Obstructive Sleep Apnea and Sleep Structure Assessed in Polysomnography and Right Ventricular Strain Parameters
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Punjabi, N.M. The epidemiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 136–143. [Google Scholar] [CrossRef]
- Khattak, H.K.; Hayat, F.; Pamboukian, S.V.; Hahn, H.S.; Schwartz, B.P.; Stein, P.K. Obstructive Sleep Apnea in Heart Failure: Review of Prevalence, Treatment with Continuous Positive Airway Pressure, and Prognosis. Tex. Heart Inst. J. 2018, 45, 151–161. [Google Scholar] [CrossRef] [PubMed]
- May, A.M.; Van Wagoner, D.R.; Mehra, R. OSA and Cardiac Arrhythmogenesis. Chest 2017, 151, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Pollicina, I.; Maniaci, A.; Lechien, J.R.; Iannella, G.; Vicini, C.; Cammaroto, G.; Cannavicci, A.; Magliulo, G.; Pace, A.; Cocuzza, S.; et al. Neurocognitive Performance Improvement after Obstructive Sleep Apnea Treatment: State of the Art. Behav. Sci. 2021, 11, 180. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Finn, M.L.; Peppard, P.E.; Szklo-Coxe, M.; Austin, M.D.; Nieto, F.J.; Stubbs, B.R.; Hla, K.M. Sleep Disordered Breathing and Mortality: Eighteen-Year Follow-up of the Wisconsin Sleep Cohort. Sleep 2008, 31, 1071–1078. [Google Scholar] [CrossRef]
- Fietze, I.; Laharnar, N.; Obst, A.; Ewert, R.; Felix, S.B.; Garcia, C.; Gläser, S.; Glos, M.; Schmidt, C.O.; Stubbe, B.; et al. Prevalence and association analysis of obstructive sleep apnea with gender and age differences—Results of SHIP-Trend. J. Sleep Res. 2019, 28, e12770. [Google Scholar] [CrossRef]
- Pace, A.; Iannella, G.; Rossetti, V.; Visconti, I.; Gulotta, G.; Cavaliere, C.; De Vito, A.; Maniaci, A.; Cocuzza, S.; Magliulo, G.; et al. Diagnosis of Obstructive Sleep Apnea in Patients with Allergic and Non-Allergic Rhinitis. Medicina 2020, 56, 454. [Google Scholar] [CrossRef]
- Adir, Y.; Humbert, M.; Chaouat, A. Sleep-related breathing disorders and pulmonary hypertension. Eur. Respir. J. 2021, 57, 2002258. [Google Scholar] [CrossRef]
- Kholdani, C.; Fares, W.H.; Mohsenin, V. Pulmonary Hypertension in Obstructive Sleep Apnea: Is it Clinically Significant? A Critical Analysis of the Association and Pathophysiology. Pulm. Circ. 2015, 5, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Sajkov, D.; McEvoy, D. Obstructive Sleep Apnea and Pulmonary Hypertension. Prog. Cardiovasc. Dis. 2009, 51, 363–370. [Google Scholar] [CrossRef]
