Transient Destabilization of Declarative Memory—Opposing Impact of Physical Exercise or Rest after Encoding in Typically Developing Children and Children with Attention Deficit Hyperactivity Disorder but No Difference after Subsequent Sleep
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
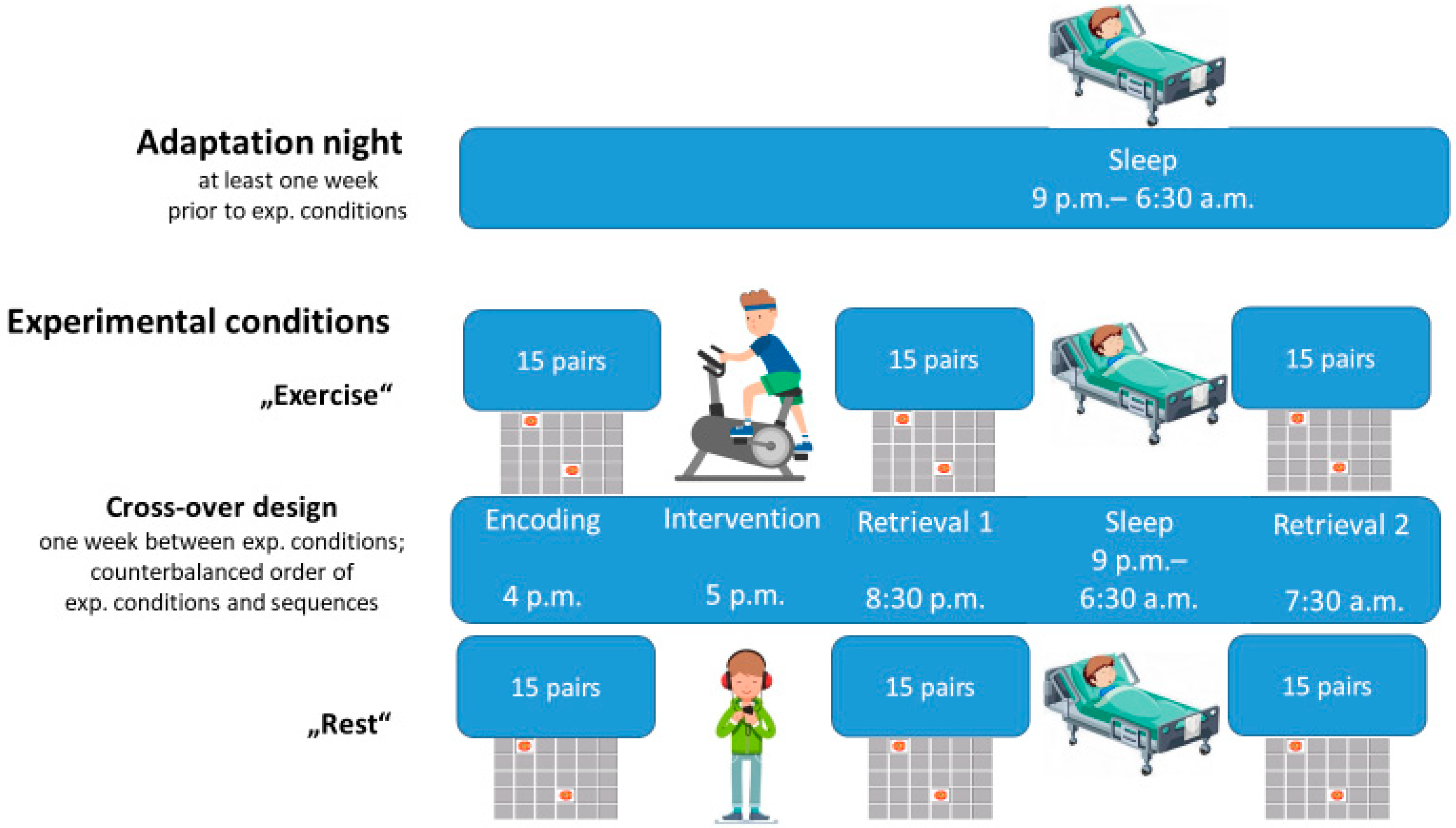
2.2. Experimental Design and Procedures
2.3. Declarative Memory Task
2.4. EEG Data Collection
2.5. Statistical Analysis
3. Results
3.1. Sleep Parameters
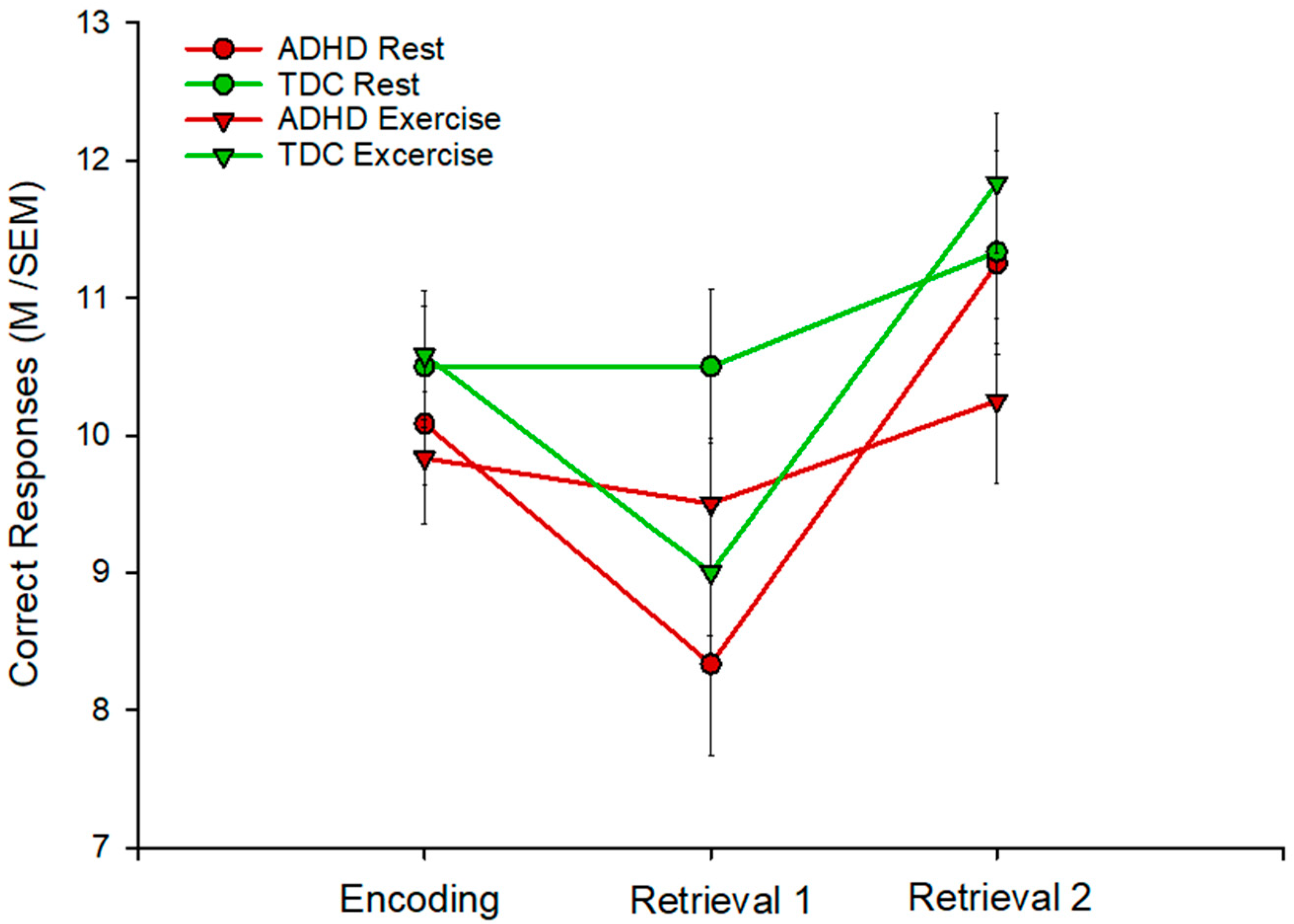
3.2. Declarative Memory
3.3. Working Memory
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasch, B.; Born, J. About Sleep’s Role in Memory. Physiol. Rev. 2013, 93, 681–766. [Google Scholar] [CrossRef]
- Rasch, B.; Büchel, C.; Gais, S.; Born, J. Odor Cues During Slow-Wave Sleep Prompt Declarative Memory Consolidation. Science 2007, 315, 1426–1429. [Google Scholar] [CrossRef] [Green Version]
- Nader, K.; Hardt, O. A single standard for memory: The case for reconsolidation. Nat. Rev. Neurosci. 2009, 10, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Diekelmann, S.; Büchel, C.; Born, J.; Rasch, B. Labile or stable: Opposing consequences for memory when reactivated during waking and sleep. Nat. Neurosci. 2011, 14, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Morris, R.G.M. Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Annu. Rev. Psychol. 2010, 61, 49–79. [Google Scholar] [CrossRef] [PubMed]
- Roig, M.; Cristini, J.; Parwanta, Z.; Ayotte, B.; Rodrigues, L.; de Las Heras, B.; Nepveu, J.F.; Huber, R.; Carrier, J.; Steib, S.; et al. Exercising the Sleepy-ing Brain: Exercise, Sleep, and Sleep Loss on Memory. Exerc. Sport Sci. Rev. 2021, 50, 38–48. [Google Scholar] [CrossRef]
- Wilhelm, I.; Diekelmann, S.; Molzow, I.; Ayoub, A.; Mölle, M.; Born, J. Sleep selectively enhances memory expected to be of future relevance. J. Neurosci. 2011, 31, 1563–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilhelm, I.; Diekelmann, S.; Born, J. Sleep in children improves memory performance on declarative but not procedural tasks. Learn. Mem. 2008, 15, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Maski, K.; Holbrook, H.; Manoach, D.; Hanson, E.; Kapur, K.; Stickgold, R. Sleep Dependent Memory Consolidation in Children with Autism Spectrum Disorder. Sleep 2015, 38, 1955–1963. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, I.; Prehn-Kristensen, A.; Born, J. Sleep-dependent memory consolidation–what can be learnt from children? Neurosci. Biobehav. Rev. 2012, 36, 1718–1728. [Google Scholar] [CrossRef]
