Evidence of Altered Functional Connectivity at Rest in the Writing Network of Children with Dyslexia
Abstract
:1. Introduction
1.1. Writing Difficulties in Dyslexia
1.2. The Contribution of fMRI Techniques to Investigate Dyslexia
1.3. The Present Study
2. Method
2.1. Participants
2.2. Procedure
2.3. Behavioural Evaluation
2.3.1. Word Reading (Accuracy and Speed)
2.3.2. Word Spelling (Accuracy)
2.3.3. Handwriting (Legibility and Speed)
2.4. MRI Scanning
2.4.1. Imaging Acquisition Parameters
2.4.2. MRI Data (Pre)Processing
2.4.3. Statistical Analyses
3. Results
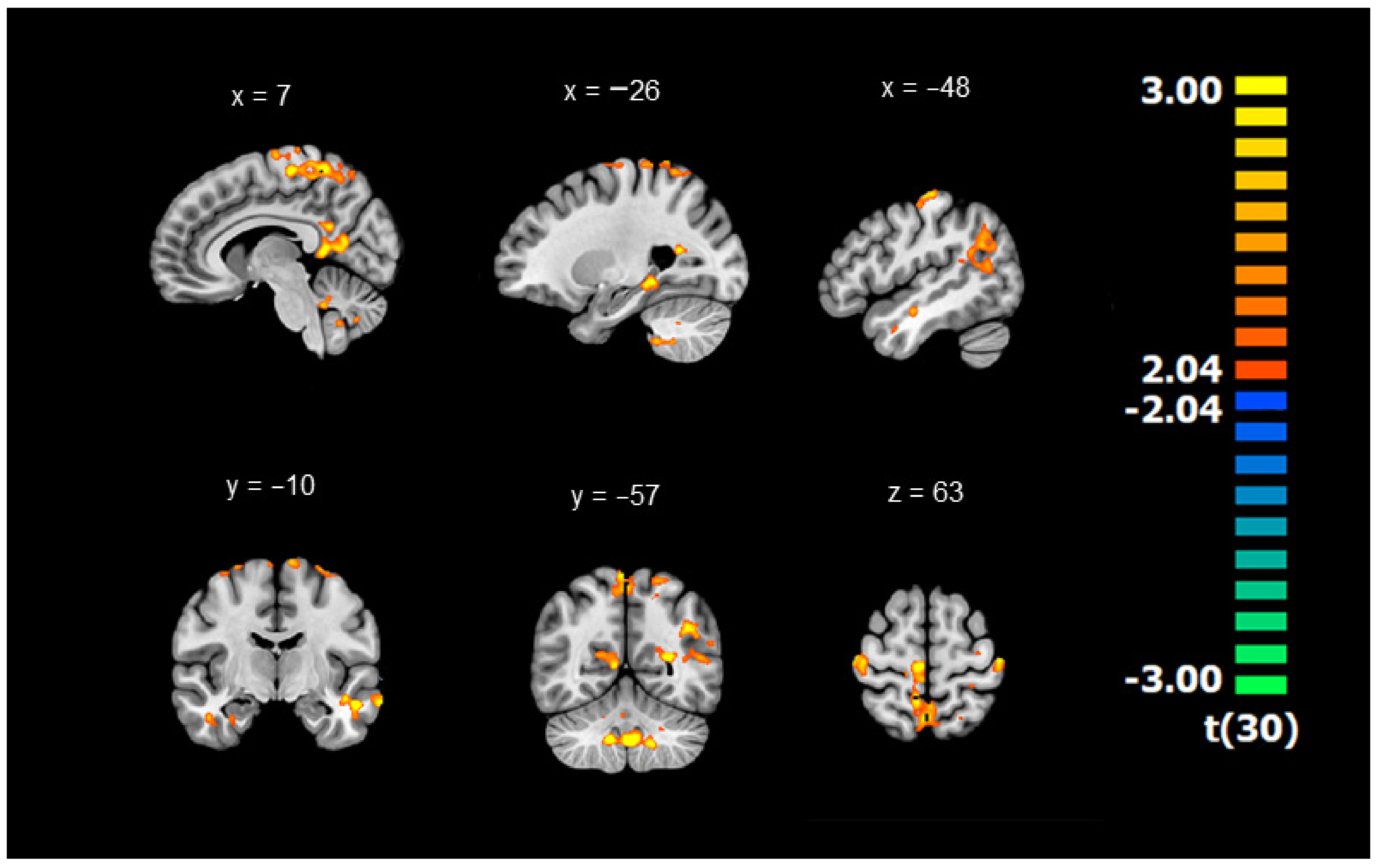
3.1. Differences in Connectivity between DYS and TD Children (Whole-Brain ANOVAs with the GMFA Seed Region)
3.2. RSFC–Behaviour Relationships (Whole-Brain ANCOVAs with the GMFA Seed Region)
4. Discussion
4.1. Connectivity Differences with the GMFA in Regions Involved in Phonological and Lexical Processes (Temporal Areas)
4.2. Connectivity Differences with the GMFA in Motor Regions (Frontoparietal and Cerebellar Areas)
4.3. Connectivity Differences with the GMFA in Regions Involved in Emotion and Behaviour Regulation (Limbic System)
4.4. Synthesis: Are There Functional Markers of Graphomotor Impairment at Rest in DYS Children?
4.5. Study Limitations and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Sprenger-Charolles, L. Developmental Dyslexia in French. In Developmental Dyslexia across Languages and Writing Systems; Perfetti, C., Pugh, K., Verhoeven, L., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 50–72. [Google Scholar]
- Snowling, M.J. Dyslexia; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Shaywitz, S.E.; Shaywitz, B.A. Dyslexia (specific reading disability). Biol. Psychiatry 2005, 57, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Tainturier, M.-J.; Rapp, B. The spelling process. In The Handbook of Cognitive Neuropsychology: What Deficits Reveal about the Human Mind; Rapp, B., Ed.; Psychology Press: Philadelphia, PA, USA, 2001; pp. 263–289. [Google Scholar]
- Lyon, G.R.; Shaywitz, S.E.; Shaywitz, B.A. A definition of dyslexia. Ann. Dyslexia 2003, 53, 1–14. [Google Scholar] [CrossRef]
- Connelly, V.; Campbell, S.; MacLean, M.; Barnes, J. Contribution of lower order skills to the written composition of college students with and without dyslexia. Dev. Neuropsychol. 2006, 29, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Lefly, D.L.; Pennington, B.F. Spelling errors and reading fluency in compensated adult dyslexics. Ann. Dyslexia 1991, 41, 141–162. [Google Scholar] [CrossRef]
- Blampain, E.; Gosse, C.; Van Reybroeck, M. Copying skills in children with and without dyslexia. Read. Writ. 2020, 34, 859–885. [Google Scholar] [CrossRef]
- Sumner, E.; Connelly, V.; Barnett, A.L. The influence of spelling ability on handwriting production: Children with and without dyslexia. J. Exp. Psychol. Learn. Mem. Cogn. 2014, 40, 1441–1447. [Google Scholar] [CrossRef]
- Gosse, C.; Van Reybroeck, M. Do children with dyslexia present a handwriting deficit? Impact of word orthographic and graphic complexity on handwriting and spelling performance. Res. Dev. Disabil. 2020, 97, 103553. [Google Scholar] [CrossRef] [PubMed]
- Sumner, E.; Connelly, V.; Barnett, A.L. Children with dyslexia are slow writers because they pause more often and not because they are slow at handwriting execution. Read. Writ. 2013, 26, 991–1008. [Google Scholar] [CrossRef]
