of post-traumatic stress disorder symptom clusters. Acta Psychiatr. Scand. 2015, 132, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Akiki, T.; Averill, C.L.; Wrocklage, K.M.; Scott, J.C.; Averill, L.A.; Schweinsburg, B.; Alexander-Bloch, A.; Martini, B.; Southwick, S.M.; Krystal, J.H.; et al. Default mode network abnormalities in posttraumatic stress disorder: A novel network-restricted topology approach. NeuroImage 2018, 176, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Daniels, J.K.; McFarlane, A.C.; Bluhm, R.L.; Moores, K.A.; Clark, C.R.; Shaw, M.E.; Williamson, P.C.; Densmore, M.; Lanius, R.A. Switching between executive and default mode networks in posttraumatic stress disorder: Alterations in functional connectivity. J. Psychiatry Neurosci. 2010, 35, 258–266. [Google Scholar] [CrossRef]
- Holmes, S.E.; Scheinost, D.; DellaGioia, N.; Davis, M.T.; Matuskey, D.; Pietrzak, R.H.; Hampson, M.; Krystal, J.H.; Esterlis, I. Cerebellar and Prefrontal Cortical Alterations in PTSD: Structural and Functional Evidence. Chronic Stress 2018, 2, 2470547018786390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bluhm, R.L.; Williamson, P.C.; Osuch, E.A.; Frewen, P.A.; Stevens, T.K.; Boksman, K.; Neufeld, R.W.J.; Théberge, J.; Lanius, R.A. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J. Psychiatry Neurosci. 2009, 34, 187–194. [Google Scholar] [PubMed]
- Rabellino, D.; Densmore, M.; Théberge, J.; McKinnon, M.C.; Lanius, R.A. The cerebellum after trauma: Resting-state functional connectivity of the cerebellum in posttraumatic stress disorder and its dissociative subtype. Hum. Brain Mapp. 2018, 39, 3354–3374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yehuda, R.; Hoge, C.W.; McFarlane, A.C.; Vermetten, E.; Lanius, R.A.; Nievergelt, C.M.; Hobfoll, S.E.; Koenen, K.C.; Neylan, T.C.; Hyman, S.E. Post-traumatic stress disorder. Nat. Rev. Dis. Prim. 2015, 1, 15057. [Google Scholar] [CrossRef] [PubMed]
- Okon-Singer, H.; Hendler, T.; Pessoa, L.; Shackman, A.J. The neurobiology of emotion-cognition interactions: Fundamental questions and strategies for future research. Front. Hum. Neurosci. 2015, 9, 58. [Google Scholar] [CrossRef] [Green Version]
- Raio, C.M.; Orederu, T.A.; Palazzolo, L.; Shurick, A.A.; Phelps, E.A. Cognitive emotion regulation fails the stress test. Proc. Natl. Acad. Sci. USA 2013, 110, 15139–15144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, J.J.; Thompson, R.A. Emotion regulation: Conceptual foundations. In Handbook of Emotion Regulation; Gross, J.J., Ed.; Guilford Press: New York, NY, USA, 2007; pp. 3–24. [Google Scholar]
- Ochsner, K.N.; Silvers, J.A.; Buhle, J.T. Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 2012, 1251, E1–E24. [Google Scholar] [CrossRef] [Green Version]
- Shurick, A.A.; Hamilton, J.R.; Harris, L.T.; Roy, A.K.; Gross, J.J.; Phelps, E.A. Durable effects of cognitive restructuring on conditioned fear. Emotion 2012, 12, 1393–1397. [Google Scholar] [CrossRef]
- Schmeichel, B.J.; Volokhov, R.N.; Demaree, H.A. Working memory capacity and the self-regulation of emotional expression and experience. J. Pers. Soc. Psychol. 2008, 95, 1526–1540. [Google Scholar] [CrossRef]
- Hofmann, W.; Schmeichel, B.J.; Baddeley, A.D. Executive functions and self-regulation. Trends Cogn. Sci. 2012, 16, 174–180. [Google Scholar] [CrossRef]
- Cisler, J.M.; Olatunji, B.O.; Feldner, M.T.; Forsyth, J.P. Emotion Regulation and the Anxiety Disorders: An Integrative Review. J. Psychopathol. Behav. Assess. 2009, 32, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Richards, J.M.; Gross, J.J. Emotion regulation and memory: The cognitive costs of keeping one’s cool. J. Personal. Soc. Psychol. 2000, 79, 410–424. [Google Scholar] [CrossRef]
- Levine, B.; Robertson, I.H.; Clare, L.; Carter, G.; Hong, J.; Wilson, B.A.; Duncan, J.S.; Stuss, D.T. Rehabilitation of executive functioning: An experimental–clinical validation of Goal Management Training. J. Int. Neuropsychol. Soc. 2000, 6, 299–312. [Google Scholar] [CrossRef]
- Robertson, I.H.; O’Connell, R. Vigilant Attention. In Attention and Time; Nobre, A.C., Coull, J.T., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 79–88. [Google Scholar]
