The Association of Self-Reported ADHD Symptoms and Sleep in Daily Life of a General Population Sample of School Children: An Inter- and Intraindividual Perspective
Abstract
:1. Introduction
1.1. Dimensionality of ADHD Symptoms
1.2. Fluctuations in ADHD Symptomatology
1.3. Importance of Sleep
1.4. The Relation between Sleep and ADHD Symptomatology
1.5. Measurement of Sleep
1.6. The Current Study
2. Method
2.1. Participants
Procedure
2.2. Measures
2.2.1. ADHD Symptoms
2.2.2. Sleep
2.2.3. Daytime Sleepiness
2.3. Statistical Analysis
3. Results
3.1. Descriptive Results
3.2. Multilevel Analyses
3.2.1. Association of Night Sleep Quality and ADHD Symptoms
3.2.2. Association of Daytime Sleepiness and ADHD Symptoms
3.3. Explorative Post-Hoc Analysis
4. Discussion
4.1. Limitations
4.2. Implications and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Hyperactivity-Impulsivity (n = 1436) | Inattention (n = 1438) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fixed Effects | Estimate | SE | p | Estimate | SE | p | |||
| Burst 1 | |||||||||
| I Intercept: initial level | γ00 | 3.12 | 2.29 | 0.17 | 1.55 | 1.24 | 0.21 | ||
| Time slope a | γ01 | −0.18 | 0.15 | 0.24 | −0.28 | * | 0.12 | 0.02 | |
| Night sleep quality, between-person differences | γ02 | −0.15 | 0.12 | 0.25 | −0.17 | * | 0.07 | 0.02 | |
| Night sleep quality, within-person fluctuations | γ03 | 0.03 | 0.03 | 0.35 | −0.06 | * | 0.02 | 0.01 | |
| Weekend effect | γ04 | −0.10 | 0.08 | 0.21 | 0.02 | 0.06 | 0.78 | ||
| Change at Burst 2, compared to Burst 1 | |||||||||
| Change in level | γ10 | −0.007 | 0.14 | 0.96 | −0.05 | 0.10 | 0.63 | ||
| Change in time slope | γ11 | −0.48 | * | 0.23 | 0.04 | −0.006 | 0.17 | 0.97 | |
| Change in effect of night sleep quality (between-person) | γ12 | 0.14 | 0.10 | 0.20 | 0.23 | ** | 0.07 | 0.001 | |
| Change in effect of night sleep quality (within-person) | γ13 | −0.06 | 0.05 | 0.23 | 0.05 | 0.04 | 0.15 | ||
| Change in weekend effect | γ14 | 0.13 | 0.12 | 0.31 | −0.03 | 0.09 | 0.74 | ||
| Change at Burst 3, compared to Burst 1 | |||||||||
| Change in level | γ20 | −0.32 | * | 0.15 | 0.03 | −0.14 | 0.11 | 0.20 | |
| Change in time slope | γ21 | −0.28 | 0.25 | 0.25 | 0.20 | 0.18 | 0.26 | ||
| Change in effect of night sleep quality (between-person) | γ22 | 0.06 | 0.12 | 0.59 | 0.20 | ** | 0.08 | 0.009 | |
| Change in effect of night sleep quality (within-person) | γ23 | −0.05 | 0.06 | 0.40 | 0.07 | 0.04 | 0.07 | ||
| Change in weekend effect | γ24 | 0.01 | 0.13 | 0.94 | −0.09 | 0.10 | 0.37 | ||
| Control variables | |||||||||
| Gender | γ30 | 0.20 | 0.20 | 0.32 | 0.02 | 0.11 | 0.82 | ||
| Age b | γ31 | −0.009 | 0.02 | 0.61 | −0.0003 | 0.01 | 0.97 | ||
| ADHD medication c | γ32 | 0.27 | 0.34 | 0.42 | 0.33 | 0.18 | 0.07 | ||
| Random Effects & Covariances | Estimate | p | Estimate | pd | |||||
| Level 2 (between-person) | |||||||||
| Intercept: initial level | SD(u0i) | 0.77 | *** | <0.001 | 0.57 | *** | <0.001 | ||
| Time slope | SD(u1i) | 0.004 | 0.77 | 0.46 | 0.37 | ||||
| Sleep quality within-person fluctuations | SD(u2i) | 0.06 | 0.77 | 0.04 | 0.96 | ||||
| Intercept and time | r(u0i,u1i) | 0.006 | 0.95 | −0.80 | *** | <0.001 | |||
| Intercept and sleep quality fluctuations | r(u0i,u2i) | −0.44 | 0.18 | −0.89 | * | 0.05 | |||