- Sajkov, D.; Wang, T.; Saunders, N.A.; Bune, A.J.; Neill, A.M.; Evoy, R.D.M.C. Daytime Pulmonary Hemodynamics in Patients with Obstructive Sleep Apnea without Lung Disease. Am. J. Respir. Crit. Care Med. 1999, 159, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- De Boer, K.; Lee, J.S. Under-recognised co-morbidities in idiopathic pulmonary fibrosis: A review. Respirology 2015, 21, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Segers, V.; Brutsaert, D.L.; De Keulenaer, G. Pulmonary hypertension and right heart failure in heart failure with preserved left ventricular ejection fraction. Curr. Opin. Cardiol. 2012, 27, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Sanner, B.M.; Doberauer, C.; Konermann, M.; Sturm, A.; Zidek, W. Pulmonary Hypertension in Patients with Obstructive Sleep Apnea Syndrome. Arch. Intern. Med. 1997, 157, 2483–2487. [Google Scholar] [CrossRef]
- Li, J.; Lin, X.; Li, H.; Lu, C.; Li, R.; Liu, W.; Wang, Z. Right ventricular diastolic dysfunction in patients with obstructive sleep apnea syndrome. Echocardiography 2020, 37, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, M. Is Right Ventricular Dysfunction in Obstructive Sleep Apnea a Potential Screening Tool? Circ. J. 2010, 74, 250–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tugcu, A.; Yildirimtürk, O.; Tayyareci, Y.; Demiroglu, C.; Aytekin, S. Evaluation of Subclinical Right Ventricular Dysfunction in Obstructive Sleep Apnea Patients Using Velocity Vector Imaging. Circ. J. 2010, 74, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Zakhama, L.; Herbegue, B.; Abouda, M.; Antit, S.; Slama, I.; Boussabah, E.; Thameur, M.; Masmoudi, M.; Abdelaali, N.; Charfi, M.R.; et al. Impact of obstructive sleep apnea on the right ventricle. Tunis Med. 2016, 94, 612–615. [Google Scholar]
- Collier, P.; Phelan, D.; Klein, A. A Test in Context: Myocardial Strain Measured by Speckle-Tracking Echocardiography. J. Am. Coll. Cardiol. 2017, 69, 1043–1056. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [Green Version]
