Functional Coupling of the Locus Coeruleus Is Linked to Successful Cognitive Control
Abstract
1. Introduction
2. Material and Methods
2.1. Participants
2.2. Stimulus Presentation
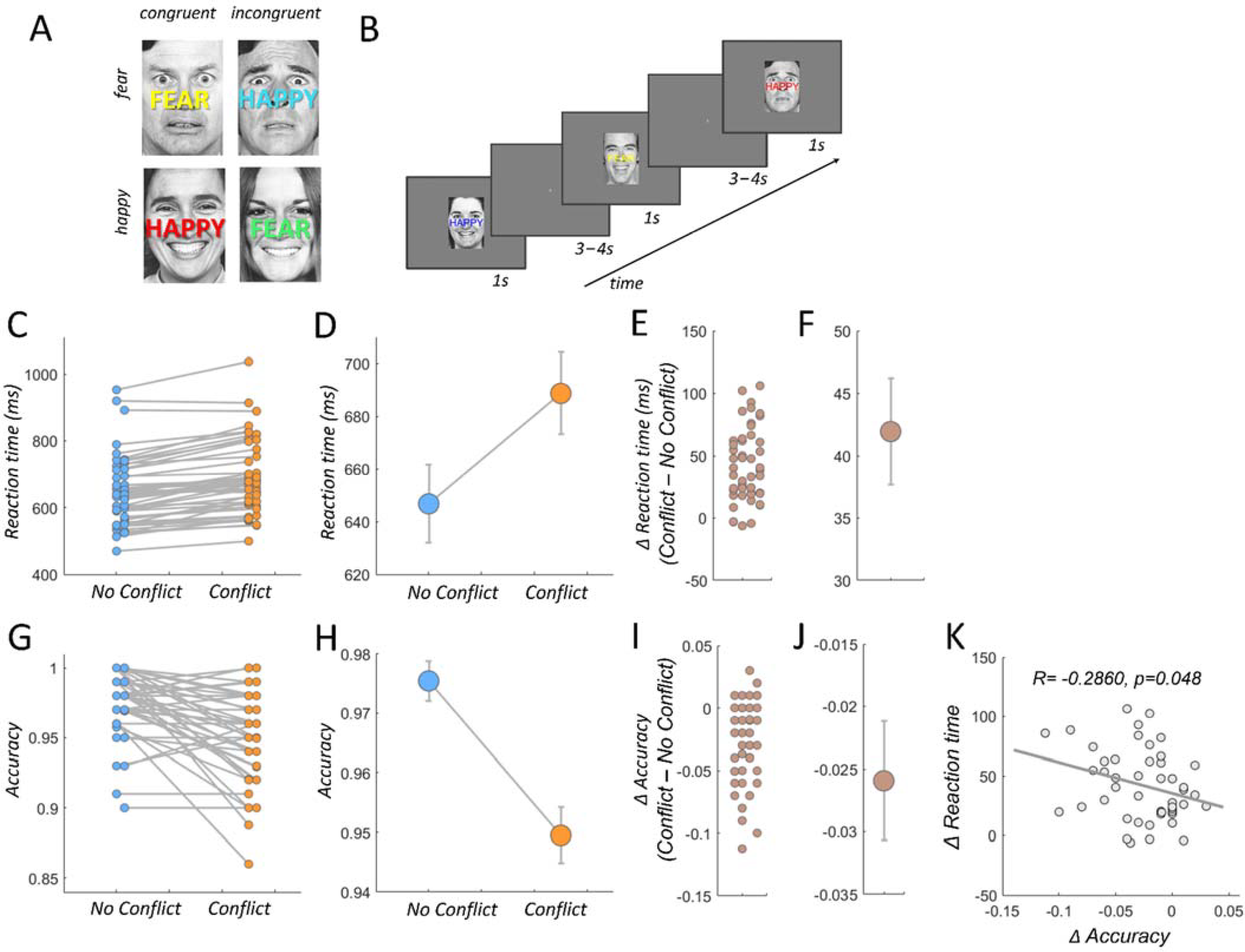
2.3. Behavioral Task
2.4. Behavioral Analyses
2.5. The fMRI Image Acquisition
2.6. The fMRI Image Preprocessing
2.7. The fMRI Data Analysis
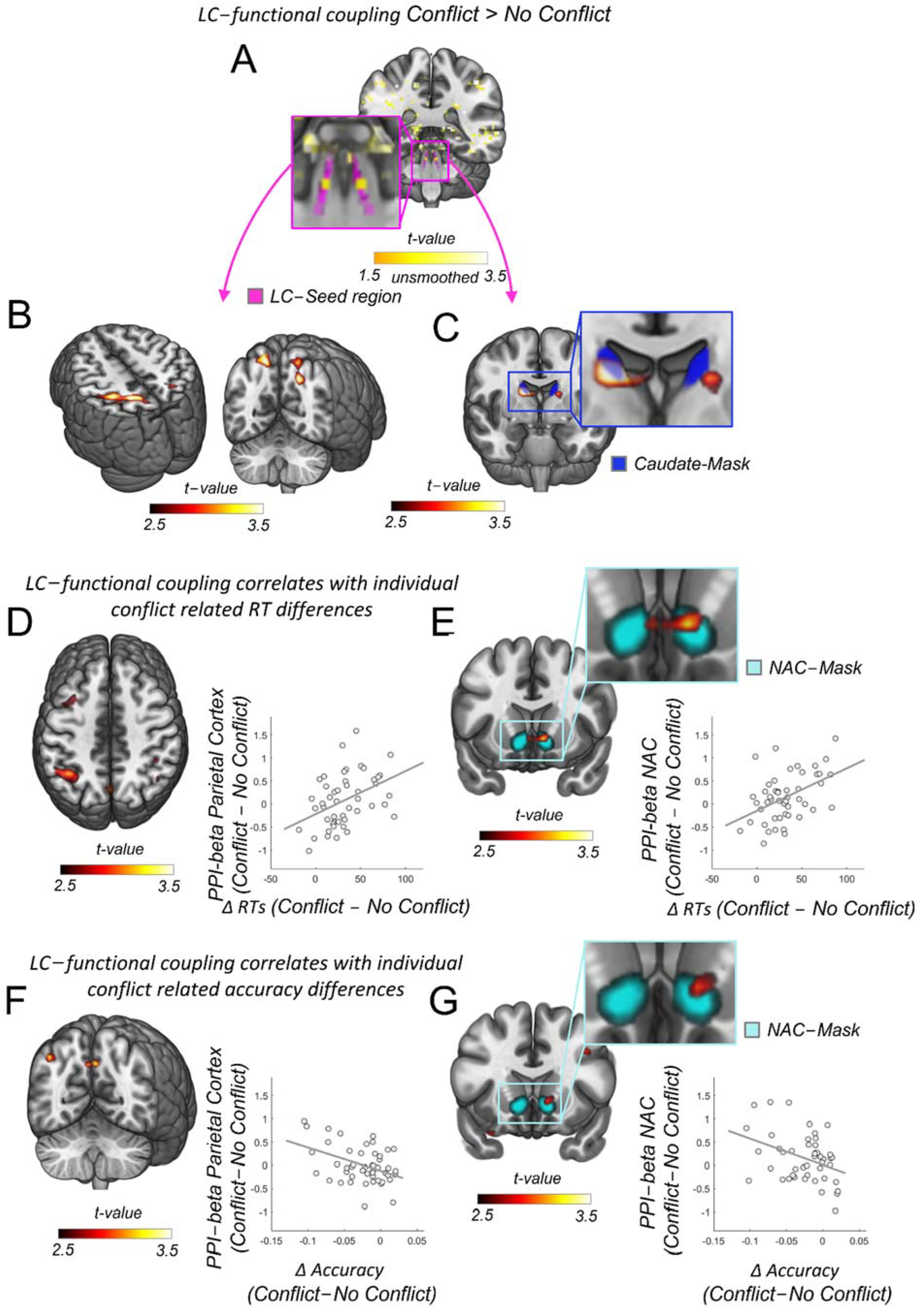
2.8. Psychophysiological Analysis (PPI)
3. Results
3.1. Behavioral Results
3.2. LC Functional Coupling Relates to Individual RTs during Conflict Resolution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manaye, K.F.; McIntire, D.D.; Mann, D.M.A.; German, D.C. Locus coeruleus cell loss in the aging human brain: A non-random process. J. Comp. Neurol. 1995, 358, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Sara, S.J.; Bouret, S. Orienting and Reorienting: The Locus Coeruleus Mediates Cognition through Arousal. Neuron 2012, 76, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Usher, M.; Cohen, J.D.; Servan-Schreiber, D.; Rajkowski, J.; Aston-Jones, G. The Role of Locus Coeruleus in the Regulation of Cognitive Performance. Science 1999, 283, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, A.; Tan, B.Z.; Ycu, E.A.; Cuevas, J.S.; Koivumaa, J.; Junyent, F.; Kremer, E.; Witten, I.B.; Deisseroth, K.; Johansen, J.P. Modular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat. Neurosci. 2017, 20, 1602–1611. [Google Scholar] [CrossRef] [PubMed]
- Dahl, M.J.; Mather, M.; Werkle-Bergner, M. Noradrenergic modulation of rhythmic neural activity shapes selective attention. Trends Cogn. Sci. 2021, 26, 38–52. [Google Scholar] [CrossRef]
- Verguts, T.; Notebaert, W. Adaptation by binding: A learning account of cognitive control. Trends Cogn. Sci. 2009, 13, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Thiele, A.; Bellgrove, M. Neuromodulation of Attention. Neuron 2018, 97, 769–785. [Google Scholar] [CrossRef]
- Egner, T. The Wiley Handbook of Cognitive Control; Egner, T., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Miller, E.K.; Cohen, J.D. An Integrative Theory of Prefrontal Cortex Function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef]
- Mansouri, F.A.; Tanaka, K.; Buckley, M.J. Conflict-induced behavioural adjustment: A clue to the executive functions of the prefrontal cortex. Nat. Rev. Neurosci. 2009, 10, 141–152. [Google Scholar] [CrossRef]
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. Gen. 1992, 121, 15–23. [Google Scholar] [CrossRef]
- Botvinick, M.M.; Braver, T.S.; Barch, D.M.; Carter, C.S.; Cohen, J.D. Conflict monitoring and cognitive control. Psychol. Rev. 2001, 108, 624–652. [Google Scholar] [CrossRef] [PubMed]
- Botvinick, M.M.; Cohen, J.D.; Carter, C.S. Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn. Sci. 2004, 8, 539–546. [Google Scholar] [CrossRef]
- Etkin, A.; Egner, T.; Kalisch, R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011, 15, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.D.; Mathias, C.J.; Josephs, O.; O’Doherty, J.; Zanini, S.; Dewar, B.; Cipolotti, L.; Shallice, T.; Dolan, R. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain 2003, 126, 2139–2152. [Google Scholar] [CrossRef]
- Egner, T.; Hirsch, J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat. Neurosci. 2005, 8, 1784–1790. [Google Scholar] [CrossRef]