- O’Connor, C.; Robertson, I.H.; Levine, B. The prosthetics of vigilant attention: Random cuing cuts processing demands. Neuropsychology 2011, 25, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.I.; Petersen, S.E. The attention system of the human brain. Annu. Rev. Neurosci. 1990, 13, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.; Emsliea, H.; Williamsa, P.; Johnsonb, R.; Freerb, C. Intelligence and the Frontal Lobe: The Organization of Goal-Directed Behavior. Cogn. Psychol. 1996, 30, 257–303. [Google Scholar] [CrossRef]
- De Braek, D.M.J.M.I.; Dijkstra, J.B.; Ponds, R.W.; Jolles, J. Goal Management Training in Adults with ADHD: An Intervention Study. J. Atten. Disord. 2012, 21, 1130–1137. [Google Scholar] [CrossRef]
- Alfonso, J.P.; Caracuel, A.; Delgado-Pastor, L.C.; Verdejo-Garcia, A. Combined goal management training and mindfulness meditation improve executive functions and decision-making performance in abstinent polysubstance abusers. Drug Alcohol Depend. 2011, 117, 78–81. [Google Scholar] [CrossRef]
- Stubberud, J.; Langenbahn, D.; Levine, B.; Stanghelle, J.; Schanke, A.-K. Goal Management Training of Executive Functions in Patients with Spina Bifida: A Randomized Controlled Trial. J. Int. Neuropsychol. Soc. 2013, 19, 672–685. [Google Scholar] [CrossRef]
- Stamenova, V.; Levine, B. Effectiveness of goal management training® in improving executive functions: A meta-analysis. Neuropsychol. Rehabil. 2018, 29, 1569–1599. [Google Scholar] [CrossRef]
- Cameron, D.H.; McCabe, R.E.; Rowa, K.; O’Connor, C.; McKinnon, M.C. A pilot study examining the use of Goal Management Training in individuals with obsessive-compulsive disorder. Pilot Feasibility Stud. 2020, 6, 151. [Google Scholar] [CrossRef]
- Boyd, J.E.; O’Connor, C.; Protopopescu, A.; Jetly, R.; Rhind, S.G.; Lanius, R.A.; McKinnon, M.C. An Open-Label Feasibility Trial Examining the Effectiveness of a Cognitive Training Program, Goal Management Training, in Individuals with Posttraumatic Stress Disorder. Chronic Stress 2019, 3, 2470547019841599. [Google Scholar] [CrossRef] [PubMed]
- Nazarov, A.; Fikretoglu, D.; Liu, A.; Thompson, M.; Zamorski, M.A. Greater prevalence of post-traumatic stress disorder and depression in deployed Canadian Armed Forces personnel at risk for moral injury. Acta Psychiatr. Scand. 2018, 137, 342–354. [Google Scholar] [CrossRef]
- Weathers, F.W.; Blake, D.D.; Schnurr, P.P.; Kaloupek, D.G.; Marx, B.P.; Keane, T.M. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). Interview Available from the National Center for PTSD. 2013. Available online: https://www.ptsd.va.gov/professional/assessment/adult-int/caps.asp#:~:text=CAPS%2D5%20requires%20the%20identification,new%20symptoms%20in%20DSM%2D5 (accessed on 5 September 2017).
- Weathers, F.W.; Litz, B.T.; Keane, T.M.; Palmieri, P.A.; Marx, B.P.; Schnurr, P.P. The PTSD Checklist for DSM-5 (PCL-5). Scale Available from the National Center for PTSD. 2013. Available online: https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp (accessed on 5 September 2017).
- Gray, M.J.; Litz, B.; Hsu, J.L.; Lombardo, T.W. Psychometric Properties of the Life Events Checklist. Assessment 2004, 11, 330–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efird, J. Blocked Randomization with Randomly Selected Block Sizes. Int. J. Environ. Res. Public Health 2011, 8, 15–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Shin, W. How to Do Random Allocation (Randomization). Clin. Orthop. Surg. 2014, 6, 103–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Balker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. S20), 22–33. [Google Scholar]
- Wechsler, D. Wechsler Test of Adult Reading (WTAR); Psychological Corporation: San Antonio, TX, USA, 2001. [Google Scholar]
- Wechsler, D. Wechsler Abbreviated Scale of Intelligence-Second Edition (WASI-II); NCS Pearson: San Antonio, TX, USA, 2011. [Google Scholar]
- Reitan, R.M.; Wolfson, D. The Halstead-Reitan Neuropsychological Battery: Theory and Clinical Interpretation; Neuropsychology Press: Tuscon, AZ, USA, 1985. [Google Scholar]