- Loredo, J.S.; Ziegler, M.G.; Ancoli-Israel, S.; Clausen, J.L.; Dimsdale, J.E. Relationship of Arousals From Sleep to Sympathetic Nervous System Activity and BP in Obstructive Sleep Apnea. Chest 1999, 116, 655–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasheghani-Farahani, A.; Kazemnejad, F.; Sadeghniiat-Haghighi, K.; Saadat, S.; Poor, P.T.; Yazdani, T.; Alidoosti, M.; Amooeian, V.G.; Ashraf, H. Obstructive sleep apnea and the severity of coronary artery disease. Casp. J. Intern. Med. 2018, 9, 276–282. [Google Scholar] [CrossRef]
- Patel, N.; Donahue, C.; Shenoy, A.; Patel, A.; El-Sherif, N. Obstructive sleep apnea and arrhythmia: A systemic review. Int. J. Cardiol. 2017, 228, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Coniglio, A.C.; Mentz, R.J. Sleep Breathing Disorders in Heart Failure. Heart Fail. Clin. 2020, 16, 45–51. [Google Scholar] [CrossRef]
- Kessler, R.; Chaouat, A.; Weitzenblum, E.; Oswald, M.; Ehrhart, M.; Apprill, M.; Krieger, J. Pulmonary hypertension in the obstructive sleep apnoea syndrome: Prevalence, causes and therapeutic consequences. Eur. Respir. J. 1996, 9, 787–794. [Google Scholar] [CrossRef] [Green Version]
- Nokes, B.; Raza, H.; Malhotra, A. Pulmonary hypertension and obstructive sleep apnea. J. Clin. Sleep Med. 2020, 16, 649. [Google Scholar] [CrossRef]
- Mesarwi, O.; Malhotra, A. Obstructive sleep apnea and pulmonary hypertension: A bidirectional relationship. J. Clin. Sleep Med. 2020, 16, 1223–1224. [Google Scholar] [CrossRef]
- Wang, S.; Cui, H.; Ji, K.; Ren, C.; Guo, H.; Zhu, C.; Lai, Y.; Wang, S. Effect of obstructive sleep apnea on right ventricular ejection fraction in patients with hypertrophic obstructive cardiomyopathy. Clin. Cardiol. 2020, 43, 1186–1193. [Google Scholar] [CrossRef]
- Patel, R.; Li, E.; Benefield, B.C.; Swat, S.A.; Polsinelli, V.B.; Carr, J.C.; Shah, S.; Markl, M.; Collins, J.D.; Freed, B.H. Diffuse right ventricular fibrosis in heart failure with preserved ejection fraction and pulmonary hypertension. ESC Heart Fail. 2020, 7, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Chu, A.-A.; Yu, H.-M.; Yang, H.; Tian, L.-M.; Hu, Z.-Y.; Jiang, N.; Xie, W.-X.; Huang, Y. Evaluation of right ventricular performance and impact of continuous positive airway pressure therapy in patients with obstructive sleep apnea living at high altitude. Sci. Rep. 2020, 10, 20186. [Google Scholar] [CrossRef]