| Time and sleep quality fluctuations | r(u1i,u2i) | −0.02 | 0.84 | −0.81 | 0.93 | ||||
| Level 1 (within-person) | |||||||||
| Residual | SD(εit) | 0.90 | 0.65 | ||||||
| Autocorrelation | ρ | 0.33 | *** | <0.001 | 0.27 | *** | <0.001 | ||
| Hyperactivity-Impulsivity (n = 5541) | Inattention (n = 5525) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fixed Effects | Estimate | SE | p | Estimate | SE | p | |||
| Burst 1 | |||||||||
| I Intercept: initial level | γ00 | 2.63 | * | 1.32 | 0.05 | 1.19 | 1.03 | 0.24 | |
| Time slope a | γ01 | −0.19 | 0.10 | 0.05 | −0.14 | 0.08 | 0.09 | ||
| Daytime sleepiness, between-person differences | γ02 | 0.04 | 0.12 | 0.71 | 0.27 | ** | 0.09 | 0.002 | |
| Daytime sleepiness, within-person fluctuations | γ03 | −0.07 | *** | 0.02 | <0.001 | −0.01 | 0.02 | 0.48 | |
| Weekend effect | γ04 | 0.09 | * | 0.05 | 0.03 | 0.03 | 0.03 | 0.45 | |
| Change at Burst 2, compared to Burst 1 | |||||||||
| Change in level | γ10 | −0.19 | * | 0.07 | 0.01 | −0.08 | 0.06 | 0.16 | |
| Change in time slope | γ11 | −0.31 | * | 0.12 | 0.01 | 0.18 | 0.11 | 0.10 | |
| Change in effect of daytime sleepiness (between-person) | γ12 | −0.006 | 0.08 | 0.94 | −0.23 | *** | 0.06 | <0.001 | |
| Change in effect of daytime sleepiness (within-person) | γ13 | 0.01 | 0.03 | 0.66 | 0.02 | 0.02 | 0.39 | ||
| Change in weekend effect | γ14 | −0.12 | 0.07 | 0.09 | −0.09 | 0.05 | 0.08 | ||
| Change at Burst 3, compared to Burst 1 | |||||||||
| Change in level | γ20 | −0.38 | *** | 0.08 | <0.001 | −0.12 | 0.06 | 0.06 | |
| Change in time slope | γ21 | −0.15 | 0.13 | 0.25 | 0.27 | * | 0.12 | 0.02 | |
| Change in effect of daytime sleepiness (between-person) | γ22 | 0.08 | 0.08 | 0.35 | −0.15 | * | 0.06 | 0.01 | |
| Change in effect of daytime sleepiness (within-person) | γ23 | 0.02 | 0.03 | 0.44 | 0.04 | 0.02 | 0.06 | ||
| Change in weekend effect | γ24 | −0.17 | * | 0.07 | 0.03 | −0.01 | 0.06 | 0.81 | |
| Control variables | |||||||||
| Gender | γ30 | 0.06 | 0.12 | 0.64 | −0.02 | 0.09 | 0.84 | ||
| Age b | γ31 | −0.006 | 0.01 | 0.55 | 0.002 | 0.008 | 0.83 | ||
| ADHD medication c | γ32 | 0.69 | *** | 0.19 | <0.001 | 0.46 | ** | 0.15 | 0.003 |
| Random Effects & Covariances | Estimate | pb | Estimate | pd | |||||
| Level 2 (between-person) | |||||||||
| Intercept: initial level | SD(u0i) | 0.68 | *** | <0.001 | 0.46 | *** | <0.001 | ||
| Time slope | SD(u1i) | 0.45 | *** | <0.001 | 0.46 | *** | <0.001 | ||
| Sleepiness within-person fluctuations | SD(u2i) | 0.11 | *** | <0.001 | 0.08 | *** | <0.001 | ||
| Intercept and time | r(u0i,u1i) | −0.17 | 0.19 | −0.61 | *** | <0.001 | |||
| Intercept and sleepiness fluctuations | r(u0i,u2i) | −0.93 | *** | <0.001 | −0.29 | 0.17 | |||
| Time and sleepiness fluctuations | r(u1i,u2i) | 0.02 | 0.84 | 0.10 | 0.73 | ||||
| Level 1 (within-person) | |||||||||
| Residual | SD(εit) | 0.85 | 0.62 | ||||||
| Autocorrelation | ρ | 0.24 | *** | <0.001 | 0.27 | *** | <0.001 | ||
Appendix B
| Fixed Effects | Estimate | SE | p | ||
|---|---|---|---|---|---|
| Burst 1 | |||||
| I Intercept: initial level | γ00 | 1.96 | 1.96 | 0.32 | |
| Time slope a | γ01 | −0.36 | * | 0.16 | 0.03 |
| Night sleep quality, between-person differences | γ02 | −0.24 | 0.12 | 0.06 | |
