COVID-19 Still Surprising Us—A Rare Movement Disorder Induced by Infection
Abstract
:1. Background
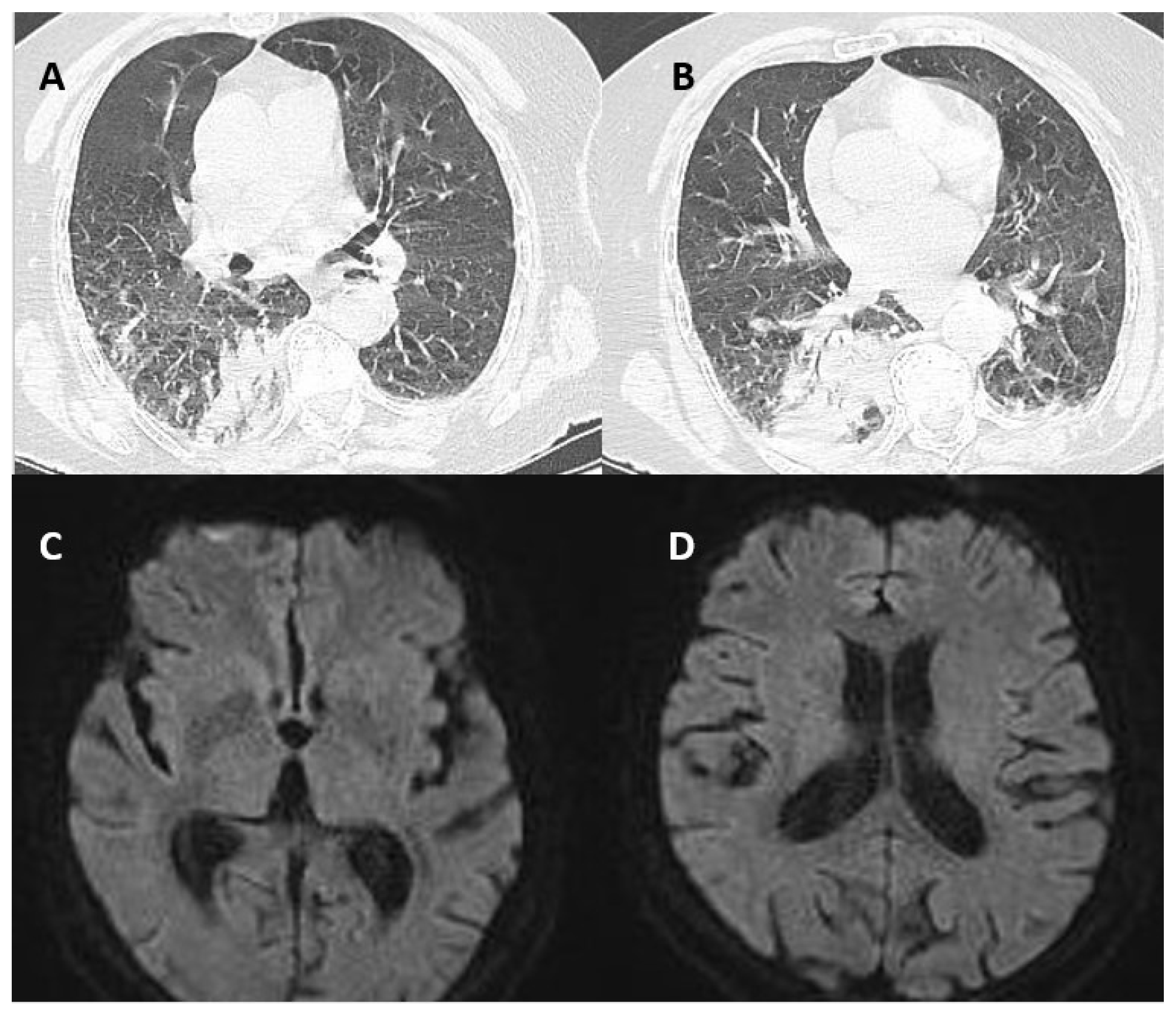
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Paterson, R.W.; Brown, R.L.; Benjamin, L.; Nortley, R.; Wiethoff, S.; Bharucha, T.; Jayaseelan, D.L.; Kumar, G.; Raftopoulos, R.E.; Zambreanu, L.; et al. The emerging spectrum of COVID-19 neurology: Clinical, radiological and laboratory findings. Brain 2020, 143, 3104–3120. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.A.; Sirbu, C.A.; Ghinescu, M.C.; Plesa, C.F.; Sirbu, A.M.; Mitrica, M.; Ionita-Radu, F. SARS-CoV-2, multiple sclerosis, and focal deficit in a postpartum woman: A case report. Exp. Ther. Med. 2021, 21, 92. [Google Scholar] [CrossRef] [PubMed]
- Acar, T.; Demirel, E.A.; Afsar, N.; Akcali, A.; Demir, G.A.; Alagoz, A.N.; Mengi, T.A.; Arsava, E.M.; Ayta, S.; Bebek, N.; et al. The COVID-19 from Neurological Overview. TJN 2020, 26, 58–108. [Google Scholar] [CrossRef]
- Al-Ramadan, A.; Rabab’h, O.; Shah, J.; Gharaibeh, A. Acute and Post-Acute Neurological Complications of COVID-19. Neurol. Int. 2021, 13, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Heidarpour, M.; Vakhshoori, M.; Haghighatpanah, M.A.; Ashrafi, L.; Khorvash, F.; Iraj, B. Rhabdomyolysis plus Hypocalcemia and Diabetic Ketoacidosis as Concurrent Rare COVID-19 Manifestations. J. Med. Case Rep. 2021, 2021, 1–6. [Google Scholar] [CrossRef]
- Tawakul, A.A.; Al-Doboke, A.W.; Altayyar, S.A.; Alsulami, S.A.; Alfahmi, A.M.; Nooh, R.T. Guillain-Barré Syndrome in the COVID-19 Pandemic. Neurol. Int. 2021, 14, 34–48. [Google Scholar] [CrossRef]
- Martinez-Hernandez, E.; Ariño, H.; McKeon, A.; Iizuka, T.; Titulaer, M.J.; Simabukuro, M.M.; Lancaster, E.; Petit-Pedrol, M.; Planagumà, J.; Blanco, Y.; et al. Clinical and Immunologic Investigations in Patients With Stiff-Person Spectrum Disorder. JAMA Neurol. 2016, 73, 714–720. [Google Scholar] [CrossRef] [Green Version]
- Manes, P.K.; Mohammad Aslam, S.; Varghese, M.; Elipulikattu, L. Stiff-Person Syndrome: A Case Report. J. Med. Case Rep. 2016, 7, 426–428. [Google Scholar] [CrossRef]
- Alexopoulos, H.; Dalakas, M.C. A critical update on the immunopathogenesis of Stiff Person Syndrome. Eur. J. Clin. Invest. 2010, 40, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Rakocevic, G.; Chen, Z.; Hoehn, G.; Semino-Mora, C.; Shi, W.; Olsen, R.; Dalakas, M.C. Autoimmunity to GABAA-receptor-associated protein in stiff-person syndrome. Brain 2006, 129 Pt 12, 3270–3276. [Google Scholar] [CrossRef]