- Feder, K.P.; Majnemer, A. Handwriting development, competency, and intervention. Dev. Med. Child Neurol. 2007, 49, 312–317. [Google Scholar] [CrossRef]
- Medwell, J.; Wray, D. Handwriting: What do we know and what do we need to know? Literacy 2007, 41, 10–15. [Google Scholar] [CrossRef]
- Alamargot, D.; Morin, M.-F.; Simard-Dupuis, E. Handwriting delay in dyslexia: Children at the end of primary school still make numerous short pauses when producing letters. J. Learn. Disabil. 2020, 53, 163–175. [Google Scholar] [CrossRef]
- Berninger, V.W.; Nielsen, K.H.; Abbott, R.D.; Wijsman, E.; Raskind, W. Writing problems in developmental dyslexia: Under-recognized and under-treated. J. Sch. Psychol. 2008, 46, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martlew, M. Handwriting and spelling: Dyslexic children’s abilities compared with children of the same chronological age and younger children of the same spelling level. Br. J. Educ. Psychol. 1992, 62, 375–390. [Google Scholar] [CrossRef]
- Di Brina, C.; Averna, R.; Rampoldi, P.; Rossetti, S.; Penge, R. Reading and writing skills in children with specific learning disabilities with and without developmental coordination disorder. Mot. Control 2018, 22, 391–405. [Google Scholar] [CrossRef]
- Martínez-García, C.; Afonso, O.; Cuetos, F.; Suárez-Coalla, P. Handwriting production in Spanish children with dyslexia: Spelling or motor difficulties? Read. Writ. 2020, 34, 565–593. [Google Scholar] [CrossRef]
- Gosse, C.; Dricot, L.; Van Reybroeck, M. Evidence of graphomotor dysfunction in children with dyslexia. A combined behavioural and fMRI experiment. Cortex 2022, 148, 68–88. [Google Scholar] [CrossRef]
- Seitzman, B.A.; Snyder, A.Z.; Leuthardt, E.C.; Shimony, J.S. The state of resting state networks. Top. Magn. Reson. Imaging TMRI 2019, 28, 189. [Google Scholar] [CrossRef]
- Koyama, M.S.; Kelly, C.; Shehzad, Z.; Penesetti, D.; Castellanos, F.X.; Milham, M.P. Reading networks at rest. Cereb. Cortex 2010, 20, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.M.; Ramdajal, R.; Peters, L.; Vandermeer, M.R.; Hayden, E.P.; Frijters, J.C.; Steinbach, K.A.; Lovett, M.W.; Archibald, L.M.; Joanisse, M.F. Resting-state functional connectivity and reading subskills in children. Neuroimage 2021, 243, 118529. [Google Scholar] [CrossRef]
- Schurz, M.; Wimmer, H.; Richlan, F.; Ludersdorfer, P.; Klackl, J.; Kronbichler, M. Resting-state and task-based functional brain connectivity in developmental dyslexia. Cereb. Cortex 2015, 25, 3502–3514. [Google Scholar] [CrossRef] [Green Version]
- Farris, E.A.; Odegard, T.N.; Miller, H.L.; Ring, J.; Allen, G.; Black, J. Functional connectivity between the left and right inferior frontal lobes in a small sample of children with and without reading difficulties. Neurocase 2011, 17, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Koyama, M.S.; Di Martino, A.; Kelly, C.; Jutagir, D.R.; Sunshine, J.; Schwartz, S.J.; Castellanos, F.X.; Milham, M.P. Cortical signatures of dyslexia and remediation: An intrinsic functional connectivity approach. PLoS ONE 2013, 8, e55454. [Google Scholar] [CrossRef] [Green Version]