| Night sleep quality, within-person fluctuations | γ03 | −0.05 | 0.03 | 0.09 | |
| Weekend effect | γ04 | −0.03 | 0.07 | 0.70 | |
| Change at Burst 2, compared to Burst 1 | |||||
| Change in level | γ10 | −0.08 | 0.11 | 0.45 | |
| Change in time slope | γ11 | −0.16 | 0.20 | 0.42 | |
| Change in effect of night sleep quality (between-person) | γ12 | 0.18 | * | 0.08 | 0.03 |
| Change in effect of night sleep quality (within-person) | γ13 | 0.02 | 0.04 | 0.57 | |
| Change in weekend effect | γ14 | 0.02 | 0.09 | 0.79 | |
| Change at Burst 3, compared to Burst 1 | |||||
| Change in level | γ20 | −0.32 | ** | 0.12 | 0.008 |
| Change in time slope | γ21 | 0.10 | 0.21 | 0.63 | |
| Change in effect of night sleep quality (between-person) | γ22 | 0.12 | 0.09 | 0.21 | |
| Change in effect of night sleep quality (within-person) | γ23 | 0.05 | 0.04 | 0.25 | |
| Change in weekend effect | γ24 | −0.08 | 0.10 | 0.40 | |
| Control variables | |||||
| Gender | γ30 | 0.20 | 0.17 | 0.26 | |
| Age b | γ31 | −0.0008 | 0.02 | 0.96 | |
| ADHD medication c | γ32 | 0.06 | 0.29 | 0.85 | |
| Intervention | γ33 | −0.02 | 0.17 | 0.92 | |
| Random Effects & Covariances | Estimate | pd | |||
| Level 2 (between-person) | |||||
| Intercept: initial level | SD(u0i) | 0.68 | *** | <0.001 | |
| Time slope | SD(u1i) | 0.56 | 0.42 | ||
| Sleep quality within-person fluctuations | SD(u2i) | 0.08 | * | 0.01 | |
| Intercept and time | r(u0i,u1i) | −0.60 | *** | <0.001 | |
| Intercept and sleep quality fluctuations | r(u0i,u2i) | −0.72 | *** | <0.001 | |
| Time and sleep quality fluctuations | r(u1i,u2i) | 0.87 | 0.90 | ||
| Level 1 (within-person) | |||||
| Residual | SD(εit) | 0.64 | |||
| Autocorrelation d | ρ | 0.37 | *** | <0.001 |
| Fixed Effects | Estimate | SE | p | ||
|---|---|---|---|---|---|
| Burst 1 | |||||
| I Intercept: initial level | γ00 | 0.94 | 1.38 | 0.50 | |
| Time slope a | γ01 | −0.21 | * | 0.10 | 0.03 |
| Daytime sleepiness, between-person differences | γ02 | 0.13 | 0.12 | 0.28 | |
| Daytime sleepiness, within-person fluctuations | γ03 | −0.05 | * | 0.02 | 0.01 |
| Weekend effect | γ04 | 0.09 | * | 0.04 | 0.03 |
| Change at Burst 2, compared to Burst 1 | |||||
| Change in level | γ10 | −0.16 | * | 0.06 | 0.01 |
| Change in time slope | γ11 | −0.06 | 0.11 | 0.58 | |
| Change in effect of daytime sleepiness (between-person) | γ12 | −0.12 | 0.06 | 0.05 | |
| Change in effect of daytime sleepiness (within-person) | γ13 | 0.02 | 0.02 | 0.47 | |
| Change in weekend effect | γ14 | −0.13 | * | 0.05 | 0.03 |
| Change at Burst 3, compared to Burst 1 | |||||
| Change in level | γ20 | −0.30 | *** | 0.07 | <0.001 |
| Change in time slope | γ21 | 0.07 | 0.12 | 0.55 | |
| Change in effect of daytime sleepiness (between-person) | γ22 | −0.06 | 0.07 | 0.38 | |
| Change in effect of daytime sleepiness (within-person) | γ23 | 0.02 | 0.02 | 0.38 | |
| Change in weekend effect | γ24 | −0.13 | * | 0.06 | 0.04 |
| Control variables | |||||
| Gender | γ30 | 0.08 | 0.12 | 0.54 | |
| Age b | γ31 | 0.006 | 0.01 | 0.57 | |
| ADHD medication c | γ32 | 0.48 | * | 0.20 | 0.02 |
| Intervention | γ33 | −0.006 | 0.12 | 0.96 | |
| Random Effects & Covariances | Estimate | pd | |||
| Level 2 (between-person) | |||||
| Intercept: initial level | SD(u0i) | 0.53 | *** | <0.001 | |
