Effects of Vibrotactile Biofeedback Providing Real-Time Pressure Information on Static Balance Ability and Weight Distribution Symmetry Index in Patients with Chronic Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design and Process
2.3. Various Biofeedback Conditions
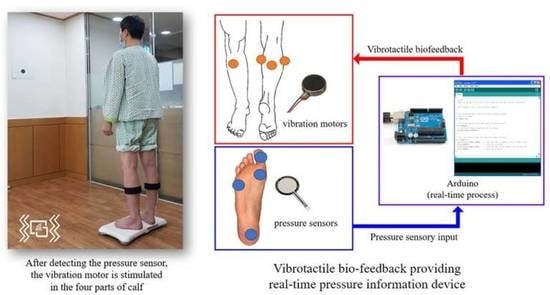
2.3.1. Pressure Sensor-Based Vibrotactile Biofeedback
2.3.2. Visual Biofeedback Providing Posture Information
2.3.3. Normal Standing Position with No Biofeedback
2.4. Outcome Measures
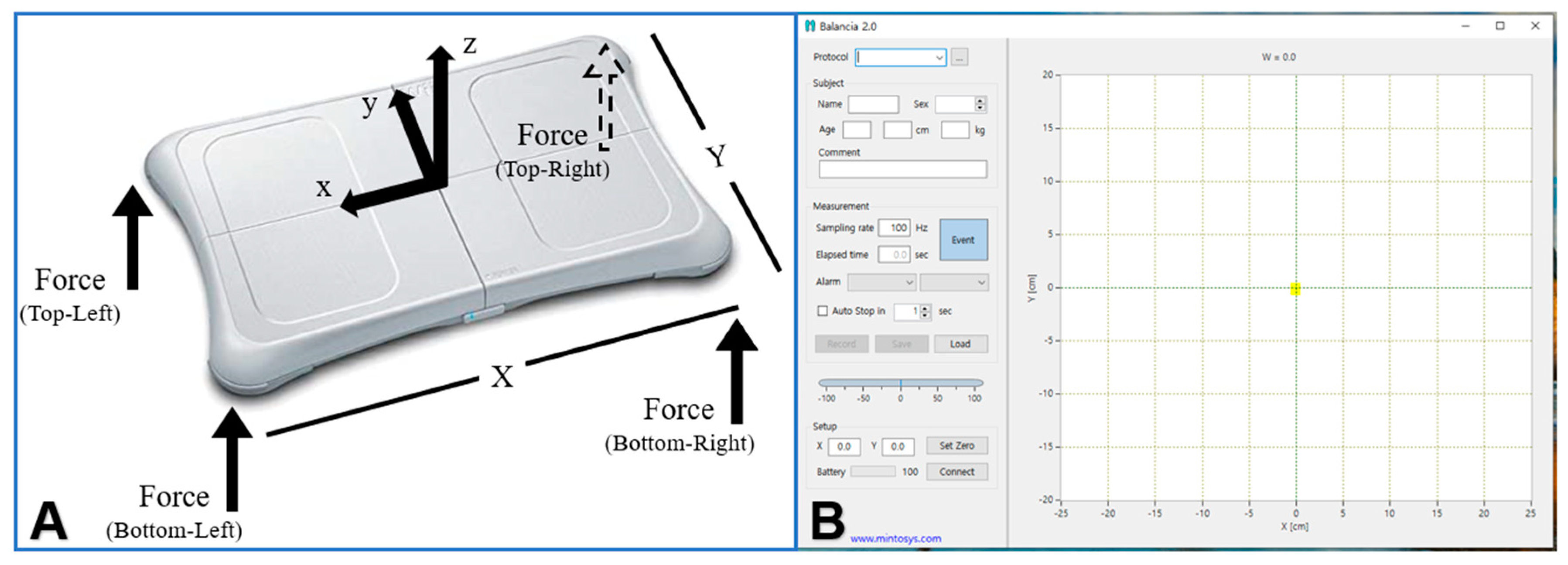
2.4.1. Static Balance Ability Assessment
2.4.2. Weight-Distribution Symmetry Index
2.5. Data Analysis
3. Results
3.1. General Characteristics of the Participants
3.2. Difference in Static Balance Ability According to Various Biofeedback Conditions
3.2.1. Sway Length
3.2.2. Sway Velocity
3.2.3. Weight-Distribution Symmetry Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; DeCarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular Contributions to Cognitive Impairment and Dementia: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, L.; Broetz, D.; Karnath, H.O. Leg orientation as a clinical sign for pusher syndrome. BMC Neurol. 2006, 6, 30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patterson, K.K.; Parafianowicz, I.; Danells, C.J.; Closson, V.; Verrier, M.C.; Staines, W.R.; Black, S.E.; William, E.; McIlroy, W.E. Gait asymmetry in community-ambulating stroke survivors. Arch. Phys. Med. Rehabil. 2008, 89, 304–310. [Google Scholar] [CrossRef]
- Michael, K.M.; Allen, J.K.; Macko, R.F. Reduced ambulatory activity after stroke: The role of balance, gait, and cardiovascular fitness. Arch. Phys. Med. Rehabil. 2005, 86, 1552–1556. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.O.; Maki, B.E.; Williams, J.I.; Holliday, P.J.; Wood-Dauphinee, S.L. Clinical and laboratory measures of postural balance in an elderly population. Arch. Phys. Med. Rehabil. 1992, 73, 1073–1080. [Google Scholar]
- Laufer, Y.; Sivan, D.; Schwarzmann, R.; Sprecher, E. Standing balance and functional recovery of patients with right and left hemiparesis in early stages of rehabilitation. Neurorehabilit. Neural Repair 2003, 17, 207–213. [Google Scholar] [CrossRef]
- Fong, K.N.; Chan, C.C.; Au, D.K. Relationship of motor and cognitive abilities to functional performance in stroke rehabilitation. Brain Inj. 2001, 15, 443–453. [Google Scholar] [CrossRef]
- Patel, M.; Gomez, S.; Lush, D.; Fransson, P.A. Adaptation and vision change th relationship between muscle activity of the lower limbs and body movement during human balance perturbations. Clin. Neurophysiol. 2009, 120, 601–609. [Google Scholar] [CrossRef]
- de Haart, M.; Geurts, A.C.; Dault, M.C.; Nienhuis, B.; Duysens, J. Restoration of weight-shifting capacity in patients with postacute stroke: A rehabilitation cohort study. Arch. Phys. Med. Rehabil. 2005, 86, 755–762. [Google Scholar] [CrossRef]
- Dozza, M.; Horak, F.B.; Chiari, L. Auditory biofeedback substitutes for loss of sensory information in maintaining stance. Exp. Brain Res. 2007, 178, 37–48. [Google Scholar] [CrossRef]