- Roux, F.E.; Dufor, O.; Giussani, C.; Wamain, Y.; Draper, L.; Longcamp, M.; Démonet, J.F. The graphemic/motor frontal area: Exner’s area revisited. Ann. Neurol. 2009, 66, 537–545. [Google Scholar] [CrossRef]
- Planton, S.; Jucla, M.; Roux, F.-E.; Démonet, J.-F. The “handwriting brain”: A meta-analysis of neuroimaging studies of motor versus orthographic processes. Cortex 2013, 49, 2772–2787. [Google Scholar] [CrossRef] [PubMed]
- Ramus, F.; Szenkovits, G. What phonological deficit? Q. J. Exp. Psychol. 2008, 61, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Castles, A.; Coltheart, M. Varieties of developmental dyslexia. Cognition 1993, 47, 149–180. [Google Scholar] [CrossRef]
- Cook, L. Misspelling analysis in dyslexia: Observation of developmental strategy shifts. Bull. Orton Soc. 1981, 31, 123–134. [Google Scholar] [CrossRef]
- Andreou, G.; Baseki, J. Phonological and spelling mistakes among dyslexic and non-dyslexic children learning two different languages: Greek vs English. Psychology 2012, 3, 595. [Google Scholar] [CrossRef]
- Caravolas, M.; Volín, J. Phonological spelling errors among dyslexic children learning a transparent orthography: The case of Czech. Dyslexia 2001, 7, 229–245. [Google Scholar] [CrossRef]
- Graham, S.; Berninger, V.; Weintraub, N.; Schafer, W. Development of handwriting speed and legibility in grades 1–9. J. Educ. Res. 1998, 92, 42–52. [Google Scholar] [CrossRef]
- Van Galen, G.P. Handwriting: Issues for a psychomotor theory. Hum. Mov. Sci. 1991, 10, 165–191. [Google Scholar] [CrossRef]
- Christensen, C.A.; Jones, D. Handwriting: An underestimated skill in the development of written language. Handwrit. Today 2000, 2, 56–69. [Google Scholar]
- Palmis, S.; Velay, J.-L.; Fabiani, E.; Nazarian, B.; Anton, J.-L.; Habib, M.; Kandel, S.; Longcamp, M. The impact of spelling regularity on handwriting production: A coupled fMRI and kinematics study. Cortex 2019, 113, 111–127. [Google Scholar] [CrossRef] [Green Version]
- Purcell, J.J.; Turkeltaub, P.E.; Eden, G.F.; Rapp, B. Examining the central and peripheral processes of written word production through meta-analysis. Front. Psychol. 2011, 2, 239. [Google Scholar] [CrossRef] [Green Version]
- Gosse, C.; Parmentier, M.; van Reybroeck, M. How do spelling, handwriting speed, and handwriting quality develop during primary school? Cross-classified growth curve analysis of children’s writing development. Front. Psychol. 2021, 2021, 2927. [Google Scholar] [CrossRef]
- Rapp, B.; Epstein, C.; Tainturier, M.-J. The integration of information across lexical and sublexical processes in spelling. Cogn. Neuropsychol. 2002, 19, 1–29. [Google Scholar] [CrossRef]
- Kandel, S.; Lassus-Sangosse, D.; Grosjacques, G.; Perret, C. The impact of developmental dyslexia and dysgraphia on movement production during word writing. Cogn. Neuropsychol. 2017, 34, 219–251. [Google Scholar] [CrossRef]
- Berninger, V. Language by hand: A synthesis of a decade of research on handwriting. Handwrit. Rev. 1998, 12, 11–25. [Google Scholar]
- Martin, K.; Kronbichler, M.; Richlan, F. Dyslexic brain activation abnormalities in deep and shallow orthographies: A meta-analysis of 28 functional neuroimaging studies. Hum. Brain Mapp. 2016, 37, 2676–2699. [Google Scholar] [CrossRef] [Green Version]