| Time slope | SD(u1i) | 0.40 | *** | <0.001 | |
| Sleepiness within-person fluctuations | SD(u2i) | 0.08 | *** | <0.001 | |
| Intercept and time | r(u0i,u1i) | −0.26 | 0.11 | ||
| Intercept and sleepiness fluctuations | r(u0i,u2i) | −0.80 | *** | <0.001 | |
| Time and sleepiness fluctuations | r(u1i,u2i) | 0.13 | 0.69 | ||
| Level 1 (within-person) | |||||
| Residual | SD(εit) | 0.64 | |||
| Autocorrelation | ρ | 0.31 | *** | <0.001 |
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DMS V; American Psychiatric Association: Philadelphia, PA, USA, 2013. [Google Scholar] [CrossRef]
- Sonuga-Barke, E.J.S. Psychological Heterogeneity in AD/HD–a Dual Pathway Model of Behaviour and Cognition. Behav. Brain Res. 2002, 130, 29–36. [Google Scholar] [CrossRef]
- Polanczyk, G.V.; Salum, G.A.; Sugaya, L.S.; Caye, A.; Rohde, L.A. Annual Research Review: A Meta-Analysis of the Worldwide Prevalence of Mental Disorders in Children and Adolescents. J. Child Psychol. Psychiatry 2015, 56, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Caye, A.; Swanson, J.; Thapar, A.; Sibley, M.; Arseneault, L.; Hechtman, L.; Arnold, L.E.; Niclasen, J.; Moffitt, T.; Rohde, L.A. Life Span Studies of ADHD—Conceptual Challenges and Predictors of Persistence and Outcome. Curr. Psychiatry Rep. 2016, 18, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robson, D.A.; Allen, M.S.; Howard, S.J. Self-Regulation in Childhood as a Predictor of Future Outcomes: A Meta-Analytic Review. Psychol. Bull. 2020, 146, 324–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coghill, D.; Sonuga-Barke, E.J.S. Annual Research Review: Categories versus Dimensions in the Classification and Conceptualisation of Child and Adolescent Mental Disorders–Implications of Recent Empirical Study. J. Child Psychol. Psychiatry 2012, 53, 469–489. [Google Scholar] [CrossRef]
- Larsson, H.; Anckarsater, H.; Råstam, M.; Chang, Z.; Lichtenstein, P. Childhood Attention-Deficit Hyperactivity Disorder as an Extreme of a Continuous Trait: A Quantitative Genetic Study of 8500 Twin Pairs. J. Child Psychol. Psychiatry 2012, 53, 73–80. [Google Scholar] [CrossRef]
- Swanson, J.M.; Schuck, S.; Porter, M.M.; Hartman, C.A.; Sergeant, J.A.; Clevenger, W.; Wasdell, M.; McCleary, R.; Lakes, K.; Wigal, T. Categorical and Dimensional Definitions and Evaluations of Symptoms of ADHD: History of the SNAP and the SWAN Rating Scales. Int. J. Educ. Psychol. Assess. 2012, 10, 51–70. [Google Scholar]
- Schulz-Zhecheva, Y.; Voelkle, M.; Beauducel, A.; Buch, N.; Fleischhaker, C.; Bender, S.; Saville, C.W.N.; Biscaldi, M.; Klein, C. ADHD Traits in German School-Aged Children: Validation of the German Strengths and Weaknesses of ADHS Symptoms and Normal Behavior (SWAN-DE) Scale. J. Atten. Disord. 2017, 23, 553–562. [Google Scholar] [CrossRef]
- Blume, F.; Kuehnhausen, J.; Buhr, L.; Köpke, R.; Fallgatter, A.J.; Gawrilow, C. Validation of a Self-Report Version of the German Strengths and Weaknesses of ADHD Symptoms and Normal Behavior Scale (SWAN-DE-SB). Psyarxiv 2020. preprint. [Google Scholar]
- Schmid, J.; Stadler, G.; Dirk, J.; Fiege, C.; Gawrilow, C. ADHD Symptoms in Adolescents’ Everyday Life. J. Atten. Disord. 2016, 24, 1169–1180. [Google Scholar] [CrossRef]