- D’Andrea, A.; Martone, F.; Liccardo, B.; Mazza, M.; Annunziata, A.; Di Palma, E.; Conte, M.; Sirignano, C.; D’Alto, M.; Esposito, N.; et al. Acute and Chronic Effects of Noninvasive Ventilation on Left and Right Myocardial Function in Patients with Obstructive Sleep Apnea Syndrome: A Speckle Tracking Echocardiographic Study. Echocardiography 2016, 33, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Buonauro, A.; Galderisi, M.; Santoro, C.; Canora, A.; Bocchino, M.L.; Lo Iudice, F.; Lembo, M.; Esposito, R.; Castaldo, S.; Trimarco, B.; et al. Obstructive sleep apnoea and right ventricular function: A combined assessment by speckle tracking and three-dimensional echocardiography. Int. J. Cardiol. 2017, 243, 544–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altekin, R.E.; Karakas, M.S.; Yanikoglu, A.; Ozel, D.; Ozbudak, O.; Demir, I.; Deger, N. Determination of right ventricular dysfunction using the speckle tracking echocardiography method in patients with obstructive sleep apnea. Cardiol. J. 2012, 19, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Kepez, A.; Niksarlioglu, E.Y.O.; Hazirolan, T.; Ranci, O.; Kabul, H.K.; Demir, A.U.; Kaya, E.B.; Kocabas, U.; Aytemir, K.; Sahin, A.; et al. Early Myocardial Functional Alterations in Patients with Obstructive Sleep Apnea Syndrome. Echocardiography 2009, 26, 388–396. [Google Scholar] [CrossRef]
- Tadic, M.; Cuspidi, C.; Grassi, G.; Mancia, G. Obstructive sleep apnea and cardiac mechanics: How strain could help us? Heart Fail. Rev. 2021, 26, 937–945. [Google Scholar] [CrossRef]
| X | Me | SD | Min | Max | |
|---|---|---|---|---|---|
| Age [years] | 56.36 | 59.50 | 14.77 | 23.00 | 81.00 |
| Height [cm] | 172.67 | 173.00 | 8.96 | 158.00 | 188.00 |
| Body mass [kg] | 80.27 | 82.00 | 19.04 | 53.00 | 125.00 |
| Body mass index [kg/m2] | 26.71 | 25.75 | 4.90 | 19.95 | 36.13 |
| n | % | ||||
| Age < 60 | 21 | 48.8 | |||
| Age ≥ 60 | 22 | 51.2 | |||
| Men | 29 | 67.4 | |||
| Women | 14 | 32.5 | |||
| Normal body mass | 26 | 60.5 | |||
| Overweight/obesity | 17 | 39.5 | |||
| Arterial hypertension | 26 | 60.5 | |||
| Coronary artery diseases | 4 | 9.3 | |||
| Type 2 diabetes | 5 | 11.6 | |||
| X | Me | SD | Min | Max | |
|---|---|---|---|---|---|