- Ridderinkhof, K.R.; Wildenberg, W.P.V.D.; Segalowitz, S.J.; Carter, C.S. Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004, 56, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Marek, S.; Dosenbach, N.U.F. The frontoparietal network: Function, electrophysiology, and importance of individual precision mapping. Dialog. Clin. Neurosci. 2018, 20, 133–140. [Google Scholar]
- Buschman, T.J.; Kastner, S. From Behavior to Neural Dynamics: An Integrated Theory of Attention. Neuron 2015, 88, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Safaai, H.; Neves, R.; Eschenko, O.; Logothetis, N.K.; Panzeri, S. Modeling the effect of locus coeruleus firing on cortical state dynamics and single-trial sensory processing. Proc. Natl. Acad. Sci. USA 2015, 112, 12834–12839. [Google Scholar] [CrossRef] [PubMed]
- Bari, A.; Xu, S.; Pignatelli, M.; Takeuchi, D.; Feng, J.; Li, Y.; Tonegawa, S. Differential attentional control mechanisms by two distinct noradrenergic coeruleo-frontal cortical pathways. Proc. Natl. Acad. Sci. USA 2020, 117, 29080–29089. [Google Scholar] [CrossRef]
- Lee, T.-H.; Greening, S.G.; Ueno, T.; Clewett, D.; Ponzio, A.; Sakaki, M.; Mather, M. Arousal increases neural gain via the locus coeruleus–noradrenaline system in younger adults but not in older adults. Nat. Hum. Behav. 2018, 2, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Vazey, E.M.; Moorman, D.E.; Aston-Jones, G. Phasic locus coeruleus activity regulates cortical encoding of salience information. Proc. Natl. Acad. Sci. USA 2018, 115, E9439–E9448. [Google Scholar] [CrossRef] [PubMed]
- Mather, M.; Clewett, D.; Sakaki, M.; Harley, C.W. Norepinephrine ignites local hotspots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behav. Brain Sci. 2015, 39, E200. [Google Scholar] [CrossRef]
- Berridge, C.W.; Waterhouse, B.D. The locus coeruleus–noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 2003, 42, 33–84. [Google Scholar] [CrossRef]
- Rodenkirch, C.; Liu, Y.; Schriver, B.J.; Wang, Q. Locus coeruleus activation enhances thalamic feature selectivity via norepinephrine regulation of intrathalamic circuit dynamics. Nat. Neurosci. 2018, 22, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Waschke, L.; Tune, S.; Obleser, J. Local cortical desynchronization and pupil-linked arousal differentially shape brain states for optimal sensory performance. eLife 2019, 8, e51501. [Google Scholar] [CrossRef]
- Gelbard-Sagiv, H.; Magidov, E.; Sharon, H.; Hendler, T.; Nir, Y. Noradrenaline Modulates Visual Perception and Late Visually Evoked Activity. Curr. Biol. 2018, 28, 2239–2249.e6. [Google Scholar] [CrossRef]
- Pfeffer, T.; Avramiea, A.-E.; Nolte, G.; Engel, A.K.; Linkenkaer-Hansen, K.; Donner, T.H. Catecholamines alter the intrinsic variability of cortical population activity and perception. PLoS Biol. 2018, 16, e2003453. [Google Scholar] [CrossRef]
- Sharon, O.; Fahoum, F.; Nir, Y. Transcutaneous Vagus Nerve Stimulation in Humans Induces Pupil Dilation and Attenuates Alpha Oscillations. J. Neurosci. 2020, 41, 320–330. [Google Scholar] [CrossRef]
- Pfeffer, T.; Ponce-Alvarez, A.; Tsetsos, K.; Meindertsma, T.; Gahnström, C.J.; Brink, R.L.V.D.; Nolte, G.; Engel, A.K.; Deco, G.; Donner, T.H. Circuit mechanisms for the chemical modulation of cortex-wide network interactions and behavioral variability. Sci. Adv. 2021, 7, eabf5620. [Google Scholar] [CrossRef]