- Koch, E.D.; Moukhtarian, T.R.; Skirrow, C.; Bozhilova, N.; Asherson, P.; Ebner-Priemer, U.W. Using E-Diaries to Investigate ADHD—State-of-the-Art and the Promising Feature of Just-in-Time-Adaptive Interventions. Neurosci. Biobehav. Rev. 2021, 127, 884–898. [Google Scholar] [CrossRef] [PubMed]
- Trull, T.J.; Ebner-Priemer, U.W. Ambulatory Assessment in Psychopathology Research: A Review of Recommended Reporting Guidelines and Current Practices. J. Abnorm. Psychol. 2020, 129, 56–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, U.; Gawrilow, C. Measuring and Compensating for Deficits of Self-Regulation in School Children via Ambulatory Assessment. Psychol. Russ. State Art 2019, 12, 8–22. [Google Scholar] [CrossRef]
- Ludwig, K.; Haindl, A.; Laufs, R.; Rauch, W.A.; Ludwig, K.; Haindl, A.; Laufs, R.; Rauch, W.A. Self-Regulation in Preschool Children ’ s Everyday Life: Exploring Day-to-Day Variability and the Within- and Between-Person Structure. J. Self-Regul. Regul. 2016, 2, 98–117. [Google Scholar] [CrossRef]
- Leonard, J.A.; Lydon-Staley, D.M.; Sharp, S.D.S.; Liu, H.Z.; Park, A.T.; Bassett, D.S.; Duckworth, A.L.; Mackey, A.P. Daily Fluctuations in Young Children’s Persistence. Child Dev. 2021, 93, e222–e236. [Google Scholar] [CrossRef]
- Hvolby, A. Associations of Sleep Disturbance with ADHD: Implications for Treatment. ADHD Atten. Deficit Hyperact. Disord. 2015, 7, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Gregory, A.M.; Sadeh, A. Sleep, Emotional and Behavioural Difficulties in Children and Adolescents. Sleep Med. Rev. 2012, 16, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Chokroverty, S. Overview of Normal Sleep. In Sleep Disorders Medicine; Chokroverty, S., Ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Vriend, J.L.; Davidson, F.D.; Corkum, P.V.; Rusak, B.; McLaughlin, E.N.; Chambers, C.T. Sleep Quantity and Quality in Relation to Daytime Functioning in Children. Child. Health Care 2012, 41, 204–222. [Google Scholar] [CrossRef]
- Becker, S.P. ADHD and Sleep: Recent Advances and Future Directions. Curr. Opin. Psychol. 2020, 34, 50–56. [Google Scholar] [CrossRef]
- Van der Meere, J. State Regulation and Attention Deficit Hyperactivity Disorder. In Attention Deficit Hyperactivity Disorder. From Genes to Patients; Gozal, D., Molfese, D.L., Eds.; Humana Press: Passaic, NJ, USA, 2005; pp. 413–434. [Google Scholar]
- Gruber, R.; Cassoff, J.; Frenette, S.; Wiebe, S.; Carrier, J. Impact of Sleep Extension and Restriction on Children’s Emotional Lability and Impulsivity. Pediatrics 2012, 130, e1155–e1161. [Google Scholar] [CrossRef]
- Schumacher, A.M.; Miller, A.L.; Watamura, S.E.; Kurth, S.; Lassonde, J.M.; LeBourgeois, M.K. Sleep Moderates the Association Between Response Inhibition and Self-Regulation in Early Childhood. J. Clin. Child Adolesc. Psychol. 2017, 46, 222–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiscock, H.; Sciberras, E.; Mensah, F.; Gerner, B.; Efron, D.; Khano, S.; Oberklaid, F. Impact of a Behavioural Sleep Intervention on Symptoms and Sleep in Children with Attention Deficit Hyperactivity Disorder, and Parental Mental Health: Randomised Controlled Trial. BMJ 2015, 350, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, J.; Gruber, R.; Brown, T.; Corkum, P.; Cortese, S.; O’Brien, L.; Stein, M.; Weiss, M. Future Research Directions in Sleep and ADHD: Report of a Consensus Working Group. J. Atten. Disord. 2012, 17, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, K.B.; Smits, M.G.; Gunning, W.B. Sleep-Related Disorders in ADHD: A Review. Clin. Pediatr. 2005, 44, 201–210. [Google Scholar] [CrossRef]