- Golden, C.J. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses; Stoelting Co.: Chicago, IL, USA, 1978. [Google Scholar]
- Golden, C.J.; Freshwater, S.M. Stroop Color and Word Test: Revised Examiner’s Manual; Stoelting Co.: Wood Dale, IL, USA, 2002. [Google Scholar]
- Delis, D.C.; Kaplan, E.; Kramer, D.C. Delis-Kaplan Executive Function System (D-KEFS); The Psychological Corporation: San Antonia, TX, USA, 2001. [Google Scholar]
- Wechsler, D. Wechsler Adult Intelligence Scale-Fourth Edition (WASI-IV); Psychological Corporation: San Antonio, TX, USA, 2008. [Google Scholar]
- Conners, C.K.; MHS Staff. (Eds.) Conners’ Continuous Performance Test II: Computer Program for Windows Technical Guide and Software Manual; Multi-Health Systems: North Tonawanda, NY, USA, 2000. [Google Scholar]
- Delis, D.C.; Kramer, J.H.; Kaplan, E.; Ober, B.A. California Verbal Learning Test-II (CVLT-II); The Psychological Corporation: San Antonio, TX, USA, 2000. [Google Scholar]
- Broadbent, D.E.; Cooper, P.F.; Fitzgerald, P.; Parkes, K.R. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br. J. Clin. Psychol. 1982, 21, 1–16. [Google Scholar] [CrossRef]
- Wallace, J.C.; Kass, S.J.; Stanny, C. The Cognitive Failures Questionnaire Revisited: Dimensions and Correlates. J. Gen. Psychol. 2002, 129, 238–256. [Google Scholar] [CrossRef]
- World Health Organization. Measuring Health and Disability: Manual for WHO Disability Assessment Schedule (WHODAS 2.0); World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Wortmann, J.H.; Jordan, A.H.; Weathers, F.W.; Resick, P.A.; Dondanville, K.A.; Hall-Clark, B.; Foa, E.B.; Young-McCaughan, S.; Yarvis, J.S.; Hembree, E.A.; et al. Psychometric analysis of the PTSD Checklist-5 (PCL-5) among treatment-seeking military service members. Psychol. Assess. 2016, 28, 1392–1403. [Google Scholar] [CrossRef]
- Pietrzak, R.H.; Tsai, J.; Armour, C.; Mota, N.; Harpaz-Rotem, I.; Southwick, S.M. Functional significance of a novel 7-factor model of DSM-5 PTSD symptoms: Results from the National Health and Resilience in Veterans Study. J. Affect. Disord. 2015, 174, 522–526. [Google Scholar] [CrossRef]
- Briere, J. Multiscale Dissociation Inventory; Psychological Assessment Resources: Odessa, FL, USA, 2002. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Beck Depression Inventory-II (BDI-II); Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W.F. Comparison of Beck Depression Inventories-IA and-II in Psychiatric Outpatients. J. Pers. Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A. Beck Anxiety Inventory; Psychological Corporation: San Antonio, TX, USA, 1993. [Google Scholar]
- De Ayala, R.J.; Vonderharr-Carlson, D.J.; Kim, D. Assessing the Reliability of the Beck Anxiety Inventory Scores. Educ. Psychol. Meas. 2005, 65, 742–756. [Google Scholar] [CrossRef]
- Raudenbush, S.W.; Bryk, A.S. Hierarchical Linear Models: Applications and Data Analysis Methods, 2nd ed.; Sage Publications: Thousand Oaks, CA, USA, 2002. [Google Scholar]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 6th ed.; Pearson: Boston, MA, USA, 2013. [Google Scholar]
- Levine, B.; Stuss, D.T.; Winocur, G.; Binns, M.A.; Fahy, L.; Mandic, M.; Bridges, K.; Robertson, I.H. Cognitive rehabilitation in the elderly: Effects on strategic behavior in relation to goal management. J. Int. Neuropsychol. Soc. 2007, 13, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C. Posttraumatic stress disorder: The burden to the individual and to society. J. Clin. Psychiatry 2000, 61, 4–14. [Google Scholar] [PubMed]
- Kessler, R.C.; Frank, R.G. The impact of psychiatric disorders on work loss days. Psychol. Med. 1997, 27, 861–873. [Google Scholar] [CrossRef] [Green Version]
- Olatunji, B.O.; Cisler, J.M.; Tolin, D.F. Quality of life in the anxiety disorders: A meta-analytic review. Clin. Psychol. Rev. 2007, 27, 572–581. [Google Scholar] [CrossRef]
- Silverberg, N.D.; Wojtowicz, M.; Bui, E.; Wershba, R.; Zafonte, R.; Laifer, L.M.; Simon, N.M.; Iverson, G.L. Contribution of Perceived Cognitive Functioning to Quality of Life in Service Members and Veterans With Posttraumatic Stress Disorder. J. Trauma. Stress 2017, 30, 318–322. [Google Scholar] [CrossRef]