- Weng, T.B.; Pierce, G.L.; Darling, W.G.; Falk, D.; Magnotta, V.A.; Voss, M.W. The Acute Effects of Aerobic Exercise on the Functional Connectivity of Human Brain Networks. Brain Plast. 2017, 2, 171–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolb, B.; Harker, A.; Gibb, R. Principles of plasticity in the developing brain. Dev. Med. Child Neurol. 2017, 59, 1218–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremblay, M.S.; Warburton, D.E.; Janssen, I.; Paterson, D.H.; Latimer, A.E.; Rhodes, R.E.; Kho, M.E.; Hicks, A.; Leblanc, A.G.; Zehr, L.; et al. New Canadian physical activity guidelines. Appl. Physiol. Nutr. Metab. 2011, 36, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.E.; Hillman, C.H.; Castelli, D.; Etnier, J.L.; Lee, S.; Tomporowski, P.; Lambourne, K.; Szabo-Reed, A.N. Physical Activity, Fitness, Cognitive Function, and Academic Achievement in Children: A Systematic Review. Med. Sci. Sports Exerc. 2016, 48, 1197–1222. [Google Scholar] [CrossRef] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Barkley, R.A. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol. Bull. 1997, 121, 65–94. [Google Scholar] [CrossRef] [Green Version]
- Willcutt, E.G.; Doyle, A.E.; Nigg, J.T.; Faraone, S.V.; Pennington, B.F. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biol. Psychiatry 2005, 57, 1336–1346. [Google Scholar] [CrossRef]
- Skodzik, T.; Holling, H.; Pedersen, A. Long-Term Memory Performance in Adult ADHD. J. Atten. Disord. 2017, 21, 267–283. [Google Scholar] [CrossRef]
- Verster, J.C.; Bekker, E.M.; Kooij, J.J.; Buitelaar, J.K.; Verbaten, M.N.; Volkerts, E.R.; Olivier, B. Methylphenidate significantly improves declarative memory functioning of adults with ADHD. Psychopharmacology 2010, 212, 277–281. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, S.M.; Coghill, D.R.; Matthews, K. Neuropsychological functioning in stimulant-naive boys with hyperkinetic disorder. Psychol. Med. 2005, 35, 1109–1120. [Google Scholar] [CrossRef]
- Prehn-Kristensen, A.; Göder, R.; Chirobeja, S.; Bressmann, I.; Ferstl, R.; Baving, L. Sleep in children enhances preferentially emotional declarative but not procedural memories. J. Exp. Child Psychol. 2009, 104, 132–139. [Google Scholar] [CrossRef]