- Vuillerme, N.; Pinsault, N.; Chenu, O.; Demongeot, J.; Payan, Y.; Danilov, Y. Sensory supplementation system based on electrotactile tongue biofeedback of head position for balance control. Neurosci. Lett. 2008, 431, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Yi, C.H.; Goo, Y.R. A study on the effects of weight-transfer training upon the gait patterns of hemiplegic patients through visual and auditory feedback. Phys. Ther. Korea 1995, 2, 9–23. [Google Scholar]
- Winstein, C.J.; Gardner, E.R.; McNeal, D.R.; Barto, P.S.; Nicholson, D.E. Standing balance training: Effect on balance and locomotion in hemiparetic adults. Arch. Phys. Med. Rehabil. 1989, 70, 755–762. [Google Scholar] [PubMed]
- Tsaih, P.L.; Chiu, M.J.; Luh, J.J.; Yang, Y.R.; Lin, J.J.; Hu, M.H. Practice Variability Combined with Task-Oriented Electromyographic Biofeedback Enhances Strength and Balance in People with Chronic Stroke. Behav. Neurol. 2018, 2018, 7080218. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Beebe, D.J.; Kramer, A.F. Comparsion of tactile and visual feedback for a multi- state input mechanism. In Proceedings of the 19th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. ‘Magnificent Milestones and Emerging Opportunities in Medical Engineering’, Chicago, IL, USA, 30 October–2 November 1997; Volume 30, pp. 1697–1700. [Google Scholar]
- Kim, D.R.; Hur, H.K. The Effects of Somatosensory Stimulation on Cognitive Function and ADL of Patients after Stroke. Korean J. Adult Nurs. 2008, 20, 239–250. [Google Scholar]
- Kil, K.S.; Kim, H.; Shin, W.S. Effects of Vibrotactile Bio-Feedback Providing Pressure Information in Real Time on Static Balance and Weight Bearing Rate in Chronic Stroke Patients—Pilot Study. J. Korean Soc. Integr. Med. 2021, 9, 41–48. [Google Scholar]
- Quattrocchi, G.; Greenwood, R.; Rothwell, J.C.; Galea, J.M.; Bestmann, S. Reward and punishment enhance motor adaptation in stroke. J. Neurol. Neurosurg. Psychiatry 2017, 88, 730–736. [Google Scholar] [CrossRef]
- Toosizadeh, N.; Mohler, J.; Marlinski, V. Low intensity vibration of ankle muscles improves balance in elderly persons at high risk of falling. PLoS ONE 2018, 13, e0194720. [Google Scholar] [CrossRef]
- Park, J.H.; Chung, Y. The effects of providing visual feedback and auditory stimulation using a robotic device on balance and gait abilities in persons with stroke: A pilot study. Phys. Ther. Rehabil. Sci. 2016, 5, 125–131. [Google Scholar] [CrossRef]
- Thieme, H.; Mehrholz, J.; Pohl, M.; Behrens, J.; Dohle, C. Mirror therapy for improving motor function after stroke. Stroke 2013, 44, e1–e2. [Google Scholar] [CrossRef]
- Sackley, C.M.; Lincoln, N.B. Single blind randomized controlled trial of visual feedback after stroke: Effects on stance symmetry and function. Disabil. Rehabil. 1997, 19, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Choi, H.S.; Shin, W.S. Effects of real-time feedback training on weight shifting during golf swinging on golf performance in amateur golfers. Phys. Ther. Rehabil. Sci. 2017, 6, 189–195. [Google Scholar] [CrossRef][Green Version]
- Park, D.S.; Lee, D.Y.; Choi, S.J.; Shin, W.S. Reliability and validity of the balancia using wii balance board for assessment of balance with stroke patients. J. Korea Acad. Ind. Coop. Soc. 2013, 14, 2767–2772. [Google Scholar]