- Maier, S.U.; Grueschow, M. Pupil dilation predicts individual self-regulation success across domains. Sci. Rep. 2021, 11, 14342 . [Google Scholar] [CrossRef]
- Kurniawan, I.T.; Grueschow, M.; Ruff, C.C. Anticipatory Energization Revealed by Pupil and Brain Activity Guides Human Effort-Based Decision Making. J. Neurosci. 2021, 41, 6328–6342. [Google Scholar] [CrossRef] [PubMed]
- Grueschow, M.; Kleim, B.; Ruff, C.C. Role of the locus coeruleus arousal system in cognitive control. J. Neuroendocr. 2020, 32, e12890. [Google Scholar] [CrossRef] [PubMed]
- Grueschow, M.; Stenz, N.; Thörn, H.; Ehlert, U.; Breckwoldt, J.; Maeder, M.B.; Exadaktylos, A.K.; Bingisser, R.; Ruff, C.C.; Kleim, B. Real-world stress resilience is associated with the responsivity of the locus coeruleus. Nat. Commun. 2021, 12, 2275. [Google Scholar] [CrossRef] [PubMed]
- Köhler, S.; Bär, K.-J.; Wagner, G. Differential involvement of brainstem noradrenergic and midbrain dopaminergic nuclei in cognitive control. Hum. Brain Mapp. 2016, 37, 2305–2318. [Google Scholar] [CrossRef] [PubMed]
- Köhler, S.; Wagner, G.; Bär, K. Activation of brainstem and midbrain nuclei during cognitive control in medicated patients with schizophrenia. Hum. Brain Mapp. 2018, 40, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Manger, P.R.; Eschenko, O. The Mammalian Locus Coeruleus Complex—Consistencies and Variances in Nuclear Organization. Brain Sci. 2021, 11, 1486. [Google Scholar] [CrossRef]
- Berridge, C.W. Noradrenergic modulation of arousal. Brain Res. Rev. 2008, 58, 1–17. [Google Scholar] [CrossRef]
- Poe, G.R.; Foote, S.; Eschenko, O.; Johansen, J.P.; Bouret, S.; Aston-Jones, G.; Harley, C.W.; Manahan-Vaughan, D.; Weinshenker, D.; Valentino, R.; et al. Locus coeruleus: A new look at the blue spot. Nat. Rev. Neurosci. 2020, 21, 644–659. [Google Scholar] [CrossRef]
- McGinley, M.J.; Vinck, M.; Reimer, J.; Batista-Brito, R.; Zagha, E.; Cadwell, C.; Tolias, A.S.; Cardin, J.A.; McCormick, D.A. Waking State: Rapid Variations Modulate Neural and Behavioral Responses. Neuron 2015, 87, 1143–1161. [Google Scholar] [CrossRef]
- McCormick, D.A.; Nestvogel, D.B.; He, B. Neuromodulation of Brain State and Behavior. Annu. Rev. Neurosci. 2020, 43, 391–415. [Google Scholar] [CrossRef] [PubMed]
- Reimer, J.; Froudarakis, E.; Cadwell, C.; Yatsenko, D.; Denfield, G.H.; Tolias, A.S. Pupil Fluctuations Track Fast Switching of Cortical States during Quiet Wakefulness. Neuron 2014, 84, 355–362. [Google Scholar] [CrossRef]
- Zerbi, V.; Floriou-Servou, A.; Markicevic, M.; Vermeiren, Y.; Sturman, O.; Privitera, M.; von Ziegler, L.; Ferrari, K.D.; Weber, B.; De Deyn, P.P.; et al. Rapid Reconfiguration of the Functional Connectome after Chemogenetic Locus Coeruleus Activation. Neuron 2019, 103, 702–718.e5. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.; Yizhar, O.; Chikahisa, S.; Nguyen, H.; Adamantidis, A.; Nishino, S.; Deisseroth, K.; De Lecea, L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 2010, 13, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Aston-Jones, G. Locus Coeruleus, A5 and A7 Noradrenergic Cell Groups. In The Rat Nervous System, 3rd ed.; Paxinos, G., Ed.; Academic Press: Cambridge, MA, USA, 2004; pp. 259–294. [Google Scholar] [CrossRef]
- Pickel, V.M.; Segal, M.; Bloom, F.E. A radioautographic study of the efferent pathways of the nucleus locus coeruleus. J. Comp. Neurol. 1974, 155, 15–41. [Google Scholar] [CrossRef]