- Kordon, A.; Kahl, K.G.; Wahl, K. A new understanding of attention-deficit disorders—beyond the age-at-onset criterion of DSM-IV. Eur. Arch. Psychiatry. Clin. Neurosci. 2006, 256 (Suppl. S1), i47–i54. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Sanders, S.; Doust, J.; Beller, E.; Glasziou, P. Prevalence of attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Pediatrics 2015, 135, e994–e1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prehn-Kristensen, A.; Göder, R.; Fischer, J.; Wilhelm, I.; Seeck-Hirschner, M.; Aldenhoff, J.; Baving, L. Reduced sleep-associated consolidation of declarative memory in attention-deficit/hyperactivity disorder. Sleep Med. 2011, 12, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Prehn-Kristensen, A.; Munz, M.; Molzow, I.; Wilhelm, I.; Wiesner, C.D.; Baving, L. Sleep promotes consolidation of emotional memory in healthy children but not in children with attention-deficit hyperactivity disorder. PLoS ONE 2013, 8, e65098. [Google Scholar] [CrossRef] [Green Version]
- Prehn-Kristensen, A.; Munz, M.; Göder, R.; Wilhelm, I.; Korr, K.; Vahl, W.; Wiesner, C.D.; Baving, L. Transcranial oscillatory direct current stimulation during sleep improves declarative memory consolidation in children with attention-deficit/hyperactivity disorder to a level comparable to healthy controls. Brain Stimul. 2014, 7, 793–799. [Google Scholar] [CrossRef] [Green Version]
- Prehn-Kristensen, A.; Ngo, H.V.; Lentfer, L.; Berghäuser, J.; Brandes, L.; Schulze, L.; Göder, R.; Mölle, M.; Baving, L. Acoustic closed-loop stimulation during sleep improves consolidation of reward-related memory information in healthy children but not in children with attention-deficit hyperactivity disorder. Sleep 2018, 43, zsaa017. [Google Scholar] [CrossRef] [PubMed]
- Roig, M.; Thomas, R.; Mang, C.S.; Snow, N.J.; Ostadan, F.; Boyd, L.A.; Lundbye-Jensen, J. Time-Dependent Effects of Cardiovascular Exercise on Memory. Exerc. Sport Sci. Rev. 2016, 44, 81–88. [Google Scholar] [CrossRef]
- Dworak, M.; Wiater, A.; Alfer, D.; Stephan, E.; Hollmann, W.; Struder, H.K. Increased slow wave sleep and reduced stage 2 sleep in children depending on exercise intensity. Sleep Med. 2008, 9, 266–272. [Google Scholar] [CrossRef]