- Barlati, S.; Deste, G.; De Peri, L.; Ariu, C.; Vita, A. Cognitive Remediation in Schizophrenia: Current Status and Future Perspectives. Schizophr. Res. Treat. 2013, 2013, 156084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wykes, T.; Reeder, C.; Corner, J.; Williams, C.; Everitt, B. The Effects of Neurocognitive Remediation on Executive Processing in Patients with Schizophrenia. Schizophr. Bull. 1999, 25, 291–307. [Google Scholar] [CrossRef] [Green Version]
- Cahn-Weiner, D.A.; Farias, S.T.; Julian, L.; Harvey, D.J.; Kramer, J.H.; Reed, B.R.; Mungas, D.; Wetzel, M.; Chui, H. Cognitive and neuroimaging predictors of instrumental activities of daily living. J. Int. Neuropsychol. Soc. 2007, 13, 747–757. [Google Scholar] [CrossRef]
- Parlar, M.; Frewen, P.A.; Oremus, C.; Lanius, R.A.; McKinnon, M.C. Dissociative symptoms are associated with reduced neuropsychological performance in patients with recurrent depression and a history of trauma exposure. Eur. J. Psychotraumatol. 2016, 7, 29061. [Google Scholar] [CrossRef] [PubMed]
- Llera, S.J.; Newman, M.G. Worry impairs the problem-solving process: Results from an experimental study. Behav. Res. Ther. 2020, 135, 103759. [Google Scholar] [CrossRef]
- Hallion, L.S.; Ruscio, A.M.; Jha, A.P. Fractionating the role of executive control in control over worry: A preliminary investigation. Behav. Res. Ther. 2014, 54, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hallion, L.S.; Tolin, D.F.; Assaf, M.; Goethe, J.; Diefenbach, G.J. Cognitive Control in Generalized Anxiety Disorder: Relation of Inhibition Impairments to Worry and Anxiety Severity. Cogn. Ther. Res. 2017, 41, 610–618. [Google Scholar] [CrossRef]
- Stefanopoulou, E.; Hirsch, C.R.; Hayes, S.; Adlam, A.; Coker, S. Are attentional control resources reduced by worry in generalized anxiety disorder? J. Abnorm. Psychol. 2014, 123, 330–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallion, L.S.; Steinman, S.A.; Kusmierski, S.N. Difficulty concentrating in generalized anxiety disorder: An evaluation of incremental utility and relationship to worry. J. Anxiety Disord. 2018, 53, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Eysenck, M.W.; Derakhshan, N.; Santos, R.; Calvo, M. Anxiety and cognitive performance: Attentional control theory. Emotion 2007, 7, 336–353. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, C.R.; Mathews, A. A cognitive model of pathological worry. Behav. Res. Ther. 2012, 50, 636–646. [Google Scholar] [CrossRef] [Green Version]
- Beaudreau, S.A.; MacKay-Brandt, A.; Reynolds, J. Application of a cognitive neuroscience perspective of cognitive control to late-life anxiety. J. Anxiety Disord. 2013, 27, 559–566. [Google Scholar] [CrossRef] [Green Version]
- Joormann, J.; Vanderlind, W.M. Emotion Regulation in Depression: The role of biased cognition and reduced cognitive control. Clin. Psychol. Sci. 2014, 2, 402–421. [Google Scholar] [CrossRef]
- Serra-Blasco, M.; Torres, I.J.; Vicent-Gil, M.; Goldberg, X.; Ventura, G.N.; Aguilar, E.; Via, E.; Portella, M.J.; Figuereo, I.; Palao, D.; et al. Discrepancy between objective and subjective cognition in major depressive disorder. Eur. Neuropsychopharmacol. 2019, 29, 46–56. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | WL (n = 18) | GMT (n = 22) |
|---|---|---|
| Demographic characteristics | ||
| Sex (female: male) | 3:15 | 10:12 |
| Age (Mean, (SD)) | 44.61 (8.54) | 43.95 (6.73) |
| Race (Caucasian/Aboriginal/Hispanic) | 15:2:1 | 22:1:0 |
| Marital Status | % of Sample | |
| Single | 5.6 | 13.6 |
| Married or common law | 72.2 | 77.3 |
| Separated/Divorced | 16.7 | 0 |
| Long-term relationship | 5.6 | 9.1 |
| Highest Level of Education Completed | ||
| Completed high school | 5.6 | 0 |
| Some college or university | 5.6 | 9.1 |
| Completed college or university | 77.8 | 77.3 |
| Some graduate level education | 5.6 | 4.5 |
| Completed graduate degree | 5.6 | 9.1 |
| Current Employment Status | ||
| Working full time | 44.4 | 27.3 |
| Working part time | 5.6 | 0 |
| Medical Leave/Long-term Disability | 22.2 | 22.7 |
| Modified duties/Return to work | 0 | 4.6 |
| On leave/WSIB | 22.2 | 27.3 |