- Richlan, F. The functional neuroanatomy of developmental dyslexia across languages and writing systems. Front. Psychol. 2020, 11, 155. [Google Scholar] [CrossRef]
- Richlan, F.; Kronbichler, M.; Wimmer, H. Functional abnormalities in the dyslexic brain: A quantitative meta-analysis of neuroimaging studies. Hum. Brain Mapp. 2009, 30, 3299–3308. [Google Scholar] [CrossRef] [Green Version]
- Richlan, F.; Kronbichler, M.; Wimmer, H. Meta-analyzing brain dysfunctions in dyslexic children and adults. Neuroimage 2011, 56, 1735–1742. [Google Scholar] [CrossRef]
- Maurer, U.; Schulz, E.; Brem, S.; van der Mark, S.; Bucher, K.; Martin, E.; Brandeis, D. The development of print tuning in children with dyslexia: Evidence from longitudinal ERP data supported by fMRI. Neuroimage 2011, 57, 714–722. [Google Scholar] [CrossRef]
- Biswal, B.B.; Kylen, J.V.; Hyde, J.S. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. Int. J. Devoted Dev. Appl. Magn. Reson. In Vivo 1997, 10, 165–170. [Google Scholar] [CrossRef]
- Guell, X.; Schmahmann, J.D.; Gabrieli, J.D.; Ghosh, S.S. Functional gradients of the cerebellum. eLife 2018, 7, e36652. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Schmahmann, J.D. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. Neuroimage 2009, 44, 489–501. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Valera, E.M.; Schmahmann, J.D. Functional topography of the cerebellum for motor and cognitive tasks: An fMRI study. Neuroimage 2012, 59, 1560–1570. [Google Scholar] [CrossRef] [Green Version]
- Anderson, S.; Damasio, A.R.; Damasio, H. Troubled letters but not numbers: Domain specific cognitive impairments following focal damage in frontal cortex. Brain 1990, 113, 749–766. [Google Scholar] [CrossRef]
- Roux, F.E.; Draper, L.; Köpke, B.; Démonet, J.F. Who actually read Exner? Returning to the source of the frontal “writing centre” hypothesis. Cortex 2010, 46, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Longcamp, M.; Anton, J.-L.; Roth, M.; Velay, J.-L. Visual presentation of single letters activates a premotor area involved in writing. Neuroimage 2003, 19, 1492–1500. [Google Scholar] [CrossRef]
- Rapcsak, S.Z.; Beeson, P.; Henry, M.; Leyden, A.; Kim, E.; Rising, K.; Andersen, S.; Cho, H. Phonological dyslexia and dysgraphia: Cognitive mechanisms and neural substrates. Cortex 2009, 45, 575–591. [Google Scholar] [CrossRef] [Green Version]
- Palmis, S.; Velay, J.; Habib, M.; Anton, J.; Nazarian, B.; Sein, J.; Longcamp, M. The handwriting brain in middle childhood. Dev. Sci. 2021, 24, e13046. [Google Scholar] [CrossRef]
- Pontart, V.; Bidet-Ildei, C.; Lambert, E.; Morisset, P.; Flouret, L.; Alamargot, D. Influence of handwriting skills during spelling in primary and lower secondary grades. Front. Psychol. 2013, 4, 818. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, V.E.; Malone, S.A.; Hulme, C. Early handwriting ability predicts the growth of children’s spelling, but not reading, skills. Sci. Stud. Read. 2020, 25, 304–318. [Google Scholar] [CrossRef]
- Bosga-Stork, I.M.; Bosga, J.; Ellis, J.L.; Meulenbroek, R.G. Developing interactions between language and motor skills in the first three years of formal handwriting education. Br. J. Educ. Soc. Behav. Sci. 2016, 12, 1–13. [Google Scholar] [CrossRef]
- Wechsler, D. Échelle D’intelligence de Wechsler Pour Enfants: WISC-IV; ECPA, Les Éditions du Centre de Psychologie Appliquée: Paris, France, 2005. [Google Scholar]