- Jones, B.E.; Moore, R.Y. Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study. Brain Res. 1977, 127, 25–53. [Google Scholar] [CrossRef]
- Nomura, S.; Bouhadana, M.; Morel, C.; Faure, P.; Cauli, B.; Lambolez, B.; Hepp, R.; Hepp, R. Noradrenalin and dopamine receptors both control cAMP-PKA signaling throughout the cerebral cortex. Front. Cell. Neurosci. 2014, 8, 247. [Google Scholar] [CrossRef]
- Berridge, C.W.; Stratford, T.L.; Foote, S.L.; Kelley, A.E. Distribution of dopamine beta-hydroxylase-like immunoreactive fibers within the shell subregion of the nucleus accumbens. Synapse 1997, 27, 230–241. [Google Scholar] [CrossRef]
- Schroeter, S.; Apparsundaram, S.; Wiley, R.G.; Miner, L.H.; Sesack, S.R.; Blakely, R.D. Immunolocalization of the cocaine- and antidepressant-sensitive l-norepinephrine transporter. J. Comp. Neurol. 2000, 420, 211–232. [Google Scholar] [CrossRef]
- Verguts, T.; Notebaert, W. Hebbian learning of cognitive control: Dealing with specific and nonspecific adaptation. Psychol. Rev. 2008, 115, 518–525. [Google Scholar] [CrossRef]
- Notebaert, W.; Verguts, T. Cognitive control acts locally. Cognition 2008, 106, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Li, Y.; Kalwani, R.M.; Gold, J.I. Relationships between Pupil Diameter and Neuronal Activity in the Locus Coeruleus, Colliculi, and Cingulate Cortex. Neuron 2015, 89, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Gold, J.I. Pupil Size as a Window on Neural Substrates of Cognition. Trends Cogn. Sci. 2020, 24, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rodenkirch, C.; Moskowitz, N.; Schriver, B.; Wang, Q. Dynamic Lateralization of Pupil Dilation Evoked by Locus Coeruleus Activation Results from Sympathetic, Not Parasympathetic, Contributions. Cell Rep. 2017, 20, 3099–3112. [Google Scholar] [CrossRef] [PubMed]
- Reimer, J.; McGinley, M.J.; Liu, Y.; Rodenkirch, C.; Wang, Q.; McCormick, D.A.; Tolias, A.S. Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat. Commun. 2016, 7, 13289. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.R.; O’Connell, R.G.; O’Sullivan, M.; Robertson, I.H.; Balsters, J.H. Pupil diameter covaries with BOLD activity in human locus coeruleus. Hum. Brain Mapp. 2014, 35, 4140–4154. [Google Scholar] [CrossRef] [PubMed]
- De Gee, J.W.; Colizoli, O.; Kloosterman, N.A.; Knapen, T.; Nieuwenhuis, S.; Donner, T.H. Dynamic modulation of decision biases by brainstem arousal systems. eLife 2017, 6, e23232. [Google Scholar] [CrossRef]
- Unsworth, N.; Robison, M.K. A locus coeruleus-norepinephrine account of individual differences in working memory capacity and attention control. Psychon. Bull. Rev. 2017, 24, 1282–1311. [Google Scholar] [CrossRef]
- Unsworth, N.; Robison, M.K. The importance of arousal for variation in working memory capacity and attention control: A latent variable pupillometry study. J. Exp. Psychol. Learn. Mem. Cogn. 2017, 43, 1962–1987. [Google Scholar] [CrossRef]
- Manohar, S.G.; Chong, T.; Apps, M.; Batla, A.; Stamelou, M.; Jarman, P.R.; Bhatia, K.P.; Husain, M. Reward Pays the Cost of Noise Reduction in Motor and Cognitive Control. Curr. Biol. 2015, 25, 1707–1716. [Google Scholar] [CrossRef]