- Balint, B.; Meinck, H.M. Pragmatic Treatment of Stiff Person Spectrum Disorders. Mov. Disord. Clin. 2018, 5, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Kasperek, S.; Zebrowski, S. Stiff-Man Syndrome and Encephalomyelitis: Report of a Case. Arch. Neurol. 1971, 24, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.F.; Ghani, M.R.; Cox, Á.M.; Tambo, W.; Bashir, F.; Wirth, M.; Moya, G. Stiff-Person Syndrome: A Treatment Update and New Directions. Cureus 2020, 12, e11995. [Google Scholar] [CrossRef]
- Levy, L.M.; Levy-Reis, I.; Fujii, M.; Dalakas, M.C. Brain γ-Aminobutyric Acid Changes in Stiff-Person Syndrome. Arch. Neurol. 2005, 62, 970–974. [Google Scholar] [CrossRef]
- Hassin-Baer, S.; Kirson, E.D.; Shulman, L.; Buchman, A.S.; Bin, H.; Hindiyeh, M.; Markevich, L.; Mendelson, E. Stiff-Person Syndrome Following West Nile Fever. Arch. Neurol. 2004, 61, 938. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.; Meinck, H.M.; Schulte-Mattler, W.; Ricker, K.; Mertens, H.G. Borrelia burgdorferi myelitis presenting as a partial stiff man syndrome. J. Neurol. 1990, 237, 51–54. [Google Scholar] [CrossRef]
- Requena, I.; Arias, M.; Pardo, J.; Portela, M.; Alvarez, J.A. Syndromes of continuous muscular activity: Report of a central case (stiff-man) and a peripheral case (neuromyotonia) associated with neuroborreliosis. Rev. Neurol. 1995, 23, 129–133. [Google Scholar]
- Bolay, H.; Söylemezoǧlu, F.; Nurlu, G.; Tuncer, S.; Vari, K. PCR detected hepatitis C virus genome in the brain of a case with progressive encephalomyelitis with rigidity. Clin. Neurol. Neurosurg. 1996, 98, 305–308. [Google Scholar] [CrossRef]
- Magira, E.E.; Alexopoulos, H.; Charitatos, E.; Michas, D.; Dalakas, M.C. Progressive encephalomyelitis with rigidity and myoclonus (PERM): Brucellosis as a possible triggering factor and long-term follow-up therapy with rituximab. Ther. Adv. Neurol. Disord. 2016, 9, 69–73. [Google Scholar] [CrossRef] [Green Version]
- Chmiela, T.; Rzepka, M.; Krzystanek, E.; Gorzkowska, A. A 50-Year-Old Patient with Guillain–Barré Syndrome after COVID-19: A Case Report. Medicina 2021, 57, 775. [Google Scholar] [CrossRef]
- Chroni, E.; Papadimitriou, A.; Avramidis, T.; Terentiou, A.E.; Tzioras, C.; Divari, R. Stiff-person like syndrome in a patient with multiple pituitary hormone deficiencies. Acta Neurol. Scand. 2000, 102, 403–405. [Google Scholar] [CrossRef]