- Dunn, L.M.; Dunn, L.M.; Theriault-Whalen, C. Échelle de Vocabulaire en Images Peabody: EVIP; Psycan: Toronto, ON, Canada, 1983. [Google Scholar]
- Vander Stappen, C.; Dricot, L.; van Reybroeck, M. RAN training in dyslexia: Behavioral and brain correlates. Neuropsychologia 2020, 146, 107566. [Google Scholar] [CrossRef]
- Jacquier-Roux, M.; Lequette, C.; Pouget, G.; Valdois, S.; Zorman, M. BALE: Batterie Analytique du Langage Écrit; Groupe Cogni-Sciences, Laboratoire de Psychologie et NeurCognition: Grenoble, France, 2010. [Google Scholar]
- Charles, M.; Soppelsa, R.; Albaret, J.-M. BHK: Échelle D’évaluation Rapide de L’écriture Chez L’enfant; Ecpa: Paris, France, 2004. [Google Scholar]
- Coltheart, M.; Rastle, K.; Perry, C.; Langdon, R.; Ziegler, J.C. DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychol. Rev. 2001, 108, 204. [Google Scholar] [CrossRef] [PubMed]
- Dehaene, S.; Cohen, L. The unique role of the visual word form area in reading. Trends Cogn. Sci. 2011, 15, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Finn, E.S.; Shen, X.; Holahan, J.M.; Scheinost, D.; Lacadie, C.; Papademetris, X.; Shaywitz, S.E.; Shaywitz, B.A.; Constable, R. Disruption of functional networks in dyslexia: A whole-brain, data-driven analysis of connectivity. Biol. Psychiatry 2014, 76, 397–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.H.; Yang, Y.; Chen, B.G.; Zhang, Y.W.; Bi, H.Y. Anomalous cerebellar anatomy in Chinese children with dyslexia. Front. Psychol. 2016, 7, 324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulesu, E.; Danelli, L.; Berlingeri, M. Reading the dyslexic brain: Multiple dysfunctional routes revealed by a new meta-analysis of PET and fMRI activation studies. Front. Hum. Neurosci. 2014, 8, 830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaywitz, S.E.; Shaywitz, B.A.; Pugh, K.R.; Fulbright, R.K.; Constable, R.T.; Mencl, W.E.; Shankweiler, D.P.; Liberman, A.M.; Skudlarski, P.; Fletcher, J.M.; et al. Functional disruption in the organization of the brain for reading in dyslexia. Proc. Natl. Acad. Sci. USA 1998, 95, 2636–2641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugh, K.R.; Mencl, W.E.; Shaywitz, B.A.; Shaywitz, S.E.; Fulbright, R.K.; Constable, R.T.; Skudlarski, P.; Marchione, K.E.; Jenner, A.R.; Fletcher, J.M.; et al. The angular gyrus in developmental dyslexia: Task-specific differences in functional connectivity within posterior cortex. Psychol. Sci. 2000, 11, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, R.I.; Fawcett, A.J. Fawcett, Dyslexia, dysgraphia, procedural learning and the cerebellum. Cortex 2011, 47, 117–127. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Stein, J.F. The cerebellum and dyslexia. Cortex 2011, 47, 101–116. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Stein, J.F. Cerebellar function in developmental dyslexia. Cerebellum 2013, 12, 267–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pernet, C.R.; Poline, J.B.; Demonet, J.F.; Rousselet, G. Brain classification reveals the right cerebellum as the best biomarker of dyslexia. BMC Neurosci. 2009, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Mugnaini, D.; Lassi, S.; La Malfa, G.; Albertini, G. Internalizing correlates of dyslexia. World J. Pediatrics 2009, 5, 255–264. [Google Scholar] [CrossRef]