- Heitz, R.P. The speed-accuracy tradeoff: History, physiology, methodology, and behavior. Front. Neurosci. 2014, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Polania, R.; Krajbich, I.; Grueschow, M.; Ruff, C. Neural Oscillations and Synchronization Differentially Support Evidence Accumulation in Perceptual and Value-Based Decision Making. Neuron 2014, 82, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.; Eagle, D.; Mar, A.C.; Bari, A.; Banerjee, G.; Jiang, X.; Dalley, J.; Robbins, T. Similar Effects of the Selective Noradrenaline Reuptake Inhibitor Atomoxetine on Three Distinct Forms of Impulsivity in the Rat. Neuropsychopharmacology 2007, 33, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Bari, A.; Mar, A.C.; Theobald, D.E.; Elands, S.A.; Oganya, K.C.N.A.; Eagle, D.M.; Robbins, T.W. Prefrontal and Monoaminergic Contributions to Stop-Signal Task Performance in Rats. J. Neurosci. 2011, 31, 9254–9263. [Google Scholar] [CrossRef]
- Pattij, T.; Schetters, D.; Schoffelmeer, A.N.M.; Van Gaalen, M.M. On the improvement of inhibitory response control and visuospatial attention by indirect and direct adrenoceptor agonists. Psychopharmacology 2011, 219, 327–340. [Google Scholar] [CrossRef]
- Navarra, R.; Graf, R.; Huang, Y.; Logue, S.; Comery, T.; Hughes, Z.; Day, M. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2008, 32, 34–41. [Google Scholar] [CrossRef]
- Bari, A.; Robbins, T.W. Noradrenergic versus dopaminergic modulation of impulsivity, attention and monitoring behaviour in rats performing the stop-signal task. Psychopharmacology 2013, 230, 89–111. [Google Scholar] [CrossRef]
- Etkin, A.; Egner, T.; Peraza, D.M.; Kandel, E.R.; Hirsch, J. Resolving Emotional Conflict: A Role for the Rostral Anterior Cingulate Cortex in Modulating Activity in the Amygdala. Neuron 2006, 51, 871–882. [Google Scholar] [CrossRef]
- Egner, T.; Etkin, A.; Gale, S.; Hirsch, J. Dissociable Neural Systems Resolve Conflict from Emotional versus Nonemotional Distracters. Cereb. Cortex 2007, 18, 1475–1484. [Google Scholar] [CrossRef]
- Robinson, O.J.; Letkiewicz, A.; Overstreet, C.; Ernst, M.; Grillon, C. The effect of induced anxiety on cognition: Threat of shock enhances aversive processing in healthy individuals. Cogn. Affect. Behav. Neurosci. 2011, 11, 217–227. [Google Scholar] [CrossRef]
- Braem, S.; King, J.; Korb, F.; Krebs, R.M.; Notebaert, W.; Egner, T. The Role of Anterior Cingulate Cortex in the Affective Evaluation of Conflict. J. Cogn. Neurosci. 2017, 29, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Dreisbach, G.; Fischer, R. Conflicts as Aversive Signals for Control Adaptation. Curr. Dir. Psychol. Sci. 2015, 24, 255–260. [Google Scholar] [CrossRef]
- Egner, T. Congruency sequence effects and cognitive control. Cogn. Affect. Behav. Neurosci. 2007, 7, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Monti, J.M.; Weintraub, S.; Egner, T. Differential age-related decline in conflict-driven task-set shielding from emotional versus non-emotional distracters. Neuropsychologia 2010, 48, 1697–1706. [Google Scholar] [CrossRef]
- Keren, N.I.; Lozar, C.T.; Harris, K.; Morgan, P.; Eckert, M.A. In vivo mapping of the human locus coeruleus. NeuroImage 2009, 47, 1261–1267. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K. Unified segmentation. NeuroImage 2005, 26, 839–851. [Google Scholar] [CrossRef]
- Dimigen, O.; Valsecchi, M.; Sommer, W.; Kliegl, R. Human Microsaccade-Related Visual Brain Responses. J. Neurosci. 2009, 29, 12321–12331. [Google Scholar] [CrossRef]