- De Crescenzo, F.; Licchelli, S.; Ciabattini, M.; Menghini, D.; Armando, M.; Alfieri, P.; Mazzone, L.; Pontrelli, G.; Livadiotti, S.; Foti, F.; et al. The Use of Actigraphy in the Monitoring of Sleep and Activity in ADHD: A Meta-Analysis. Sleep Med. Rev. 2014, 26, 9–20. [Google Scholar] [CrossRef]
- Gaina, A.; Sekine, M.; Kanayama, H.; Sengoku, K.; Yamagami, T.; Kagamimori, S. Short-Long Sleep Latency and Associated Factors in Japanese Junior High School Children. Sleep Biol. Rhythm. 2005, 3, 162–165. [Google Scholar] [CrossRef]
- Dewald, J.F.; Meijer, A.M.; Oort, F.J.; Kerkhof, G.A.; Bögels, S.M. The Influence of Sleep Quality, Sleep Duration and Sleepiness on School Performance in Children and Adolescents: A Meta-Analytic Review. Sleep Med. Rev. 2010, 14, 179–189. [Google Scholar] [CrossRef]
- Lidzba, K.; Christiansen, H.; Drechsler, R. Conners Skalen Zu Aufmerksamkeit Und Verhalten—3 (Conners 3®). Deutschsprachige Adaption Der Conners 3rd Edition® (Conners 3®) von C. Keith Conners; Verlag Hans Huber, Hogrefe AG: Bern, Switzerland, 2013. [Google Scholar]
- Shrout, P.E.; Lane, S.P. Psychometrics. In Handbook of Research Methods for Studying Daily Life; Mehl, M.R., Conner, T.S., Eds.; The Guilford Press: New York, NY, USA, 2012; pp. 302–320. [Google Scholar]
- Könen, T.; Dirk, J.; Schmiedek, F. Cognitive Benefits of Last Night’s Sleep: Daily Variations in Children’s Sleep Behavior Are Related to Working Memory Fluctuations. J. Child Psychol. Psychiatry Allied Discip. 2015, 56, 171–182. [Google Scholar] [CrossRef]
- Steyer, R.; Schwenkmezger, P.; Notz, P.; Eid, M. Der Mehrdimensionale Befindlichkeitsfragebogen (MDBF); Hogrefe: Göttingen, Germany, 1997. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Bolger, N.; Laurenceau, J.-P. Intensive Longitudinal Methods. An Introduction to Diary and Experience Sampling Research; The Guilford Press: New York, NY, USA, 2013. [Google Scholar]
- Cohen, J. A Power Primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Faraone, S.V.; Biederman, J.; Mick, E. The Age-Dependent Decline of Attention Deficit Hyperactivity Disorder: A Meta-Analysis of Follow-up Studies. Psychol. Med. 2006, 36, 159–165. [Google Scholar] [CrossRef]
- Döpfner, M.; Hautmann, C.; Görtz-Dorten, A.; Klasen, F.; Ravens-Sieberer, U. Long-Term Course of ADHD Symptoms from Childhood to Early Adulthood in a Community Sample. Eur. Child Adolesc. Psychiatry 2015, 24, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Shrout, P.E.; Stadler, G.; Lane, S.P.; McClure, M.J.; Jackson, G.L.; Clavél, F.D.; Iida, M.; Gleason, M.E.J.; Xu, J.H.; Bolger, N. Initial Elevation Bias in Subjective Reports. Proc. Natl. Acad. Sci. USA 2018, 115, E15–E23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paavonen, E.J.; Raikkonen, K.; Lahti, J.; Komsi, N.; Heinonen, K.; Pesonen, A.-K.; Järvenpää, A.-L.; Strandberg, T.; Kajantie, E.; Porkka-Heiskanen, T. Short Sleep Duration and Behavioral Symptoms of Attention-Deficit/ Hyperactivity Disorder in Healthy 7-to 8-Year-Old Children. Pediatrics 2009, 123, 857–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fricke-Oerkermann, L.; Plück, J.; Schredl, M.; Heinz, K.; Mitschke, A.; Wiater, A.; Lehmkuhl, G. Prevalence and Course of Sleep Problems in Childhood. Sleep 2007, 30, 1371–1377. [Google Scholar] [CrossRef] [Green Version]