- Delmo, C.; Weiffenbach, O.; Gabriel, M.; Bölte, S.; Marchio, E.; Poustka, F. Kiddie-SADS-Present and Lifetime Version (K-SADS-PL), 3rd ed.; Clinic of Child and Adolescent Psychiatry: Frankfurt, Germany, 2000. [Google Scholar]
- Kaufman, J.; Birmaher, B.; Brent, D.; Rao, U.; Flynn, C.; Moreci, P.; Williamson, D.; Ryan, N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 980–988. [Google Scholar] [CrossRef]
- Achenbach, T.M. Manual for the Child Behavior Checklist/4-18 and 1991 Profile; University of Vermont, Department of Psychiatry: Burlington, VT, USA, 1991. [Google Scholar]
- Weiß, R.; Albinus, B.; Arzt, D. Grundintelligenztest Skala 2-Revision (CFT 20-R); Hogrefe: Göttingen, Germany, 2006. [Google Scholar]
- Weidlich, S.; Lamberti, G. Diagnosticum für Cerebralschädigung (DCS); Huber: Bern, Switzerland, 2001. [Google Scholar]
- Petersen, A.C.; Crockett, L.; Richards, M.; Boxer, A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J. Youth Adolesc. 1988, 17, 117–133. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Schwerdtle, B.; Roeser, K.; Kübler, A.; Schlarb, A.A. Validierung und psychometrische Eigenschaften der deutschen version des sleepself report (SSR-DE). Somnologie 2010, 14, 267–274. [Google Scholar] [CrossRef]
- Owens, J.A.; Spirito, A.; McGuinn, M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep 2000, 23, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.M.I.I.I.; Naughton, J.P.; Haskell, W.L. Physical activity and the prevention of coronary heart disease. Ann. Clin. Res. 1971, 3, 404–432. [Google Scholar] [CrossRef] [Green Version]
- Munz, M.; Baving, L.; Prehn-Kristensen, A. Sleep following intense physical exercise stabilizes motor learning in typically developing boys. Ment. Health Phys. Act. 2021, 20, 100365. [Google Scholar] [CrossRef]
- Marshall, L.; Helgadottir, H.; Molle, M.; Born, J. Boosting slow oscillations during sleep potentiates memory. Nature 2006, 444, 610–613. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events; American Academy of Sleep Medicine: Darien, IL, USA, 2007. [Google Scholar]
- Yerkes, R.M.; Dodson, J.D. The relation of strength of stimulus to rapidity of habit-formation. J. Comp. Neurol. Psychol. 1908, 18, 459–482. [Google Scholar] [CrossRef] [Green Version]
- Arnold, L.E.; Hodgkins, P.; Kahle, J.; Madhoo, M.; Kewley, G. Long-Term Outcomes of ADHD: Academic Achievement and Performance. J. Atten. Disord. 2020, 24, 73–85. [Google Scholar] [CrossRef]
| ADHD M(SEM) | TDC M(SEM) | F(22) | p | |