| Not currently employed | 5.6 | 9.1 |
| Other | 0 | 9.1 |
| Military, Veteran, or Public Safety Personnel Status | ||
| Military | 0 | 18.2 |
| Veteran | 5.6 | 13.6 |
| Public Safety Personnel | 94.4 | 95.5 |
| Both | 0 | 27.3 |
| Clinical characteristics | ||
| CAPS-5—Severity Score (Mean, (SD)) | 36.00 (14.33) | 40.86 (12.67) |
| CAPS-5—PTSD Criteria Met (% of Sample) | 77.8 | 90 |
| Additional M.I.N.I. 7.0.2 diagnoses | % of Sample | |
| Major Depressive Disorder | 61.1 | 63.6 |
| Generalized Anxiety Disorder | 27.8 | 54.5 |
| Social Anxiety Disorder | 27.8 | 50.0 |
| Panic Disorder | 16.7 | 27.3 |
| Agoraphobia | 11.1 | 40.9 |
| Alcohol Use Disorder, Past 12 Months | 5.6 | 13.64 |
| Substance Use Disorder, Past 12 Months | 0 | 4.55 |
| Binge Eating Disorder | 15.0 | 4.55 |
| IQ | Mean (SD) | |
| WTAR Estimated IQ | 113.44 (6.08) | 113.32 (5.38) |
| WASI-II FSIQ | 105.56 (16.00) | 108.45 (14.13) |
| Variable | Group | n | Mean | SD |
|---|---|---|---|---|
| COWAT | ||||
| FAS T Score | WL | 18 | 42.28 | 8.98 |
| GMT | 22 | 41.41 | 10.25 | |
| Animals T Score | WL | 18 | 48.28 | 12.84 |
| GMT | 22 | 49.32 | 12.81 | |
| Stroop Color and Word Test | ||||
| Word T-Score | WL | 18 | 36.78 | 10.64 |
| GMT | 22 | 37.55 | 11.28 | |
| Color T-Score | WL | 18 | 39.17 | 10.43 |
| GMT | 22 | 41.14 | 10.74 | |
| Color-Word Trial T Score | WL | 18 | 43.44 | 8.00 |
| GMT | 22 | 44.09 | 11.17 | |
| Interference T-Score | WL | 18 | 49.56 | 6.78 |
| GMT | 22 | 49.41 | 6.96 | |
| DKEFS Tower Test | ||||
| Total Scaled Score | WL | 18 | 11.22 | 2.53 |
| GMT | 22 | 11.45 | 2.18 | |
| First Move Time Scaled Score | WL | 18 | 10.61 | 2.57 |
| GMT | 22 | 9.23 | 2.58 | |
| Time Per Move Scaled Score | WL | 18 | 9.61 | 2.85 |
| GMT | 22 | 8.82 | 3.51 | |
| Move Accuracy Scaled Score | WL | 18 | 9.17 | 2.68 |
| GMT | 22 | 9.50 | 3.40 | |
| Rule Violations | WL | 18 | 0.78 | 1.16 |
| GMT | 22 | 0.45 | 0.67 | |
| WAIS-IV | ||||
| Digit Symbol Coding Scaled Score | WL | 18 | 9.78 | 3.00 |
| GMT | 22 | 9.36 | 2.26 | |
| TMT | ||||
| TMT Part A T-Score | WL | 17 | 50.00 | 9.25 |
| GMT | 21 | 47.43 | 11.47 | |
| TMT Part B T-Score | WL | 16 | 46.88 | 9.16 |
| GMT | 21 | 44.67 | 10.73 | |
| CPT 3.0 a | ||||
| Omissions T Score | WL | 18 | 47.94 | 8.35 |
| GMT | 22 | 47.95 | 5.10 | |
| Commissions T Score | WL | 18 | 51.89 | 7.75 |
| GMT | 22 | 52.27 | 8.95 | |
| Detectability T Score | WL | 18 | 48.94 | 6.03 |
| GMT | 22 | 50.27 | 8.40 | |
| Hit Reaction Time T Score | WL | 18 | 46.83 | 8.58 |
| GMT | 22 | 43.68 | 7.93 | |
| Variability T Score | WL | 18 | 50.50 | 11.13 |
| GMT | 22 | 52.00 | 7.80 | |
| Perseverations T Score | WL | 18 | 45.94 | 3.12 |
| GMT | 22 | 48.55 | 7.58 | |
| CVLT-II | ||||
| Trial 1 Z Score | WL | 18 | −0.22 | 1.73 |
| GMT | 22 | 0.52 | 1.15 | |
| Trials 5 Z Score | WL | 18 | −0.14 | 0.78 |
| GMT | 22 | −0.21 | 0.97 | |
| Trial 1-15 Z Score | WL | 18 | 50.61 | 10.88 |
| GMT | 22 | 55.68 | 10.37 | |
| Trial B Z Score | WL | 18 | 0.14 | 1.61 |
| GMT | 22 | 0.02 | 0.88 | |
| Short-Delay Free Recall Z Score | WL | 18 | 0.17 | 0.73 |
| GMT | 22 | −0.14 | 1.20 | |
| Short-Delay Cued Recall Z Score | WL | 18 | 0.17 | 0.82 |
| GMT | 22 | 0.09 | 1.07 | |
| Long-Delay Free Recall Z Score | WL | 18 | 0.11 | 0.96 |
| GMT | 22 | −0.07 | 1.15 | |
| Long-Delay Cued Recall Z Score | WL | 18 | −0.08 | 0.77 |
| GMT | 22 | −0.09 | 0.93 | |
| Repetitions Z Score | WL | 18 | 0.14 | 1.30 |
| GMT | 22 | 0.07 | 1.13 | |
| Intrusions Z Score | WL | 18 | 0.23 | 1.53 |
| GMT | 22 | −0.16 | 1.00 | |
| Discriminability Z Score | WL | 18 | −0.06 | 1.14 |
| GMT | 22 | 0.25 | 1.09 |
| Tests of Executive Functioning, Processing Speed, and Attention | |||||
|---|---|---|---|---|---|
| Neuropsychological Test | Neuropsychological Subtest | Source | F | p | η2p |
| COWAT a | FAS T Score | Time | 9.044 | 0.006 ** | 0.251 |
| Condition | 0.373 | 0.547 | 0.014 | ||
| Time * Condition | 0.149 | 0.703 | 0.005 | ||
| Animals T Score | Time | 0.932 | 0.343 | 0.033 | |
| Condition | 0.664 | 0.422 | 0.024 | ||
| Time * Condition | 0.022 | 0.883 | 0.001 | ||
| Stroop Color and Word Test a | Word T Score | Time | 3.352 | 0.078 | 0.110 |