- Tse, P.U.; Baumgartner, F.J.; Greenlee, M.W. Event-related functional MRI of cortical activity evoked by microsaccades, small visually-guided saccades, and eyeblinks in human visual cortex. NeuroImage 2010, 49, 805–816. [Google Scholar] [CrossRef]
- Grueschow, M.; Polania, R.; Hare, T.; Ruff, C.C. Automatic versus Choice-Dependent Value Representations in the Human Brain. Neuron 2015, 85, 874–885. [Google Scholar] [CrossRef]
- Schumann, A.; Köhler, S.; de la Cruz, F.; Güllmar, D.; Reichenbach, J.R.; Wagner, G.; Bär, K.-J. The Use of Physiological Signals in Brainstem/Midbrain fMRI. Front. Neurosci. 2018, 12, 718. [Google Scholar] [CrossRef]
- Bazin, P.-L.; Alkemade, A.; van der Zwaag, W.; Caan, M.W.; Mulder, M.; Forstmann, B.U. Denoising High-Field Multi-Dimensional MRI With Local Complex PCA. Front. Neurosci. 2019, 13, 1066. [Google Scholar] [CrossRef] [PubMed]
- Nichols, T.E.; Holmes, A.P. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum. Brain Mapp. 2002, 15, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Kerns, J.G.; Cohen, J.D.; MacDonald, A.W.; Cho, R.Y.; Stenger, V.A.; Carter, C.S. Anterior Cingulate Conflict Monitoring and Adjustments in Control. Science 2004, 303, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.Y.; Marijatta, F.; Hämmerer, D.; Acosta-Cabronero, J.; Düzel, E.; Howard, R.J. Magnetic resonance imaging of the human locus coeruleus: A systematic review. Neurosci. Biobehav. Rev. 2017, 83, 325–355. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, S.R.; Robbins, T. Noradrenergic modulation of cognition: Therapeutic implications. J. Psychopharmacol. 2013, 27, 694–718. [Google Scholar] [CrossRef]
- Chamberlain, S.R.; Muller, U.; Blackwell, A.D.; Clark, L.; Robbins, T.W.; Sahakian, B.J. Neurochemical Modulation of Response Inhibition and Probabilistic Learning in Humans. Science 2006, 311, 861–863. [Google Scholar] [CrossRef]
- Schwarz, L.A.; Miyamichi, K.; Gao, X.J.; Beier, K.T.; Weissbourd, B.; Deloach, K.E.; Ren, J.; Ibanes, S.; Malenka, R.C.; Kremer, E.J.; et al. Viral-genetic tracing of the input–output organization of a central noradrenaline circuit. Nature 2015, 524, 88–92. [Google Scholar] [CrossRef]
- Arnsten, A.; Goldman-Rakic, P. Selective prefrontal cortical projections to the region of the locus coeruleus and raphe nuclei in the rhesus monkey. Brain Res. 1984, 306, 9–18. [Google Scholar] [CrossRef]
- Porrino, L.J.; Goldman-Rakic, P.S. Brainstem innervation of prefrontal and anterior cingulate cortex in the rhesus monkey revealed by retrograde transport of HRP. J. Comp. Neurol. 1982, 205, 63–76. [Google Scholar] [CrossRef]
- Tervo, D.G.; Proskurin, M.; Manakov, M.; Kabra, M.; Vollmer, A.; Branson, K.; Karpova, A.Y. Behavioral Variability through Stochastic Choice and Its Gating by Anterior Cingulate Cortex. Cell 2014, 159, 21–32. [Google Scholar] [CrossRef]
- Chandler, D.J.; Waterhouse, B.D.; Gao, W.-J. New perspectives on catecholaminergic regulation of executive circuits: Evidence for independent modulation of prefrontal functions by midbrain dopaminergic and noradrenergic neurons. Front. Neural Circuits 2014, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, B.; Chandler, D.; Prouty, E.; Gao, W.J. Electrophysiological Properties of Locus Coeruleus-Prefrontal Cortical Projection Neurons in Normal and Inattentive Rats. Neuropsychopharmacol 2014, 39, S167. [Google Scholar]