- Goh, K.G.; Yusof Khan, A.H.K.; Nasruddin, A. Stiff Person-Like Syndrome: An Unusual Presentation of Pituitary Macroadenoma with Panhypopituitarism. Case Rep. Neurol 2022, 14, 157–161. [Google Scholar] [CrossRef]
- Anton, E. Hipopituitarism due to primary empty sella and uncommon muscular symptoms. Rheumatology 2012, 32, 565–566. [Google Scholar] [CrossRef]
- Borku Uysal, B.; Ikitimur, H.; Yavuzer, S.; Islamoglu, M.S.; Cengiz, M. Case Report: A COVID-19 Patient Presenting with Mild Rhabdomyolysis. Am. J. Trop. Med. 2020, 103, 847–850. [Google Scholar] [CrossRef]
- Paliwal, V.K.; Garg, R.K.; Gupta, A.; Tejan, N. Neuromuscular presentations in patients with COVID-19. Neurol. Sci. 2020, 41, 3039–3056. [Google Scholar] [CrossRef]
- Meegada, S.; Muppidi, V.; Wilkinson, D.C.; Siddamreddy, S.; Katta, S.K. Coronavirus Disease 2019-Induced Rhabdomyolysis. Cureus 2020, 12, e10123. [Google Scholar] [CrossRef]
- Piscitelli, D.; Perin, C.; Tremolizzo, L.; Peroni, F.; Cerri, C.G.; Cornaggia, C.M. Functional movement disorders in a patient with COVID-19. Neurol. Sci. 2020, 41, 2343–2344. [Google Scholar] [CrossRef]
- Ercoli, T.; Lutzoni, L.; Orofino, G.; Muroni, A.; Defazio, G. Functional neurological disorder after COVID-19 vaccination. Neurol. Sci. 2021, 42, 3989–3990. [Google Scholar] [CrossRef]
- Stone, J.; Carson, A.; Duncan, R.; Roberts, R.; Warlow, C.; Hibberd, C.; Coleman, R.; Cull, R.; Murray, G.; Pelosi, A.; et al. Who is referred to neurology clinics?--the diagnoses made in 3781 new patients. Clin. Neurol. Neurosurg. 2010, 112, 747–751. [Google Scholar] [CrossRef]
- Lidstone, S.C.; Costa-Parke, M.; Robinson, E.J.; Ercoli, T.; Stone, J. FMD GAP Study Group. Functional movement disorder gender, age and phenotype study: A systematic review and individual patient meta-analysis of 4905 cases. J. Neurol. Neurosurg. Psychiatry 2022, 93, 609–616. [Google Scholar] [CrossRef]
| Day of Hospitalization | CK Value | Grade of Rigidity |
|---|---|---|
| Day 1 | 253 U/L | + |
| Day 3 | 597 U/L | ++ |
| Day 4 | 12,030 U/L | ++ |
| Day 5 | 9797 U/L | + |
| Day 8 | 457 U/L | No rigidity |
| Associated Pathogen | Clinical Manifestation | Paraclinical Findings | Evolution |
|---|---|---|---|
| West Nile Virus | Increased muscle tone in left arm and legs (stiffness particularly in the arms and shoulder girdle area) Stiff appearance when walking Bradykinesia Hyperreflexia Plantar response extension bilaterally | Positive IgM and IgG antibodies to WNV in serum and CSF Positive serum anti-GAD antibodies Elevated Creatin-Kinase values CSF biochemistry, cellular count, and cultures Oligoclonal IgG antibodies in CSF Cervical and Brain MRI were normal Nerve conduction studies, repetitive nerve stimulation, and electromyography were normal | Complete resolution of a clinical picture after 3 months |