| Condition | 0.002 | 0.968 | 0.000 | ||
| Time * Condition | 0.056 | 0.815 | 0.002 | ||
| Color T Score | Time | 5.137 | 0.032 * | 0.160 | |
| Condition | 0.101 | 0.754 | 0.004 | ||
| Time * Condition | 1.564 | 0.222 | 0.055 | ||
| Color-Word T Score | Time | 12.477 | 0.002 ** | 0.316 | |
| Condition | 0.016 | 0.901 | 0.001 | ||
| Time * Condition | 2.903 | 0.100 | 0.097 | ||
| Interference T Score | Time | 9.402 | 0.005 ** | 0.258 | |
| Condition | 0.076 | 0.785 | 0.003 | ||
| Time * Condition | 0.953 | 0.338 | 0.034 | ||
| DKEFS Tower Test b | Total Score Scaled Score | Time | 3.557 | 0.071 | 0.120 |
| Condition | 0.251 | 0.621 | 0.010 | ||
| Time * Condition | 0.002 | 0.964 | 0.000 | ||
| First Move Time Scaled Score | Time | 11.186 | 0.003 ** | 0.301 | |
| Condition | 6.559 | 0.017 * | 0.201 | ||
| Time * Condition | 1.945 | 0.175 | 0.070 | ||
| Time Per Move Scaled Score | Time | 16.065 | 0.000 ** | 0.382 | |
| Condition | 1.550 | 0.224 | 0.056 | ||
| Time * Condition | 4.066 | 0.054 | 0.135 | ||
| Move Accuracy Scaled Score | Time | 0.035 | 0.854 | 0.001 | |
| Condition | 0.812 | 0.376 | 0.030 | ||
| Time * Condition | 0.730 | 0.401 | 0.027 | ||
| Rule Violations | Time | 5.935 | 0.022 * | 0.186 | |
| Condition | 1.335 | 0.258 | 0.049 | ||
| Time * Condition | 3.393 | 0.077 | 0.115 | ||
| WAIS-IV a | Digit Symbol Coding Scaled Score | Time | 12.675 | 0.001 ** | 0.319 |
| Condition | 1.054 | 0.314 | 0.038 | ||
| Time * Condition | 0.400 | 0.533 | 0.015 | ||
| TMT c | TMT Part A T Score | Time | 0.604 | 0.445 | 0.024 |
| Condition | 0.223 | 0.641 | 0.009 | ||
| Time * Condition | 0.198 | 0.660 | 0.008 | ||
| TMT Part B T Score | Time | 7.581 | 0.011 * | 0.233 | |
| Condition | 0.025 | 0.875 | 0.001 | ||
| Time * Condition | 0.056 | 0.815 | 0.002 | ||
| CPT 3.0 b | Omissions T Score | Time | 0.018 | 0.895 | 0.001 |
| Condition | 0.005 | 0.946 | 0.000 | ||
| Time * Condition | 2.133 | 0.156 | 0.076 | ||
| Commissions T Score | Time | 17.009 | 0.000 ** | 0.395 | |
| Condition | 0.012 | 0.915 | 0.000 | ||
| Time * Condition | 0.148 | 0.703 | 0.006 | ||
| Detectability T Score | Time | 4.912 | 0.036* | 0.159 | |
| Condition | 0.328 | 0.572 | 0.012 | ||
| Time * Condition | 1.147 | 0.294 | 0.042 | ||
| Hit Reaction Time T Score | Time | 0.235 | 0.632 | 0.009 | |
| Condition | 0.107 | 0.746 | 0.004 | ||
| Time * Condition | 2.078 | 0.161 | 0.074 | ||
| Variability T Score | Time | 0.450 | 0.508 | 0.017 | |
| Condition | 1.086 | 0.307 | 0.040 | ||
| Time * Condition | 0.375 | 0.546 | 0.014 | ||
| Perseverations T Score | Time | 6.259 | 0.019 * | 0.194 | |
| Condition | 0.916 | 0.347 | 0.034 | ||
| Time * Condition | 0.263 | 0.613 | 0.010 | ||
| Tests of Declarative Memory | |||||
| CVLT-II a | Trial 1 Z Score | Time | 0.032 | 0.860 | 0.001 |
| Condition | 2.997 | 0.095 | 0.100 | ||
| Time * Condition | 0.228 | 0.637 | 0.008 | ||
| Trial 5 Z Score | Time | 14.603 | 0.001 ** | 0.351 | |
| Condition | 0.293 | 0.593 | 0.011 | ||
| Time * Condition | 0.690 | 0.413 | 0.025 | ||
| Trial 1-5 T Score | Time | 0.592 | 0.448 | 0.021 | |
| Condition | 1.668 | 0.207 | 0.058 | ||
| Time * Condition | 1.247 | 0.274 | 0.044 | ||
| Trial B Z Score | Time | 0.944 | 0.340 | 0.034 | |
| Condition | 0.494 | 0.488 | 0.018 | ||
| Time * Condition | 2.976 | 0.096 | 0.099 | ||
| Short Delay Free Recall Z Score | Time | 0.129 | 0.722 | 0.005 | |
| Condition | 0.356 | 0.556 | 0.013 | ||
| Time * Condition | 7.963 | 0.009 ** | 0.228 | ||
| Short Delay Cued Recall Z Score | Time | 0.217 | 0.645 | 0.008 | |
| Condition | 0.880 | 0.357 | 0.032 | ||
| Time * Condition | 3.530 | 0.071 | 0.116 | ||
| Long Delay Free Recall Z Score | Time | 0.475 | 0.496 | 0.017 | |
| Condition | 0.426 | 0.519 | 0.016 | ||
| Time * Condition | 1.955 | 0.173 | 0.068 | ||
| Long Delay Cued Recall Z Score | Time | 0.824 | 0.372 | 0.030 | |
| Condition | 1.621 | 0.214 | 0.057 | ||
| Time * Condition | 2.372 | 0.135 | 0.081 | ||
| Repetitions Z Score | Time | 0.107 | 0.746 | 0.004 | |
| Condition | 0.884 | 0.355 | 0.032 | ||
| Time * Condition | 0.604 | 0.444 | 0.022 | ||
| Intrusions Z Score | Time | 2.495 | 0.126 | 0.085 | |