- Chandler, D.J.; Gao, W.-J.; Waterhouse, B.D. Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices. Proc. Natl. Acad. Sci. USA 2014, 111, 6816–6821. [Google Scholar] [CrossRef] [PubMed]
- Redmond, D.; Huang, Y., II. New evidence for a locus coeruleus-norepinephrine connection with anxiety. Life Sci. 1979, 25, 2149–2162. [Google Scholar] [CrossRef]
- Weiss, J.M.; Stout, J.; Aaron, M.F.; Quan, N.; Owens, M.J.; Butler, P.D.; Nemeroff, C.B. Depression and anxiety: Role of the locus coeruleus and corticotropin-releasing factor. Brain Res. Bull. 1994, 35, 561–572. [Google Scholar] [CrossRef]
- Itoi, K.; Sugimoto, N. The Brainstem Noradrenergic Systems in Stress, Anxiety and Depression. J. Neuroendocr. 2010, 22, 355–361. [Google Scholar] [CrossRef]
- McCall, J.G.; Siuda, E.R.; Bhatti, D.L.; Lawson, L.A.; McElligott, Z.A.; Stuber, G.D.; Bruchas, M.R. Locus coeruleus to basolateral amygdala noradrenergic projections promote anxiety-like behavior. eLife 2017, 6, e18247. [Google Scholar] [CrossRef]
- Tanaka, M.; Yoshida, M.; Emoto, H.; Ishii, H. Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: Basic studies. Eur. J. Pharmacol. 2000, 405, 397–406. [Google Scholar] [CrossRef]
- Zhu, M.-Y.; Klimek, V.; Dilley, G.E.; Haycock, J.W.; Stockmeier, C.; Overholser, J.C.; Meltzer, H.Y.; Ordway, G.A. Elevated levels of tyrosine hydroxylase in the locus coeruleus in major depression. Biol. Psychiatry 1999, 46, 1275–1286. [Google Scholar] [CrossRef]
- Naegeli, C.; Zeffiro, T.; Piccirelli, M.; Jaillard, A.; Weilenmann, A.; Hassanpour, K.; Schick, M.; Rufer, M.; Orr, S.P.; Mueller-Pfeiffer, C. Locus Coeruleus Activity Mediates Hyperresponsiveness in Posttraumatic Stress Disorder. Biol. Psychiatry 2018, 83, 254–262. [Google Scholar] [CrossRef]
- Southwick, S.M.; Bremner, J.D.; Rasmusson, A.; Morgan, C.A.; Arnsten, A.; Charney, D.S. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol. Psychiatry 1999, 46, 1192–1204. [Google Scholar] [CrossRef]
- Grueschow, M.; Jelezarova, I.; Westphal, M.; Ehlert, U.; Kleim, B. Emotional conflict adaptation predicts intrusive memories. PLoS ONE 2020, 15, e0225573. [Google Scholar] [CrossRef] [PubMed]
- McCall, J.G.; Al-Hasani, R.; Siuda, E.R.; Hong, D.Y.; Norris, A.; Ford, C.P.; Bruchas, M.R. CRH Engagement of the Locus Coeruleus Noradrenergic System Mediates Stress-Induced Anxiety. Neuron 2015, 87, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Bassett, D.S. Dynamic representations in networked neural systems. Nat. Neurosci. 2020, 23, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, F.; Kaufmann, J.; Hoffmann, M.; Tempelmann, C.; Kluge, C.; Rampp, S.; Voges, J.; Heinze, H.; Buentjen, L.; Grueschow, M. Case Report: Practicability of functionally based tractography of the optic radiation during presurgical epilepsy work up. Neurosci. Lett. 2014, 568, 56–61. [Google Scholar] [CrossRef][Green Version]
- Loewe, K.; Grueschow, M.; Stoppel, C.M.; Kruse, R.; Borgelt, C. Fast construction of voxel-level functional connectivity graphs. BMC Neurosci. 2014, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.S.; Zurn, P.; Gold, J.I. On the nature and use of models in network neuroscience. Nat. Rev. Neurosci. 2018, 19, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.G.; Kindermann, S.S.; Siegle, G.J.; Granholm, E.; Wong, E.C.; Buxton, R.B. Brain activation and pupil response during covert performance of the Stroop Color Word task. J. Int. Neuropsychol. Soc. 1999, 5, 308–319. [Google Scholar] [CrossRef]