|---|---|---|---|---|
| Age | 10.7 (0.22) | 10.2 (0.23) | 2.068 | 0.153 |
| estimation of IQ (CFT-20R, part one) | 104.1 (2.0) | 111.4 (3.1) | 3.820 | 0.064 |
| DCS | 53.8 (6.5) | 51.0 (7.0) | 0.090 | 0.767 |
| PDS | 3.33 (0.3) | 3.08 (0.1) | 0.529 | 0.475 |
| ASEBA (T-values) | ||||
| withdrawn/depressed | 60.25 (2.1) | 52.8 (1.3) | 9.480 | 0.005 |
| somatic complaints | 56.4 (2.4) | 56.5 (2.6) | 0.001 | 0.981 |
| anxious/depressed | 59.6 (2.4) | 56.3 (1.8) | 1.166 | 0.292 |
| social problems | 59.3 (2.9) | 51.7 (1.0) | 5.947 | 0.023 |
| thought problems | 54.0 (2.6) | 53.0 (2.0) | 0.094 | 0.763 |
| attention problems | 67.4 (2.2) | 51.0 (0.9) | 47,124 | <0.0001 |
| rule breaking behavior | 61.5 (2.6) | 51.1 (0.6) | 15,046 | >0.001 |
| aggressive behavior | 62.3 (2.9) | 50.1 (0.7) | 14,865 | >0.001 |
| internalizing problems | 60.2 (2.4) | 54.1 (2.2) | 3.396 | 0.079 |
| externalizing problems | 61.4 (2.9) | 43.9 (2.2) | 22,713 | <0.0001 |
| Total | 64.0 (2.4) | 48.4 (1.8) | 27,007 | <0.0001 |
| Sleep | ||||
| SSR | 23.4 (0.9) | 21.6 (0.7) | 2.776 | 0.110 |
| CSQH | 43.3 (0.7) | 39.4 (1.0) | 5.801 | 0.025 |
| physical examination | ||||
| resting heart rate (bpm) | 76.2 (2.7) | 69.4 (2.6) | 3.247 | 0.086 |
| systolic blood pressure (mmHg) | 112.1 (1.1) | 111.3 (0.9) | 0.328 | 0.572 |
| diastolic blood pressure (mmHg) | 80.8 (1.0) | 79.6 (0.4) | 1.253 | 0.275 |
| calculated maximal heart rate (bpm) | 209.8 (0.22) | 210.4 (0.2) | 5.254 | 0.032 |
| max_power (W) | 125 (5.2) | 122.3 (7.2) | 0.094 | 0.762 |
| ADHD | TDC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exercise | Rest | Exercise | Rest | Main Effect of Condition | p | Main Effect of Group | p | Group × Condition Interaction | p | |
| M (SEM) | M (SEM) | M (SEM) | M (SEM) | F(1.22) | F(1.21) | F(1.21) | ||||
| TST (min) | 496.1 (6.7) | 506.6 (11.3) | 484.6 (7.9) | 499.4 (13.8) | 2.08 | 0.164 | 00.62 | 0.439 | 0.06 | 0.831 |
| SE (%) | 91.2 (0.9) | 89.8 (1.4) | 86.8 (1.9) | 89.9 (2.1) | 0.28 | 0.602 | 10.50 | 0.235 | 2.21 | 0.152 |
| SOL (min) | 23.1 (3.2) | 26.7 (3.9) | 38.0 (7.4) | 32.2 (10.2) | 0.03 | 0.866 | 10.80 | 0.194 | 0.55 | 0.468 |
| REM-L(min) | 154.9 (12.6) | 110.3 (15.7) | 103.2 (9.4) | 159.8 (13.9) | 24.57 | <0.001 * | 00.005 | 0.943 | 0.35 | 0.563 |
| WASO (min) | 24.0 (4.4) | 29.7 (5.8) | 34.7 (7.8) | 22.6 (6.3) | 0.28 | 0.604 | 00.08 | 0.779 | 2.08 | 0.164 |