| Condition | 0.480 | 0.494 | 0.017 | ||
| Time * Condition | 0.782 | 0.384 | 0.028 | ||
| Discriminability Z Score | Time | 1.838 | 0.186 | 0.064 | |
| Condition | 1.161 | 0.291 | 0.041 | ||
| Time * Condition | 0.472 | 0.498 | 0.017 | ||
| Measures | Group (n) a | Pre-Intervention | Post-Intervention | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| CFQ | WL (11) | 50.82 | 12.55 | 49.09 | 12.79 |
| GMT (18) | 54.50 | 20.54 | 46.50 | 15.74 | |
| WHODAS Score | WL (11) | 31.44 | 11.35 | 32.39 | 14.51 |
| GMT (17) | 42.03 | 19.67 | 33.70 | 13.79 | |
| PCL-5 Total Score | WL (11) | 38.55 | 15.92 | 39.82 | 15.77 |
| GMT (14) | 38.71 | 12.08 | 32.71 | 14.90 | |
| DERS Total Score | WL (11) | 94.09 | 23.79 | 90.91 | 24.00 |
| GMT (17) | 101.53 | 23.87 | 90.53 | 23.55 | |
| MDI Total Score | WL (11) | 50.36 | 15.48 | 52.82 | 16.67 |
| GMT (18) | 55.61 | 16.68 | 49.56 | 12.52 | |
| BDI-II Total Score | WL (10) | 25.30 | 8.31 | 21.40 | 6.62 |
| GMT (18) | 28.11 | 12.10 | 21.11 | 11.21 | |
| BAI Total Score | WL (9) | 26.33 | 14.20 | 23.56 | 12.08 |
| GMT (18) | 25.44 | 12.55 | 18.50 | 10.15 | |
| Measures | Source | F | p | η2p |
|---|---|---|---|---|
| CFQ Total Score | Time | 2.997 | 0.095 | 0.100 |
| Condition | 0.009 | 0.924 | 0.000 | |
| Time * Condition | 1.246 | 0.274 | 0.044 | |
| WHODAS Score | Time | 3.516 | 0.072 | 0.119 |
| Condition | 1.092 | 0.306 | 0.040 | |
| Time * Condition | 5.552 | 0.026 | 0.176 | |
| PCL-5 Total Score | Time | 1.538 | 0.227 | 0.063 |
| Condition | 0.388 | 0.539 | 0.017 | |
| Time * Condition | 3.641 | 0.069 | 0.137 | |
| DERS Total Score | Time | 4.473 | 0.044 | 0.147 |
| Condition | 0.167 | 0.686 | 0.006 | |
| Time * Condition | 1.359 | 0.254 | 0.050 | |
| MDI Total Score | Time | 0.567 | 0.458 | 0.021 |
| Condition | 0.035 | 0.854 | 0.001 | |
| Time * Condition | 3.164 | 0.087 | 0.105 | |
| BDI-II Total Score | Time | 9.797 | 0.004 | 0.274 |
| Condition | 0.115 | 0.737 | 0.004 | |
| Time * Condition | 0.792 | 0.382 | 0.030 | |
| BAI Total Score | Time | 5.088 | 0.033 | 0.169 |
| Condition | 0.456 | 0.506 | 0.018 | |
| Time * Condition | 0.935 | 0.343 | 0.036 |
| CFQ Total Score | ||||||
| Effect | b | SE | t | df | p | d |
| Initial CFQ Severity (Intercept) | 53.90 | 2.58 | 20.88 | 33 | <0.001 | |
| CFQ Severity Over Time (Slope) | −1.44 | 0.53 | −2.72 | 33 | 0.010 | −0.47 |
| PCL-5 Total Score | ||||||
| Effect | b | SE | t | df | p | d |
| Initial PCL-5 Severity (Intercept) | 43.13 | 2.48 | 17.40 | 33 | <0.001 | |
| PCL-5 Severity Over Time (Slope) | −2.23 | 3.74 | −5.97 | 33 | <0.001 | −1.02 |
| DERS Total Score | ||||||
| Effect | b | SE | t | df | p | d |
| Initial DERS Severity (Intercept) | 103.02 | 3.67 | 28.11 | 33 | <0.001 | |
| DERS Severity Over Time (Slope) | −2.36 | 0.61 | −3.89 | 33 | <0.001 | −0.67 |
| MDI Total Score | ||||||
| Effect | b | SE | t | df | p | d |
| Initial MDI Severity (Intercept) | 55.95 | 2.71 | 20.64 | 33 | <0.001 | |
| MDI Severity Over Time (Slope) | −1.24 | 0.34 | −3.70 | 33 | <0.001 | −0.63 |
| BDI-II Total Score | ||||||
| Effect | b | SE | t | df | p | d |
| Initial BDI-II Severity (Intercept) | 28.07 | 20.2 | 13.90 | 33 | <0.001 | |
| BDI-II Severity Over Time (Slope) | −1.39 | 0.31 | −4.56 | 33 | <0.001 | −0.78 |
| BAI Total Score | ||||||
| Effect | b | SE | t | df | p | d |
| Initial BAI Severity (Intercept) | 25.12 | 2.00 | 12.57 | 33 | <0.001 | |
| BAI Severity Over Time (Slope) | −1.33 | 0.31 | −4.25 | 33 | <0.001 | −0.73 |
| Effect of Baseline PCL-5 on CFQ Total Score Trajectory | ||||||
| Effect | b | SE | t | df | p | d |
| Initial CFQ Severity (Intercept) | 48.49 | 2.30 | 21.05 | 27 | <0.001 | |
| PCL-5 Total | 0.57 | 0.15 | 3.82 | 27 | <0.001 | 0.71 |
| CFQ Severity Over Time (Slope) | −0.97 | 0.59 | −1.65 | 27 | 0.110 | −0.31 |
| PCL-5 Total | −0.04 | −0.05 | −0.92 | 27 | 0.365 | −0.17 |
| Effect of Baseline DERS on CFQ total score trajectory | ||||||
| Effect | b | SE | t | df | p | d |
| Initial CFQ Severity (Intercept) | 51.61 | 2.27 | 22.79 | 30 | <0.001 | |
| DERS Total | 0.34 | 0.09 | 3.64 | 30 | <0.001 | 0.64 |
| CFQ Severity Over Time (Slope) | −1.25 | 0.56 | −2.25 | 30 | 0.032 | −0.40 |