- Carroll, J.M.; Iles, J.E. An assessment of anxiety levels in dyslexic students in higher education. Br. J. Educ. Psychol. 2006, 76, 651–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nachshon, O.; Farah, R.; Horowitz-Kraus, T. Decreased functional connectivity between the left amygdala and frontal regions interferes with reading, emotional, and executive functions in children with reading difficulties. Front. Hum. Neurosci. 2020, 14, 104. [Google Scholar] [CrossRef]
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain 2013, 137, 12–32. [Google Scholar] [CrossRef] [Green Version]
- Vandermosten, M.; Vanderauwera, J.; Theys, C.; De Vos, A.; Vanvooren, S.; Sunaert, S.; Wouters, J.; Ghesquière, P. A DTI tractography study in pre-readers at risk for dyslexia. Dev. Cogn. Neurosci. 2015, 14, 8–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, C.A. The role of orthographic–motor integration in the production of creative and well-structured written text for students in secondary school. Educ. Psychol. 2005, 25, 441–453. [Google Scholar] [CrossRef]
- Bartoň, M.; Fňašková, M.; Rektorová, I.; Mikl, M.; Mareček, R.; Rapcsak, S.Z.; Rektor, I. The role of the striatum in visuomotor integration during handwriting: An fMRI study. J. Neural Transm. 2020, 127, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Volman, M.J.M.; van Schendel, B.M.; Jongmans, M.J. Handwriting Difficulties in primary school children: A search for underlying mechanisms. Am. J. Occup. Ther. 2006, 60, 451–460. [Google Scholar] [CrossRef] [Green Version]
- Pajares, F. Self-efficacy during childhood and adolescence. Self-Effic. Beliefs Adolesc. 2006, 5, 339–367. [Google Scholar]
- Pajares, F.; Cheong, Y.F. Achievement goal orientations in writing: A developmental perspective. Int. J. Educ. Res. 2003, 39, 437–455. [Google Scholar] [CrossRef]

| DYS | TD | Group Comparison | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 16; 5 Girls, 9 Boys) | (n = 16; 9 Girls, 5 Boys) | ||||||||||
| M | SD | Min | Max | M | SD | Min | Max | t(30) | p | ||
| Reading accuracy | |||||||||||
| Non-words raw (/20) | 12.60 | 3.52 | 7.00 | 18.00 | 16.94 | 2.05 | 12.00 | 20.00 | −4.23 | <0.001 | DYS < TD |
| Non-words standardised | −1.38 | 1.44 | −4.41 | 0.21 | −0.15 | 0.71 | −1.99 | 1.25 | −3.04 | 0.005 | DYS < TD |
| Regular words raw (/20) | 17.60 | 2.75 | 9.00 | 20.00 | 19.38 | 1.26 | 16.00 | 20.00 | −2.34 | 0.027 | DYS < TD |
| Regular words standardised | −1.36 | 1.39 | −3.56 | 0.45 | 0.01 | 0.89 | −1.88 | 0.77 | −3.27 | <0.001 | DYS < TD |
| Irregular words raw (/20) | 14.93 | 4.04 | 3.00 | 19.00 | 18.00 | 2.61 | 12.00 | 20.00 | −2.53 | 0.017 | DYS < TD |
| Irregular words standardised | −1.24 | 1.17 | −3.41 | 0.49 | 0.27 | 0.75 | −1.03 | 1.14 | −4.28 | <0.001 | DYS < TD |
| Spelling accuracy | |||||||||||
| Simple regular words raw (/10) | 6.81 | 2.66 | 0.00 | 10.00 | 8.94 | 1.18 | 7.00 | 10.00 | −2.92 | 0.010 | DYS < TD |
| Standardised | −2.04 | 2.03 | −5.48 | 0.87 | −0.11 | 0.87 | −1.78 | 0.87 | −3.49 | <0.001 | DYS < TD |
| Complex regular words raw (/10) | 5.19 | 3.10 | 0.00 | 10.00 | 7.81 | 2.26 | 4.00 | 10.00 | −2.74 | 0.010 | DYS < TD |
| Standardised | −2.52 | 2.32 | −8.46 | 0.95 | −0.46 | 1.13 | −1.93 | 0.95 | −3.18 | <0.001 | DYS < TD |