| N1 (min) | 44.7 (4.8) | 34.5 (2.7) | 44.7 (4.8) | 43.2 (3.9) | 3.68 | 0.07 | 10.14 | 0.298 | 1.77 | 0.198 |
| N2 (min) | 234.6 (8.3) | 233.8 (10.2) | 226.8 (7.5) | 230.0 (9.3) | 0.03 | 0.872 | 00.32 | 0.578 | 0.08 | 0.786 |
| N3 (min) | 119.7 (7.8) | 128.5 (6.8) | 120.0 (6.0) | 127.0 (6.8) | 2.61 | 0.121 | 00.005 | 0.944 | 0.03 | 0.862 |
| NREM (min) | 399.0 (6.3) | 396.8 (6.5) | 391.8 (8.5) | 400.2 (10.4) | 0.14 | 0.717 | 00.04 | 0.844 | 0.10 | 0.535 |
| REM (min) | 97.1 (5.7) | 109.9 (5.9) | 92.8 (4.2) | 99.2 (8.3) | 4.00 | 0.058 | 00.46 | 0.507 | 1.02 | 0.324 |
| REST | EXERCISE | |||||
|---|---|---|---|---|---|---|
| E | R1 | R2 | E | R1 | R2 | |
| Adhd M(SEM) | 10.1 (0.50) | 8.3 (0.66) * | 11.3 (0.48) | 9.8 (0.30) | 9.5 (0.54) | 10.3 (0.60) |
| Tdc M(SEM) | 10.5 (0.44) | 10.5 (0.56) | 11.3 (0.74) | 10.6 (0.46) | 9.00 (0.46) ** | 11.8 (0.50) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munz, M.; Baving, L.; Prehn-Kristensen, A. Transient Destabilization of Declarative Memory—Opposing Impact of Physical Exercise or Rest after Encoding in Typically Developing Children and Children with Attention Deficit Hyperactivity Disorder but No Difference after Subsequent Sleep. Brain Sci. 2022, 12, 322. https://doi.org/10.3390/brainsci12030322
Munz M, Baving L, Prehn-Kristensen A. Transient Destabilization of Declarative Memory—Opposing Impact of Physical Exercise or Rest after Encoding in Typically Developing Children and Children with Attention Deficit Hyperactivity Disorder but No Difference after Subsequent Sleep. Brain Sciences. 2022; 12(3):322. https://doi.org/10.3390/brainsci12030322
Chicago/Turabian StyleMunz, Manuel, Lioba Baving, and Alexander Prehn-Kristensen. 2022. "Transient Destabilization of Declarative Memory—Opposing Impact of Physical Exercise or Rest after Encoding in Typically Developing Children and Children with Attention Deficit Hyperactivity Disorder but No Difference after Subsequent Sleep" Brain Sciences 12, no. 3: 322. https://doi.org/10.3390/brainsci12030322
APA StyleMunz, M., Baving, L., & Prehn-Kristensen, A. (2022). Transient Destabilization of Declarative Memory—Opposing Impact of Physical Exercise or Rest after Encoding in Typically Developing Children and Children with Attention Deficit Hyperactivity Disorder but No Difference after Subsequent Sleep. Brain Sciences, 12(3), 322. https://doi.org/10.3390/brainsci12030322