| DERS Total | −0.03 | 0.02 | −1.69 | 30 | 0.102 | −0.30 |
| Effect of baseline DERS on PCL-5 total score trajectory | ||||||
| Effect | b | SE | t | df | p | d |
| Initial PCL Severity (Intercept) | 40.14 | 2.07 | 19.37 | 30 | <0.001 | |
| DERS Total | 0.41 | 0.08 | 5.08 | 30 | <0.001 | 0.90 |
| PCL Severity Over Time (Slope) | −2.46 | 0.41 | −5.98 | 30 | <0.001 | −1.06 |
| DERS Total | 0.01 | 0.02 | 0.39 | 30 | 0.702 | 0.07 |
| Effect of baseline DERS on MDI total score trajectory | ||||||
| Effect | b | SE | t | df | p | d |
| Initial MDI Severity (Intercept) | 54.04 | 2.28 | 23.73 | 30 | <0.001 | |
| DERS Total | 0.36 | 0.10 | 3.72 | 30 | <0.001 | 0.66 |
| MDI Severity Over Time (Slope) | −1.14 | 0.28 | −4.12 | 30 | <0.001 | −0.73 |
| DERS Total | −0.02 | 0.01 | −2.11 | 30 | 0.044 | −0.37 |
| Effect of baseline DERS on BDI-II total score trajectory | ||||||
| Effect | b | SE | t | df | p | d |
| Initial BDI Severity (Intercept) | 25.77 | 1.27 | 20.22 | 30 | <0.001 | |
| DERS Total | 0.36 | 0.05 | 6.80 | 30 | <0.001 | 1.20 |
| BDI Severity Over Time (Slope) | −1.47 | 0.30 | −4.89 | 30 | <0.001 | −0.86 |
| DERS Total | −0.00 | 0.01 | −0.44 | 30 | 0.664 | −0.08 |
| Effect of baseline DERS on BAI total score trajectory | ||||||
| Effect | b | SE | t | df | p | d |
| Initial BAI Severity (Intercept) | 22.60 | 1.61 | 14.01 | 30 | <0.001 | |
| DERS Total | 0.34 | 0.06 | 5.45 | 30 | <0.001 | 0.96 |
| BAI Severity Over Time (Slope) | −1.25 | 0.33 | −3.76 | 30 | <0.001 | −0.67 |
| DERS Total | −0.02 | 0.01 | −1.63 | 30 | 0.113 | −0.29 |
| Effect of baseline MDI on CFQ total score trajectory | ||||||
| Effect | b | SE | t | df | p | d |
| Initial CFQ Severity (Intercept) | 51.80 | 1.88 | 27.63 | 31 | <0.001 | |
| MDI Total | 0.62 | 0.16 | 3.88 | 31 | <0.001 | 0.68 |
| CFQ Severity Over Time (Slope) | −1.25 | 0.48 | −2.63 | 31 | 0.013 | −0.46 |
| MDI Total | −0.06 | 0.04 | −1.77 | 31 | 0.087 | −0.31 |
| Effect of baseline BDI-II on CFQ total score trajectory | ||||||
| Effect | b | SE | t | df | p | d |
| Initial CFQ Severity (Intercept) | 52.73 | 2.23 | 23.63 | 31 | <0.001 | |
| BDI Total | 0.73 | 0.14 | 5.36 | 31 | <0.001 | 0.93 |
| CFQ Severity Over Time (Slope) | −1.32 | 0.52 | −2.52 | 31 | 0.017 | −0.44 |
| BDI Total | −0.02 | 0.04 | −0.586 | 31 | 0.562 | −0.10 |
| Effect of baseline BAI on CFQ total score trajectory | ||||||
| Effect | b | SE | t | df | p | d |
| Initial CFQ Severity (Intercept) | 52.58 | 1.97 | 26.64 | 31 | <0.001 | |
| BAI Total | 0.87 | 0.14 | 6.34 | 31 | <0.001 | 1.10 |
| CFQ Severity Over Time (Slope) | −1.31 | 0.49 | −2.66 | 31 | 0.012 | −0.46 |
| BAI Total | −0.08 | 0.04 | −2.14 | 31 | 0.041 | −0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Protopopescu, A.; O’Connor, C.; Cameron, D.; Boyd, J.E.; Lanius, R.A.; McKinnon, M.C. A Pilot Randomized Controlled Trial of Goal Management Training in Canadian Military Members, Veterans, and Public Safety Personnel Experiencing Post-Traumatic Stress Symptoms. Brain Sci. 2022, 12, 377. https://doi.org/10.3390/brainsci12030377
Protopopescu A, O’Connor C, Cameron D, Boyd JE, Lanius RA, McKinnon MC. A Pilot Randomized Controlled Trial of Goal Management Training in Canadian Military Members, Veterans, and Public Safety Personnel Experiencing Post-Traumatic Stress Symptoms. Brain Sciences. 2022; 12(3):377. https://doi.org/10.3390/brainsci12030377
Chicago/Turabian StyleProtopopescu, Alina, Charlene O’Connor, Duncan Cameron, Jenna E. Boyd, Ruth A. Lanius, and Margaret C. McKinnon. 2022. "A Pilot Randomized Controlled Trial of Goal Management Training in Canadian Military Members, Veterans, and Public Safety Personnel Experiencing Post-Traumatic Stress Symptoms" Brain Sciences 12, no. 3: 377. https://doi.org/10.3390/brainsci12030377
APA StyleProtopopescu, A., O’Connor, C., Cameron, D., Boyd, J. E., Lanius, R. A., & McKinnon, M. C. (2022). A Pilot Randomized Controlled Trial of Goal Management Training in Canadian Military Members, Veterans, and Public Safety Personnel Experiencing Post-Traumatic Stress Symptoms. Brain Sciences, 12(3), 377. https://doi.org/10.3390/brainsci12030377