- Pesonen, A.K.; Räikkönen, K.; Paavonen, E.J.; Heinonen, K.; Komsi, N.; Lahti, J.; Kajantie, E.; Järvenpää, A.L.; Strandberg, T. Sleep Duration and Regularity Are Associated with Behavioral Problems in 8-Year-Old Children. Int. J. Behav. Med. 2010, 17, 298–305. [Google Scholar] [CrossRef]

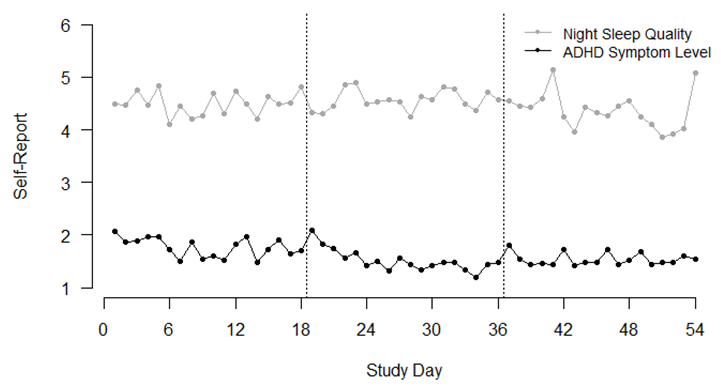
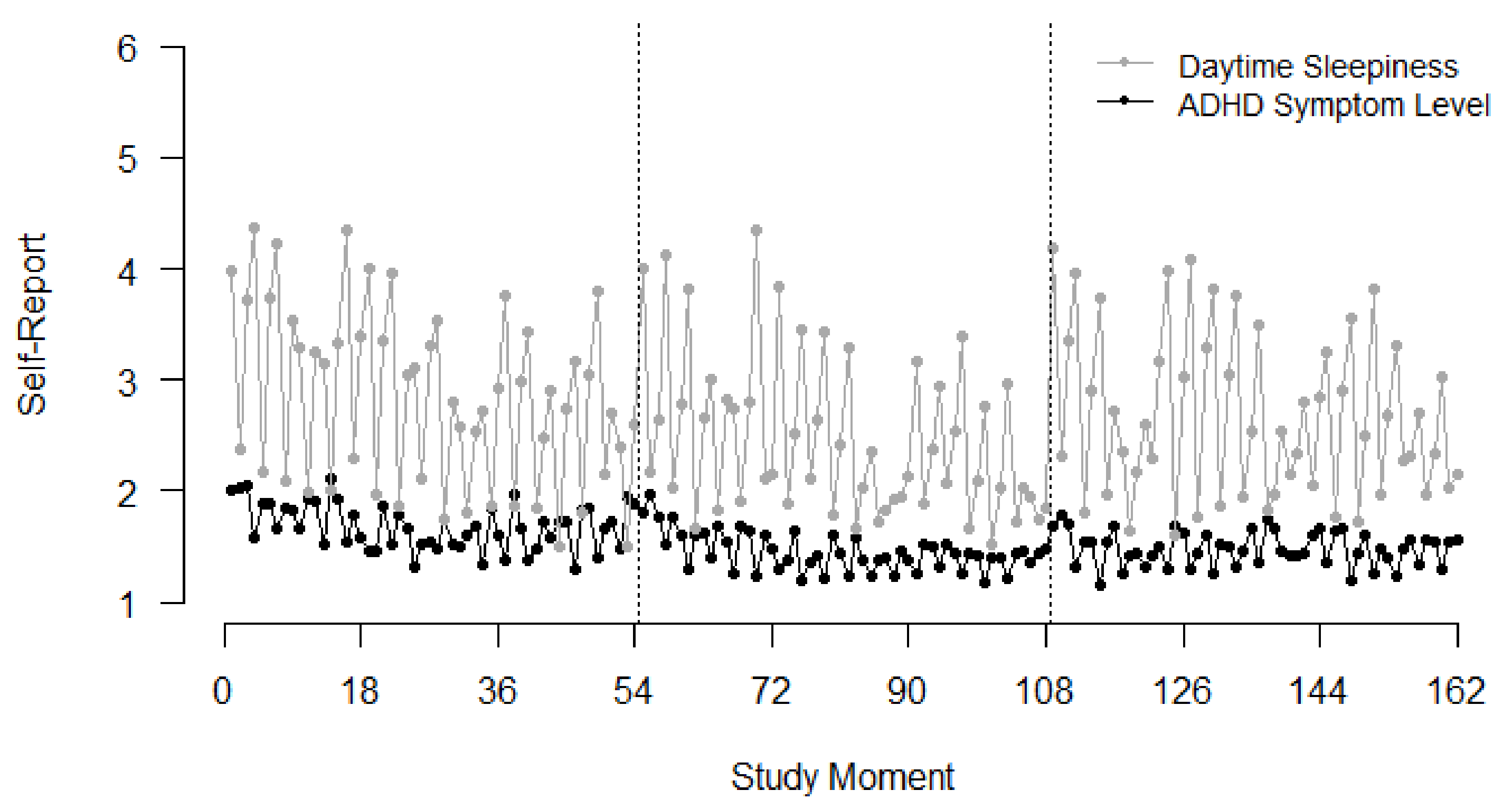
| Between-Person | Within-Person | ||||||
|---|---|---|---|---|---|---|---|
| M | SD | Range | MISD | SD | Range | ICC | |
| ADHD (afternoon) 1 | 1.62 | 0.60 | 1.00–3.64 | 0.59 | 0.41 | 0.00–1.92 | 0.44 |
| ADHD (overall) 2 | 1.52 | 0.50 | 1.00–2.91 | 0.56 | 0.37 | 0.00–1.79 | 0.43 |
| Night sleep quality | 4.58 | 0.82 | 2.23–6.00 | 1.11 | 0.57 | 0.00–2.37 | 0.43 |
| Daytime sleepiness | 2.68 | 0.98 | 1.00–4.42 | 1.48 | 0.55 | 0.00–2.28 | 0.34 |
| Fixed Effects | Estimate | SE | p | ||
|---|---|---|---|---|---|
| Burst 1 | |||||
| Intercept: initial level | γ00 | 2.07 | 1.60 | 0.20 | |
| Time slope a | γ01 | −0.27 | * | 0.13 | 0.04 |
| Night sleep quality, between-person differences | γ02 | −0.16 | 0.09 | 0.07 | |
| Night sleep quality, within-person fluctuations | γ03 | −0.02 | 0.02 | 0.40 | |
| Weekend effect | γ04 | −0.02 | 0.06 | 0.75 | |
| Change at Burst 2, compared to Burst 1 | |||||
| Change in level | γ10 | −0.03 | 0.10 | 0.74 | |
| Change in time slope | γ11 | −0.21 | 0.18 | 0.24 | |
| Change in effect of night sleep quality (between-person) | γ12 | 0.18 | * | 0.08 | 0.03 |
| Change in effect of night sleep quality (within-person) | γ13 | −0.002 | 0.04 | 0.95 | |
| Change in weekend effect | γ14 | 0.02 | 0.09 | 0.85 | |
| Change at Burst 3, compared to Burst 1 | |||||
| Change in level | γ20 | −0.25 | * | 0.11 | 0.02 |
| Change in time slope | γ21 | 0.02 | 0.19 | 0.92 | |
| Change in effect of night sleep quality (between-person) | γ22 | 0.12 | 0.09 | 0.17 | |
| Change in effect of night sleep quality (within-person) | γ23 | 0.02 | 0.04 | 0.61 | |
| Change in weekend effect | γ24 | −0.07 | 0.09 | 0.44 | |
| Control variables | |||||
| Gender | γ30 | 0.08 | 0.14 | 0.56 | |
| Age b | γ31 | −0.003 | 0.01 | 0.80 | |
| ADHD medication c | γ32 | 0.29 | 0.23 | 0.22 | |
| Random Effects & Covariances | Estimate | pd | |||
| Level 2 (between-person) | |||||
| Intercept: initial level | SD(u0i) | 0.62 | *** | <0.001 | |
| Time slope | SD(u1i) | 0.49 | 0.93 | ||
| Sleep quality within-person fluctuations | SD(u2i) | 0.06 | 0.93 | ||
| Intercept and time | r(u0i,u1i) | −0.51 | ** | 0.003 | |
| Intercept and sleep quality fluctuations | r(u0i,u2i) | −0.78 | * | 0.03 | |
| Time and sleep quality fluctuations | r(u1i,u2i) | 0.78 | 0.99 | ||
| Level 1 (within-person) | |||||
| Residual | SD(εit) | 0.66 | |||
| Autocorrelation | ρ | 0.34 | *** | <0.001 |
| Fixed Effects | Estimate | SE | p | ||
|---|---|---|---|---|---|
| Burst 1 | |||||
| Intercept: initial level | γ00 | 1.65 | 1.13 | 0.14 | |