| Borrelia Burgdorferi | Pain and stiffness in the left leg; Spasmodic jerks and painful cramps in the left leg provoked by touch or loud noises; Difficulty walking with frequent falls; Reflex myoclonus in lower extremities which could be induced by touch, loud noises, touching or tapping the leg tendons or the bed | High CK values CSF analysis showed elevated proteins and elevated cell count Borrelia Burgdorferi-specific antibodies were found in serum and CSF Electromyography showed continuous motor activity in the muscles of the left leg Normal brain and spine MRI | Resolution of symptoms after 3 months |
| Hepatitis C Virus | Abnormal posture Motor and sensory deficits in upper and lower limbs Sphincter incontinence Diffuse painful muscle spasms in the extremities which could be induced emotional factors, noise and touch Hyporeflexia | HCV-RNA positive in serum Mild pleocytosis and elevated proteins at CSF analysis; Nerve conduction studies were normal Electromyography showed continuous muscle unit activity of agonist and antagonist muscles in the extremities; Normal brain and spine MRI | Unfavorable clinical outcome |
| Brucella spp. | Restricted vertical gaze movements; bilateral horizontal gaze-evoked nystagmus; diffuse spontaneous myoclonic spasms | Positive PCR test for Brucella in CSF Antiglycine receptor antibodies in serum and CSF; Normal brain and spine MRI; Continuous muscular activity on electromyography | Clinical improvement after 12 months |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirbu, C.A.; Popescu, D.; Stefan, I.; Stefani, C.; Mitrica, M.; Anghel, D. COVID-19 Still Surprising Us—A Rare Movement Disorder Induced by Infection. Brain Sci. 2022, 12, 1733. https://doi.org/10.3390/brainsci12121733
Sirbu CA, Popescu D, Stefan I, Stefani C, Mitrica M, Anghel D. COVID-19 Still Surprising Us—A Rare Movement Disorder Induced by Infection. Brain Sciences. 2022; 12(12):1733. https://doi.org/10.3390/brainsci12121733
Chicago/Turabian StyleSirbu, Carmen Adella, Diana Popescu, Ion Stefan, Constantin Stefani, Marian Mitrica, and Daniela Anghel. 2022. "COVID-19 Still Surprising Us—A Rare Movement Disorder Induced by Infection" Brain Sciences 12, no. 12: 1733. https://doi.org/10.3390/brainsci12121733