- Patterson, K.K.; Gage, W.H.; Brooks, D.; Black, S.E.; McIlroy, W.E. Evaluation of gait symmetry after stroke: A comparison of current methods and recommendations for standardization. Gait Posture 2010, 31, 241–246. [Google Scholar] [CrossRef]
- Geiger, R.A.; Allen, J.B.; O’Keefe, J.; Hicks, R.R. Balance and mobility following stroke: Effects of physical therapy interventions with and without biofeedback/forceplate training. Phys. Ther. 2001, 81, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.; Ada, L.; Dean, C.M.; Preston, E. Biofeedback improves activities of the lower limb after stroke: A systematic review. J. Physiother. 2011, 57, 145–155. [Google Scholar] [CrossRef]
- Wu, S.W.; Fan, R.E.; Wottawa, C.R.; Fowler, E.G.; Bisley, J.W.; Grundfest, W.S.; Culjat, M.O. Torso-based tactile feedback system for patients with balance disorders. In Proceedings of the 2010 IEEE Haptics Symposium, Waltham, MA, USA, 25–26 March 2010; pp. 359–362. [Google Scholar]
- Tzorakoleftherakis, E.; Mussa-Ivaldi, F.A.; Scheidt, R.A.; Murphey, T.D. Effects of optimal tactile feedback in balancing tasks: A pilot study. In Proceedings of the 2014 American Control Conference, Portland, OR, USA, 4–6 June 2014; pp. 778–783. [Google Scholar]
- Joodaki, M.R.; Olyaei, G.R.; Bagheri, H. The effects of electrical nerve stimulation of the lower extremity on H-reflex and F-wave parameters. Electroencephalogr. Clin. Neurophysiol. 2001, 41, 23–28. [Google Scholar]
- Pérennou, D.A.; Leblond, C.; Amblard, B.; Micallef, J.P.; Hérisson, C.; Pélissier, J.Y. Transcutaneous electric nerve stimulation reduces neglect-related postural instability after stroke. Arch. Phys. Med. Rehabil. 2001, 82, 440–448. [Google Scholar] [CrossRef]
- Jung, K.S.; In, T.S.; Cho, H.Y. Effects of sit-to-stand training combined with transcutaneous electrical stimulation on spasticity, muscle strength and balance ability in patients with stroke: A randomized controlled study. Gait Posture 2017, 54, 183–187. [Google Scholar] [CrossRef]
- Yasuda, K.; Kaibuki, N.; Watanabe, S.; Harashima, H.; Iwata, H. A vibro-tactile biofeedback system supplying online center of foot pressure displacement for balance training in stroke patients. Physiotherapy 2015, 101, 1687–1688. [Google Scholar] [CrossRef]
- Afzal, M.R.; Pyo, S.; Oh, M.K.; Park, Y.S.; Yoon, J. Identifying the effects of using integrated haptic feedback for gait rehabilitation of stroke patients. In Proceedings of the 2017 International Conference on Rehabilitation Robotics (ICORR), London, UK, 17–20 July 2017; pp. 1055–1060. [Google Scholar]
- Suputtitada, A.; Yooktanan, P.; Rarerng-Ying, T. Effect of partial body weight support treadmill training in chronic stroke patients. J. Med. Assoc. Thai. 2004, 87, 107–111. [Google Scholar]
- Ward, N.S.; Brown, M.M.; Thompson, A.J.; Frackowiak, R.S. Longitudinal changes in cerebral response to proprioceptive input in individual patients after stroke: An FMRI study. Neurorehabil. Neural Repair 2006, 20, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.B. Brain plasticity and stroke rehabilitation: The Willis lecture. Stroke 2000, 31, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Kaas, J.H. Plasticity of sensory and motor maps in adult mammals. Annu. Rev. Neurosci. 1991, 14, 137–167. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wolf, S.L.; He, J. Recent developments in biofeedback for neuromotor rehabilitation. J. Neuroeng. Rehabil. 2006, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.R.; Lee, H.; Eizad, A.; Lee, C.H.; Oh, M.K.; Yoon, J. Effects of Vibrotactile Biofeedback Coding Schemes on Gait Symmetry Training of Individuals With Stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Bonan, I.V.; Yelnik, A.P.; Colle, F.M.; Michaud, C.; Normand, E.; Panigot, B.; Roth, P.; Guichard, J.P.; Vicaut, E. Reliance on visual information after stroke. Part ii: Effectiveness of a balance rehabilitation program with visual cue deprivation after stroke: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2004, 85, 274–278. [Google Scholar] [CrossRef]
- Yavuzer, G.; Selles, R.; Sezer, N.; Sutbeyaz, S.; Bussmann, J.B.; Koseoğlu, F.; Stam, H.J. Mirror therapy improves hand function in subacute stroke: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2008, 89, 393–398. [Google Scholar] [CrossRef]
- Sutbeyaz, S.; Yavuzer, G.; Sezer, N.; Koseoglu, B.F. Mirror therapy enhances lower-extremity motor recovery and motor functioning after stroke: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2007, 88, 555–559. [Google Scholar] [CrossRef]


| Variables | Values |
|---|---|
| Sex (Male/Female) | 18/6 |
| Paretic side (left/right) | 10/14 |
| Age (year) | 63.00 (12.31) |
| Height (cm) | 163.08 (8.84) |
| Weight (kg) | 59.25 (8.83) |
| Duration of onset (month) | 15.54 (9.00) |
| MMSE (score) | 26.54 (2.34) |
| BBS (score) | 34.38 (4.23) |
| Variables | Tactile Biofeedback | Visual Biofeedback | No Biofeedback | F |
|---|---|---|---|---|
| Sway Length (cm) | 91.11 (19.27) †‡ | 102.89 (28.66) ‡ | 114.59 (28.78) | 56.857 * |
| Sway Velocity (cm/s) | 3.06 (0.87) †‡ | 3.34 (1.10) ‡ | 3.68 (1.09) | 38.382 * |
| Variables | Tactile Biofeedback | Visual Biofeedback | No Biofeedback | F |
|---|---|---|---|---|
| Weight-distribution symmetric index (%) | 10.85 (9.81) †‡ | 25.89 (17.65) ‡ | 39.21 (24.42) | 41.663 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Kim, H.; Shin, W.-S. Effects of Vibrotactile Biofeedback Providing Real-Time Pressure Information on Static Balance Ability and Weight Distribution Symmetry Index in Patients with Chronic Stroke. Brain Sci. 2022, 12, 358. https://doi.org/10.3390/brainsci12030358
Kim H, Kim H, Shin W-S. Effects of Vibrotactile Biofeedback Providing Real-Time Pressure Information on Static Balance Ability and Weight Distribution Symmetry Index in Patients with Chronic Stroke. Brain Sciences. 2022; 12(3):358. https://doi.org/10.3390/brainsci12030358
Chicago/Turabian StyleKim, Ho, Hongjun Kim, and Won-Seob Shin. 2022. "Effects of Vibrotactile Biofeedback Providing Real-Time Pressure Information on Static Balance Ability and Weight Distribution Symmetry Index in Patients with Chronic Stroke" Brain Sciences 12, no. 3: 358. https://doi.org/10.3390/brainsci12030358
APA StyleKim, H., Kim, H., & Shin, W.-S. (2022). Effects of Vibrotactile Biofeedback Providing Real-Time Pressure Information on Static Balance Ability and Weight Distribution Symmetry Index in Patients with Chronic Stroke. Brain Sciences, 12(3), 358. https://doi.org/10.3390/brainsci12030358