| AHI [events/hours] | 27.09 | 23.05 | 19.76 | 0.20 | 71.30 |
| ODI [events/hours] | 27.44 | 26.30 | 19.74 | 0.20 | 73.70 |
| Snore [events/hours] | 23.43 | 11.40 | 24.93 | 0.00 | 81.60 |
| OAH [events/hours] | 6.84 | 1.70 | 10.30 | 0.00 | 38.40 |
| CAH [events/hours] | 0.80 | 0.15 | 1.66 | 0.00 | 9.10 |
| CSB [events/hours] | 1.60 | 0.00 | 4.09 | 0.00 | 19.90 |
| Average SpO2 [%] | 91.42 | 92.20 | 4.75 | 65.50 | 96.10 |
| Minimal SpO2 [%] | 80.90 | 82.00 | 7.45 | 64.00 | 94.00 |
| % SpO2 < 90 [%] | 14.75 | 5.30 | 21.29 | 0.00 | 91.00 |
| Average desaturation [%] | 6.48 | 4.50 | 11.51 | 2.00 | 77.00 |
| Average HR [bpm] | 61.63 | 61.85 | 8.16 | 45.80 | 80.00 |
| Minimal HR [bpm] | 49.33 | 50.00 | 9.31 | 24.00 | 69.00 |
| Maximal HR [bpm] | 89.85 | 89.50 | 15.65 | 62.00 | 141.00 |
| Sleep efficiency [%] | 78.16 | 81.25 | 14.55 | 25.30 | 93.60 |
| TST N1 [% sleep time] | 8.52 | 4.50 | 8.24 | 0.60 | 33.90 |
| TST N2 [% sleep time] | 54.94 | 51.60 | 27.62 | 24.40 | 98.00 |
| TST N3 [% sleep time] | 21.74 | 19.35 | 13.31 | 1.20 | 61.50 |
| TST REM [% sleep time] | 25.35 | 21.40 | 19.28 | 2.90 | 98.50 |
| n | % | ||||
| AHI < 5 (without OSA) | 10 | 23.3 | |||
| AHI ≥ 5 (OSA) | 33 | 76.7 | |||
| AHI: 5–15 (mild OSA) | 4 | 9.3 | |||
| AHI: 15–30 (moderate OSA) | 15 | 34.9 | |||
| AHI ≥ 30 (severe OSA) | 14 | 32.5 | |||
| X | Me | SD | Min | Max | |
|---|---|---|---|---|---|
| RAA [cm2] | 25.02 | 24.75 | 1.13 | 24.00 | 26.70 |
| RA major dimension [mm] | 49.40 | 44.00 | 17.64 | 24.00 | 92.00 |
| RA minor dimension [mm] | 47.60 | 43.00 | 15.93 | 23.00 | 84.00 |
| RVOT p [mm] | 31.58 | 32.00 | 4.33 | 18.00 | 40.00 |
| RVOT m [mm] | 32.86 | 33.50 | 4.45 | 22.00 | 41.00 |
| RVOT d [mm] | 27.80 | 27.00 | 4.03 | 21.00 | 37.00 |
| MPA [mm] | 26.11 | 25.00 | 1.90 | 24.00 | 29.00 |
| RVD [mm] | 36.95 | 37.00 | 4.26 | 29.00 | 51.00 |
| s’RV [cm/s] | 13.63 | 13.00 | 2.46 | 10.00 | 20.00 |
| TAPSE [mm] | 24.51 | 24.00 | 3.60 | 19.00 | 34.00 |
| RV E [cm/s] | 60.58 | 60.50 | 11.67 | 39.00 | 92.00 |
| RV A [cm/s] | 51.79 | 47.50 | 15.74 | 28.00 | 109.00 |
| RV E/A | 1.00 | 0.91 | 0.30 | 0.71 | 1.68 |
| RVEDt [ms] | 254.42 | 242.00 | 64.75 | 118.00 | 405.00 |
| RV E’ [cm/s] | 10.00 | 9.00 | 3.70 | 4.00 | 24.00 |
| RV A’ [cm/s] | 12.90 | 13.00 | 3.45 | 5.00 | 21.00 |