| Irregular words raw (/10) | 3.44 | 2.03 | 0.00 | 8.00 | 6.94 | 3.21 | 2.00 | 10.00 | −3.68 | 0.002 | DYS < TD |
| Standardised | −2.08 | 1.05 | −4.44 | −0.44 | −0.08 | 1.11 | −1.79 | 1.24 | −5.22 | 0.003 | DYS < TD |
| Handwriting legibility | |||||||||||
| Raw (number of errors)a | 15.31 | 7.81 | 39.00 | 8.00 | 9.55 | 4.26 | 2.00 | 17.00 | 2.59 | 0.015 | DYS < TD |
| Standardised | −0.68 | 1.57 | −4.44 | 0.89 | 0.82 | 1.31 | −1.29 | 3.57 | −2.93 | 0.006 | DYS < TD |
| Handwriting speed | |||||||||||
| Raw (number of words copied) | 186.75 | 63.91 | 82.00 | 273.00 | 223.38 | 83.05 | 102.00 | 393.00 | −1.40 | 0.172 | DYS = TD |
| Standardised | −0.30 | 0.73 | −1.48 | 0.62 | 0.31 | 1.28 | −1.85 | 3.18 | −1.65 | 0.110 | DYS = TD |
| Correlated Brain Area (DYS < TD) | MNI Peak | |||||||
|---|---|---|---|---|---|---|---|---|
| Size | x | y | z | t(30) | ||||
| 13,639 | Cluster 1 | Bilateral Frontoparietal | −11 | −36 | 68 | 2.43 | ||
| L | Paracentral Lobule | BA 6 | −4 | −25 | 69 | 3.04 | ||
| L | Postcentral Gyrus | BA 2 | −49 | −22 | 63 | 2.88 | ||
| L | Postcentral Gyrus | BA 5 | −18 | −35 | 76 | 3.04 | ||
| L | Postcentral Gyrus | BA 5 | −33 | −35 | 71 | 3.09 | ||
| L | Precentral Gyrus | BA 4 | −36 | −22 | 69 | 2.81 | ||
| L | Precentral Gyrus | BA 4 | −37 | −16 | 67 | 2.80 | ||
| L | Precuneus | BA 7 | 2 | −57 | 67 | 2.99 | ||
| L | Superior Frontal Gyrus | BA 6 | −21 | −3 | 76 | 2.82 | ||
| R | Medial Frontal Gyrus | BA 6 | 5 | −13 | 74 | 2.80 | ||
| R | Paracentral Lobule | BA 5 | 8 | −39 | 59 | 2.83 | ||
| R | Paracentral Lobule | BA 6 | 6 | −24 | 64 | 3.05 | ||
| R | Postcentral Gyrus | BA 7 | 25 | −49 | 74 | 3.11 | ||
| R | Precuneus | BA 7 | 8 | −47 | 65 | 3.07 | ||
| 2943 | Cluster 2 | Right Frontoparietal | 40 | −21 | 65 | 2.38 | ||
| R | Postcentral Gyrus | BA 3 | 43 | −25 | 65 | 2.85 | ||
| R | Postcentral Gyrus | BA 3 | 34 | −26 | 72 | 2.88 | ||
| R | Precentral Gyrus | BA 4 | 32 | −16 | 72 | 2.84 | ||
| 4263 | Cluster 3 | Bilateral Limbic | 6 | −52 | 13 | 2.49 | ||
| L | Posterior Cingulate | BA 29 | 0 | −46 | 7 | 3.09 | ||
| R | Posterior Cingulate | BA 30 | 19 | −62 | 14 | 2.95 | ||
| R | Posterior Cingulate | BA 30 | 7 | −60 | 12 | 2.90 | ||
| R | Posterior Cingulate | BA 30 | 6 | −51 | 24 | 2.85 | ||
| 1693 | Cluster 4 | Right Limbic | BA 34 | 37 | −3 | −28 | 2.32 | |
| R | Parahippocampal Gyrus | BA 34 | 33 | 1 | −28 | 2.85 | ||
| R | Parahippocampal Gyrus | Amygdala | 33 | −4 | −22 | 2.91 | ||
| 5940 | Cluster 5 | Left Temporolimbic | −39 | −56 | 23 | 2.39 | ||
| L | Posterior Cingulate | BA 31 | −15 | −50 | 23 | 3.05 | ||
| L | Superior Temporal Gyrus | BA 39 | −43 | −56 | 33 | 2.91 | ||
| 1766 | Cluster 6 | Left Temporolimbic | −31 | −35 | −15 | 2.44 | ||
| L | Parahippocampal Gyrus | Hippocampus | −28 | −37 | −8 | 3.12 | ||
| L | Parahippocampal Gyrus | BA 35 | −32 | −31 | −24 | 2.86 | ||
| L | Fusiform Gyrus | BA 20 | −35 | −38 | −17 | 2.83 | ||
| 3621 | Cluster 7 | Left Temporal | −58 | −9 | −18 | 2.48 | ||
| L | Superior Temporal Gyrus | BA 38 | −40 | 20 | −36 | 2.92 | ||
| L | Fusiform Gyrus | BA 20 | −40 | −5 | −27 | 3.34 | ||
| L | Sub-Gyral | BA 21 | −46 | −11 | −16 | 2.91 | ||
| L | Middle Temporal Gyrus | BA 21 | −55 | −4 | −18 | 3.10 | ||
| L | Middle Temporal Gyrus | BA 21 | −65 | −3 | −18 | 2.82 | ||