- Siegle, G.J.; Steinhauer, S.; Thase, M.E. Pupillary assessment and computational modeling of the Stroop task in depression. Int. J. Psychophysiol. 2004, 52, 63–76. [Google Scholar] [CrossRef]
- Siegle, G.J.; Ichikawa, N.; Steinhauer, S. Blink before and after you think: Blinks occur prior to and following cognitive load indexed by pupillary responses. Psychophysiology 2008, 45, 679–687. [Google Scholar] [CrossRef]
- Laeng, B.; Ørbo, M.; Holmlund, T.; Miozzo, M. Pupillary Stroop effects. Cogn. Process. 2010, 12, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Van Der Wel, P.; Van Steenbergen, H. Pupil dilation as an index of effort in cognitive control tasks: A review. Psychon. Bull. Rev. 2018, 25, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Saposnik, G.; Grueschow, M.; Oh, J.; Terzaghi, M.A.; Kostyrko, P.; Vaidyanathan, S.; Nisenbaum, R.; Ruff, C.C.; Tobler, P.N. Effect of an Educational Intervention on Therapeutic Inertia in Neurologists with Expertise in Multiple Sclerosis. JAMA Netw. Open 2020, 3, e2022227. [Google Scholar] [CrossRef] [PubMed]
- Azza, Y.; Grueschow, M.; Karlen, W.; Seifritz, E.; Kleim, B. How stress affects sleep and mental health: Nocturnal heart rate increases during prolonged stress and interacts with childhood trauma exposure to predict anxiety. Sleep 2019, 43, zsz310. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.U.; Makwana, A.B.; Hare, T.A. Acute Stress Impairs Self-Control in Goal-Directed Choice by Altering Multiple Functional Connections within the Brain’s Decision Circuits. Neuron 2015, 87, 621–631. [Google Scholar] [CrossRef]
- Boden, J.M.; McLeod, G.F.H. Resilience and psychiatric epidemiology: Implications for a conceptual framework. Behav. Brain Sci. 2015, 38, e95. [Google Scholar] [CrossRef] [PubMed]
- Pitman, R.K.; Rasmusson, A.M.; Koenen, K.; Shin, L.M.; Orr, S.P.; Gilbertson, M.W.; Milad, M.R.; Liberzon, I. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 2012, 13, 769–787. [Google Scholar] [CrossRef]
- Ruscio, A.M.; Hallion, L.S.; Lim, C.C.W.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.; Alonso, J.; Andrade, L.H.; Borges, G.; Bromet, E.J.; Bunting, B.; et al. Cross-sectional Comparison of the Epidemiology of DSM-5 Generalized Anxiety Disorder Across the Globe. JAMA Psychiatry 2017, 74, 465–475. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grueschow, M.; Kleim, B.; Ruff, C.C. Functional Coupling of the Locus Coeruleus Is Linked to Successful Cognitive Control. Brain Sci. 2022, 12, 305. https://doi.org/10.3390/brainsci12030305
Grueschow M, Kleim B, Ruff CC. Functional Coupling of the Locus Coeruleus Is Linked to Successful Cognitive Control. Brain Sciences. 2022; 12(3):305. https://doi.org/10.3390/brainsci12030305
Chicago/Turabian StyleGrueschow, Marcus, Birgit Kleim, and Christian Carl Ruff. 2022. "Functional Coupling of the Locus Coeruleus Is Linked to Successful Cognitive Control" Brain Sciences 12, no. 3: 305. https://doi.org/10.3390/brainsci12030305
APA StyleGrueschow, M., Kleim, B., & Ruff, C. C. (2022). Functional Coupling of the Locus Coeruleus Is Linked to Successful Cognitive Control. Brain Sciences, 12(3), 305. https://doi.org/10.3390/brainsci12030305