| TCO [ms] | 393.40 | 377.00 | 47.05 | 319.00 | 494.00 |
| RVET [ms] | 300.93 | 303.00 | 27.84 | 247.00 | 354.00 |
| RIMP | 0.71 | 0.71 | 0.15 | 0.60 | 0.81 |
| PAV [cm/s] | 74.35 | 73.00 | 9.88 | 55.00 | 104.00 |
| PAAT [ms] | 140.93 | 145.00 | 26.12 | 88.00 | 198.00 |
| X | Me | SD | Min | Max | |
|---|---|---|---|---|---|
| Average free wall strain [%] | −27.83 | −27.00 | 5.81 | −42.00 | −17.50 |
| Basal free wall strain [%] | −27.19 | −26.00 | 6.10 | −45.00 | −15.00 |
| Mid free wall strain [%] | −28.88 | −28.00 | 5.67 | −44.00 | −20.00 |
| Apex free wall strain [%] | −23.67 | −23.00 | 6.56 | −36.00 | −8.00 |
| Average septal strain [%] | −16.58 | −17.00 | 4.41 | −25.00 | −6.67 |
| Basal septal strain [%] | −16.47 | −17.00 | 4.73 | −26.00 | −5.00 |
| Mid septal strain [%] | −17.02 | −17.00 | 4.72 | −26.00 | −4.00 |
| Apex septal strain [%] | −16.26 | −15.00 | 5.60 | −29.00 | −4.00 |
| AHI < 5 (without OSA) | AHI ≥ 5 (OSA) | p Value | |||
|---|---|---|---|---|---|
| X | SD | X | SD | ||
| Average free wall strain [%] | −32.64 | 5.40 | −27.17 | 5.60 | 0.023 |
| Basal free wall strain [%] | −31.57 | 7.46 | −26.58 | 5.67 | 0.052 |
| Mid free wall strain [%] | −33.71 | 3.50 | −28.30 | 5.65 | 0.020 |
| Apex free wall strain [%] | −26.71 | 8.73 | −23.03 | 6.23 | 0.194 |
| Average septal strain [%] | −20.24 | 3.25 | −15.93 | 4.21 | 0.015 |
| Basal septal strain [%] | −20.14 | 3.02 | −16.06 | 4.55 | 0.030 |
| Mid septal strain [%] | −20.57 | 2.64 | −16.48 | 4.74 | 0.034 |
| Apex septal strain [%] | −20.00 | 5.42 | −15.24 | 5.26 | 0.037 |
| Average Free Wall Strain | Basal Free Wall Strain | Mid Free Wall Strain | Apex Free Wall Strain | Average Septal Strain | Basal Septal Strain | Mid Septal Strain | Apex Septal Strain | |
|---|---|---|---|---|---|---|---|---|
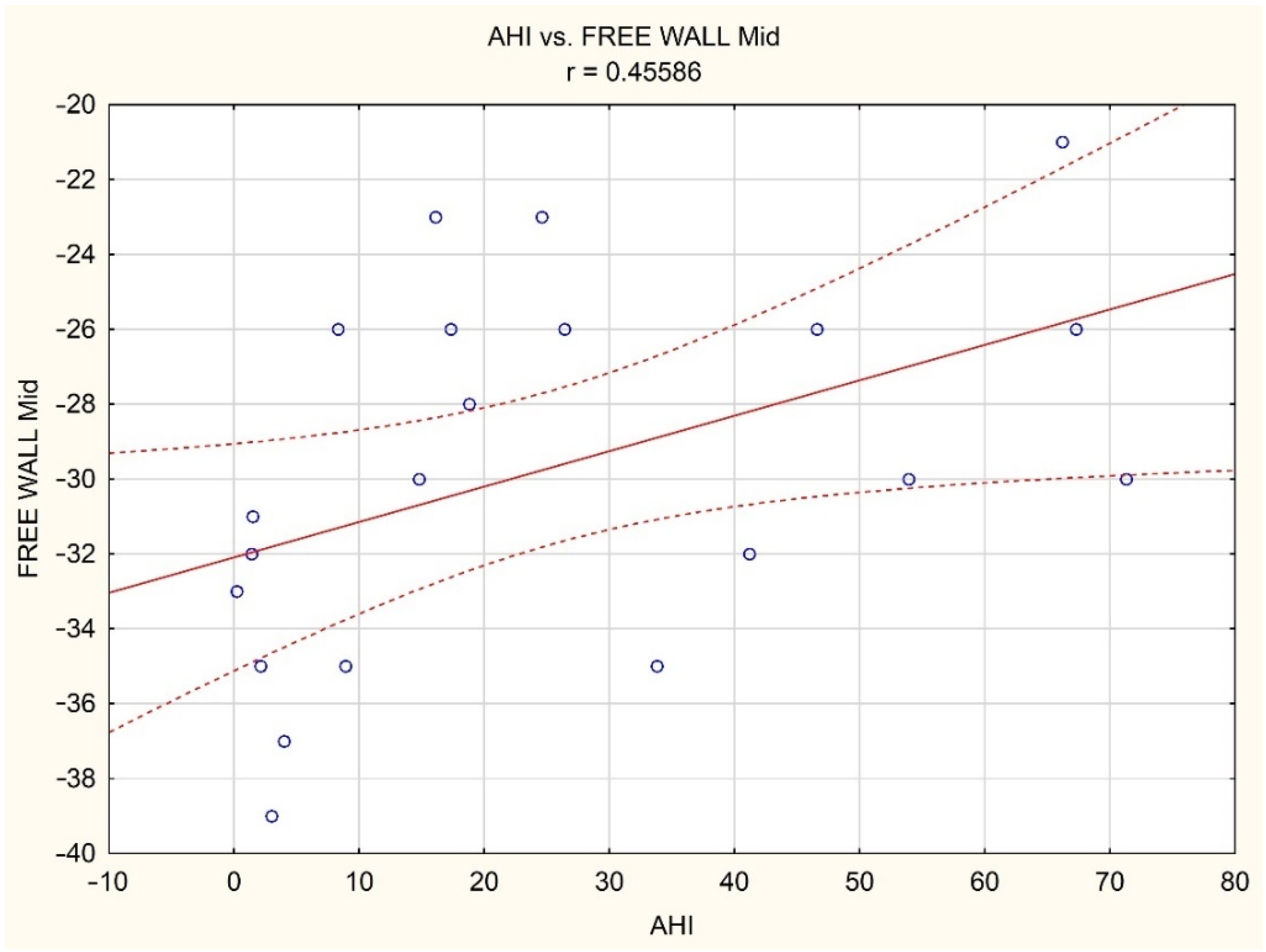
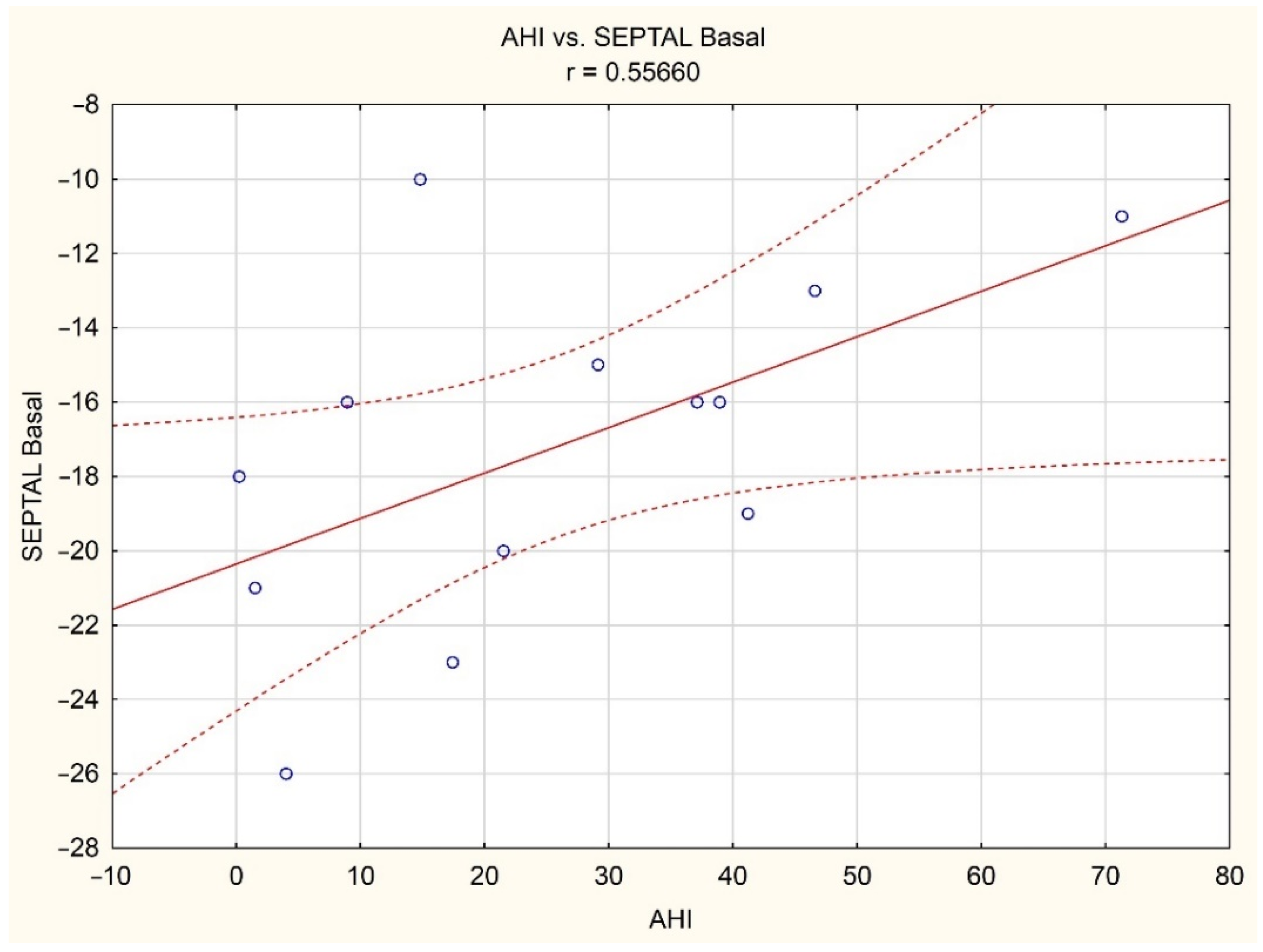
| AHI [events/hours] | 0.20 | 0.08 | 0.26 | 0.07 | −0.01 | 0.02 | −0.01 | −0.04 |
| ODI [events/hours] | 0.16 | 0.04 | 0.22 | 0.07 | 0.05 | 0.10 | 0.06 | −0.02 |
| Snore [events/hours] | 0.19 | 0.09 | 0.34 | 0.15 | 0.03 | 0.12 | 0.00 | −0.03 |
| OAH [events/hours] | 0.37 | 0.21 | 0.30 | 0.11 | −0.06 | −0.17 | −0.07 | 0.05 |
| CAH [events/hours] | 0.17 | 0.18 | 0.15 | −0.01 | 0.14 | 0.26 | 0.24 | −0.08 |