| Time slope a | γ01 | −0.23 | ** | 0.08 | 0.004 |
| Daytime sleepiness, between-person differences | γ02 | 0.16 | 0.10 | 0.11 | |
| Daytime sleepiness, within-person fluctuations | γ03 | −0.04 | * | 0.02 | 0.01 |
| Weekend effect | γ04 | 0.05 | 0.03 | 0.14 | |
| Change at Burst 2, compared to Burst 1 | |||||
| Change in level | γ10 | −0.16 | ** | 0.06 | 0.006 |
| Change in time slope | γ11 | −0.06 | 0.10 | 0.57 | |
| Change in effect of daytime sleepiness (between-person) | γ12 | −0.12 | * | 0.06 | 0.04 |
| Change in effect of daytime sleepiness (within-person) | γ13 | 0.01 | 0.02 | 0.50 | |
| Change in weekend effect | γ14 | −0.09 | 0.05 | 0.10 | |
| Change at Burst 3, compared to Burst 1 | |||||
| Change in level | γ20 | −0.28 | *** | 0.06 | <0.001 |
| Change in time slope | γ21 | 0.06 | 0.10 | 0.57 | |
| Change in effect of daytime sleepiness (between-person) | γ22 | −0.06 | 0.07 | 0.39 | |
| Change in effect of daytime sleepiness (within-person) | γ23 | 0.03 | 0.02 | 0.18 | |
| Change in weekend effect | γ24 | −0.09 | 0.06 | 0.10 | |
| Control variables | |||||
| Gender | γ30 | −0.002 | 0.10 | 0.98 | |
| Age b | γ31 | 0.0003 | 0.009 | 0.97 | |
| ADHD medication c | γ32 | 0.55 | ** | 0.17 | 0.001 |
| Random Effects & Covariances | Estimate | pd | |||
| Level 2 (between-person) | |||||
| Intercept: initial level | SD(u0i) | 0.50 | *** | <0.001 | |
| Time slope | SD(u1i) | 0.40 | *** | <0.001 | |
| Sleepiness within-person fluctuations | SD(u2i) | 0.07 | *** | <0.001 | |
| Intercept and time | r(u0i,u1i) | −0.30 | 0.05 | ||
| Intercept and sleepiness fluctuations | r(u0i,u2i) | −0.73 | *** | <0.001 | |
| Time and sleepiness fluctuations | r(u1i,u2i) | −0.04 | 0.54 | ||
| Level 1 (within-person) | |||||
| Residual | SD(εit) | 0.64 | |||
| Autocorrelation | ρ | 0.27 | *** | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buhr, L.; Moschko, T.; Eppinger Ruiz de Zarate, A.; Schwarz, U.; Kühnhausen, J.; Gawrilow, C. The Association of Self-Reported ADHD Symptoms and Sleep in Daily Life of a General Population Sample of School Children: An Inter- and Intraindividual Perspective. Brain Sci. 2022, 12, 440. https://doi.org/10.3390/brainsci12040440
Buhr L, Moschko T, Eppinger Ruiz de Zarate A, Schwarz U, Kühnhausen J, Gawrilow C. The Association of Self-Reported ADHD Symptoms and Sleep in Daily Life of a General Population Sample of School Children: An Inter- and Intraindividual Perspective. Brain Sciences. 2022; 12(4):440. https://doi.org/10.3390/brainsci12040440
Chicago/Turabian StyleBuhr, Lilly, Tomasz Moschko, Anne Eppinger Ruiz de Zarate, Ulrike Schwarz, Jan Kühnhausen, and Caterina Gawrilow. 2022. "The Association of Self-Reported ADHD Symptoms and Sleep in Daily Life of a General Population Sample of School Children: An Inter- and Intraindividual Perspective" Brain Sciences 12, no. 4: 440. https://doi.org/10.3390/brainsci12040440
APA StyleBuhr, L., Moschko, T., Eppinger Ruiz de Zarate, A., Schwarz, U., Kühnhausen, J., & Gawrilow, C. (2022). The Association of Self-Reported ADHD Symptoms and Sleep in Daily Life of a General Population Sample of School Children: An Inter- and Intraindividual Perspective. Brain Sciences, 12(4), 440. https://doi.org/10.3390/brainsci12040440