| L | Middle Temporal Gyrus | BA 21 | −68 | −13 | −16 | 3.22 | ||
| 7273 | Cluster 8 | Bilateral Cerebellum | 4 | −52 | −41 | 2.52 | ||
| L | Posterior Lobe | Lob X | −22 | −41 | −46 | 2.43 | ||
| L | Posterior Lobe | Lob IX | −6 | −55 | −42 | 2.73 | ||
| R | Posterior Lobe | Lob IX | 10 | −54 | −42 | 2.52 | ||
| R | Posterior Lobe | Dentate | 17 | −52 | −34 | 2.29 | ||
| R | Anterior lobe | Lob V | 17 | −51 | −27 | 2.20 | ||
| R | Posterior Lobe | Lob X | 24 | −39 | −44 | 2.51 | ||
| R | Posterior Lobe | Lob VIIIa | 32 | −45 | −47 | 2.50 | ||
| R | Anterior Lobe | Crus I | 44 | −42 | −37 | 2.46 | ||
| All Children (n = 32) | DYS (n = 16) | TD (n = 16) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Measures | SP | HW | READ | SP | HW | READ | SP | HW | READ |
| SP | - | - | - | ||||||
| HW | 0.514 ** | - | 0.297 | - | 0.557 * | - | |||
| READ | 0.592 ** | 0.711 ** | - | 0.411 | 0.564 * | - | 0.528 * | 0.659 ** | - |
| Cluster 1 | 0.168 | 0.302 | 0.346 | −0.480 | 0.062 | −0.125 | 0.105 | 0.051 | 0.210 |
| Cluster 2 | 0.203 | 0.274 | 0.357 * | −0.388 | −0.072 | 0.067 | 0.159 | 0.143 | 0.190 |
| Cluster 3 | 0.353 * | 0.378 * | 0.422 * | 0.104 | 0.269 | 0.217 | 0.157 | 0.029 | 0.146 |
| Cluster 4 | 0.359 * | 0.421 * | 0.390 * | 0.241 | 0.387 | 0.206 | 0.083 | 0.008 | 0.045 |
| Cluster 5 | 0.345 | 0.406 * | 0.428 * | 0.006 | 0.322 | 0.305 | 0.229 | 0.138 | 0.148 |
| Cluster 6 | 0.256 | 0.296 | 0.387 * | −0.099 | −0.036 | 0.081 | 0.106 | 0.102 | 0.158 |
| Cluster 7 | 0.360 * | 0.353 * | 0.388 * | −0.146 | −0.067 | 0.006 | 0.313 | 0.199 | 0.081 |
| Cluster 8 | 0.496 ** | 0.467 ** | 0.517 ** | 0.456 | 0.348 | 0.403 | 0.209 | 0.119 | 0.152 |
| Correlated Brain Area (DYS < TD) | Cov SP | Cov HW | Cov SP and HW | Cov READ | |
|---|---|---|---|---|---|
| t(29) | t(29) | t(28) | t(29) | ||
| Cluster 1 | Bilateral frontoparietal | 2.00 | 2.33 * | 2.04 | 1.86 |
| Cluster 2 | Right frontoparietal | 1.92 | 2.13 * | 1.87 | 1.62 |
| Cluster 3 | Bilateral limbic | 1.86 | 1.95 | 1.67 | 1.67 |
| Cluster 4 | Right limbic | 1.57 | 1.79 | 1.42 | 1.62 |
| Cluster 5 | Left temporolimbic | 1.65 | 1.84 | 1.49 | 1.51 |
| Cluster 6 | Left temporolimbic | 2.02 | 2.11 * | 1.92 | 1.73 |
| Cluster 7 | Left temporal | 2.03 | 2.03 | 1.85 | 1.92 |
| Cluster 8 | Bilateral cerebellum | 1.75 | 1.76 | 1.47 | 1.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gosse, C.; Dricot, L.; Van Reybroeck, M. Evidence of Altered Functional Connectivity at Rest in the Writing Network of Children with Dyslexia. Brain Sci. 2022, 12, 243. https://doi.org/10.3390/brainsci12020243
Gosse C, Dricot L, Van Reybroeck M. Evidence of Altered Functional Connectivity at Rest in the Writing Network of Children with Dyslexia. Brain Sciences. 2022; 12(2):243. https://doi.org/10.3390/brainsci12020243
Chicago/Turabian StyleGosse, Claire, Laurence Dricot, and Marie Van Reybroeck. 2022. "Evidence of Altered Functional Connectivity at Rest in the Writing Network of Children with Dyslexia" Brain Sciences 12, no. 2: 243. https://doi.org/10.3390/brainsci12020243
APA StyleGosse, C., Dricot, L., & Van Reybroeck, M. (2022). Evidence of Altered Functional Connectivity at Rest in the Writing Network of Children with Dyslexia. Brain Sciences, 12(2), 243. https://doi.org/10.3390/brainsci12020243