| CSB [events/hours] | −0.01 | −0.09 | 0.07 | 0.11 | 0.33 | 0.36 | 0.42 | 0.15 |
| Average SpO2 [%] | −0.28 | −0.23 | −0.22 | −0.14 | 0.07 | 0.10 | 0.11 | 0.00 |
| Minimal SpO2 [%] | −0.08 | −0.01 | −0.16 | −0.20 | −0.14 | −0.12 | −0.12 | −0.12 |
| % SpO2 < 90 [%] | 0.17 | 0.08 | 0.24 | 0.19 | 0.12 | 0.15 | 0.12 | 0.06 |
| Average desaturation [%] | 0.11 | 0.10 | 0.12 | −0.06 | −0.07 | −0.07 | −0.01 | −0.11 |
| Average HR [bpm] | 0.26 | 0.34 | 0.20 | −0.10 | 0.17 | 0.22 | 0.16 | 0.09 |
| Minimal HR [bpm] | 0.08 | 0.13 | 0.02 | −0.18 | 0.02 | 0.06 | 0.01 | −0.00 |
| Maximal HR [bpm] | 0.25 | 0.24 | 0.10 | 0.03 | 0.19 | 0.12 | 0.17 | 0.20 |
| Sleep efficiency [%] | −0.22 | −0.21 | −0.25 | −0.14 | −0.09 | −0.07 | −0.01 | −0.15 |
| TST N1 [% sleep time] | 0.20 | 0.19 | 0.16 | 0.07 | 0.02 | −0.07 | 0.01 | 0.10 |
| TST N2 [% sleep time] | −0.01 | 0.02 | −0.02 | −0.04 | 0.01 | 0.05 | 0.02 | −0.03 |
| TST N3 [% sleep time] | 0.02 | −0.01 | 0.10 | 0.11 | −0.00 | 0.05 | −0.02 | −0.03 |
| TST REM [% sleep time] | 0.21 | 0.07 | 0.02 | −0.07 | 0.07 | 0.12 | 0.12 | −0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macek, P.; Poręba, M.; Stachurska, A.; Martynowicz, H.; Mazur, G.; Gać, P.; Poręba, R. Obstructive Sleep Apnea and Sleep Structure Assessed in Polysomnography and Right Ventricular Strain Parameters. Brain Sci. 2022, 12, 331. https://doi.org/10.3390/brainsci12030331
Macek P, Poręba M, Stachurska A, Martynowicz H, Mazur G, Gać P, Poręba R. Obstructive Sleep Apnea and Sleep Structure Assessed in Polysomnography and Right Ventricular Strain Parameters. Brain Sciences. 2022; 12(3):331. https://doi.org/10.3390/brainsci12030331
Chicago/Turabian StyleMacek, Piotr, Małgorzata Poręba, Aneta Stachurska, Helena Martynowicz, Grzegorz Mazur, Paweł Gać, and Rafał Poręba. 2022. "Obstructive Sleep Apnea and Sleep Structure Assessed in Polysomnography and Right Ventricular Strain Parameters" Brain Sciences 12, no. 3: 331. https://doi.org/10.3390/brainsci12030331
APA StyleMacek, P., Poręba, M., Stachurska, A., Martynowicz, H., Mazur, G., Gać, P., & Poręba, R. (2022). Obstructive Sleep Apnea and Sleep Structure Assessed in Polysomnography and Right Ventricular Strain Parameters. Brain Sciences, 12(3), 331. https://doi.org/10.3390/brainsci12030331








