Brain AVMs-Related microRNAs: Machine Learning Algorithm for Expression Profiles of Target Genes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Inclusion Criteria
2.2. Bioinformatic Analyses
3. Results
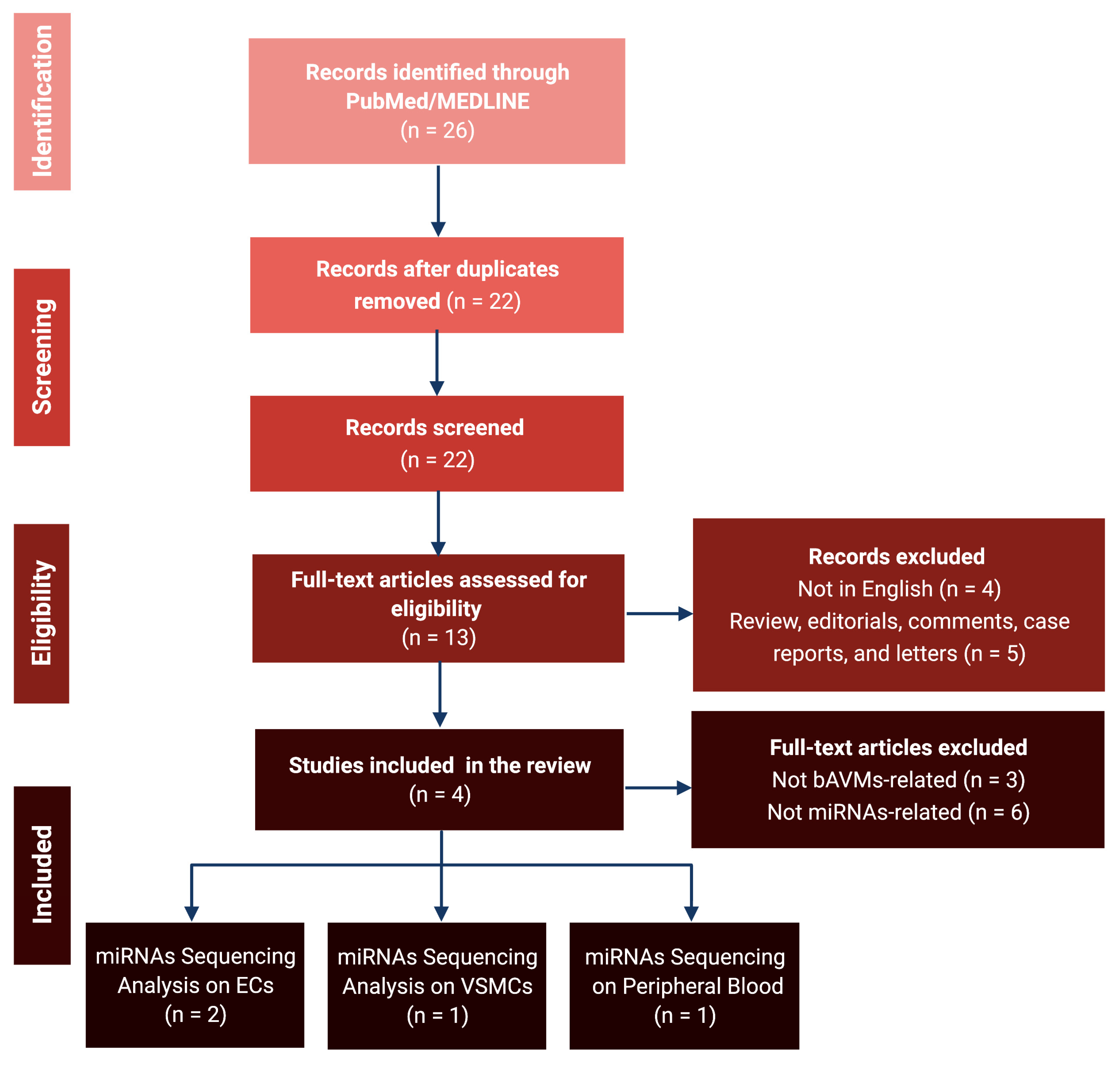
3.1. Literature Review and Data Extraction
3.2. MicroRNA Sequencing Analyses
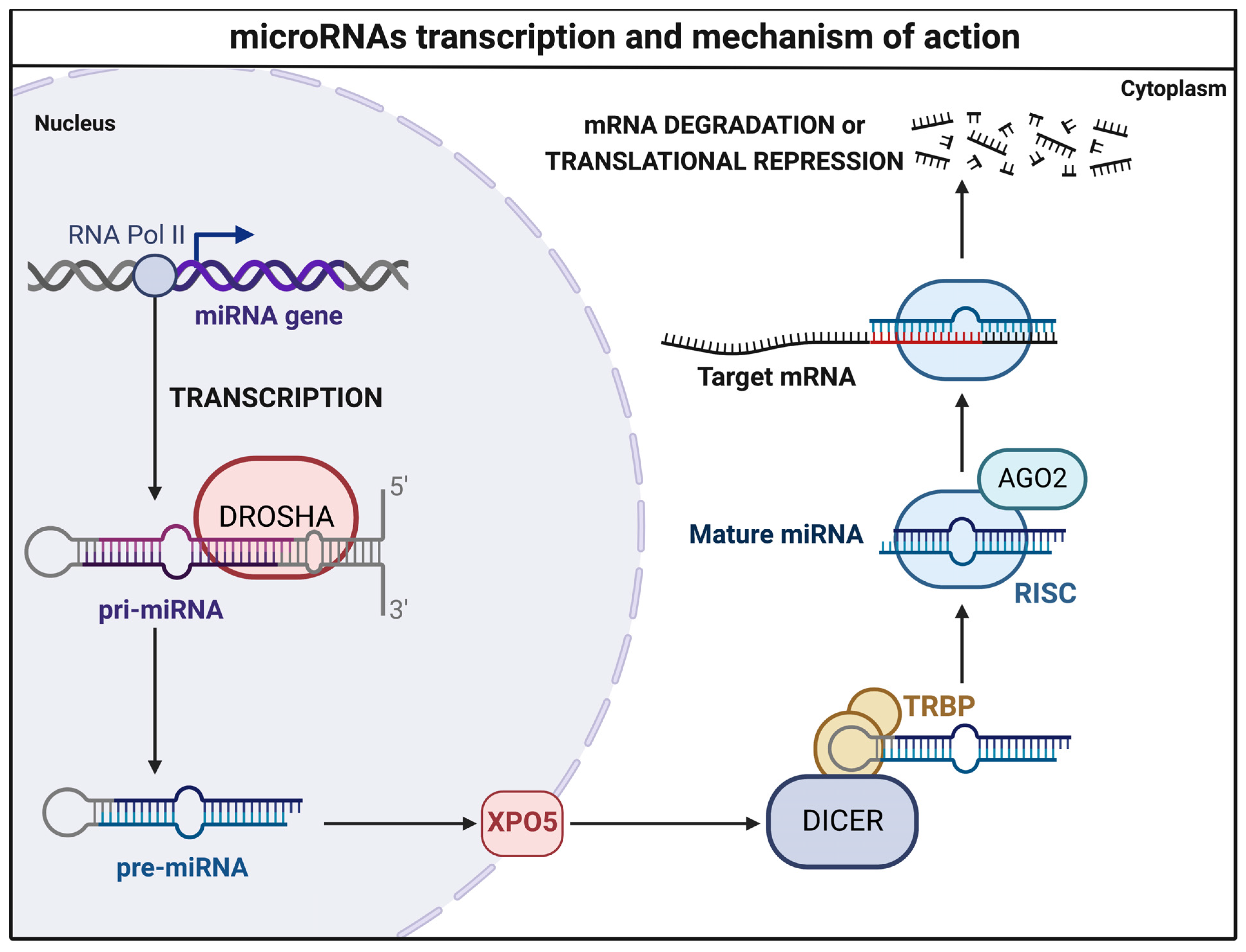
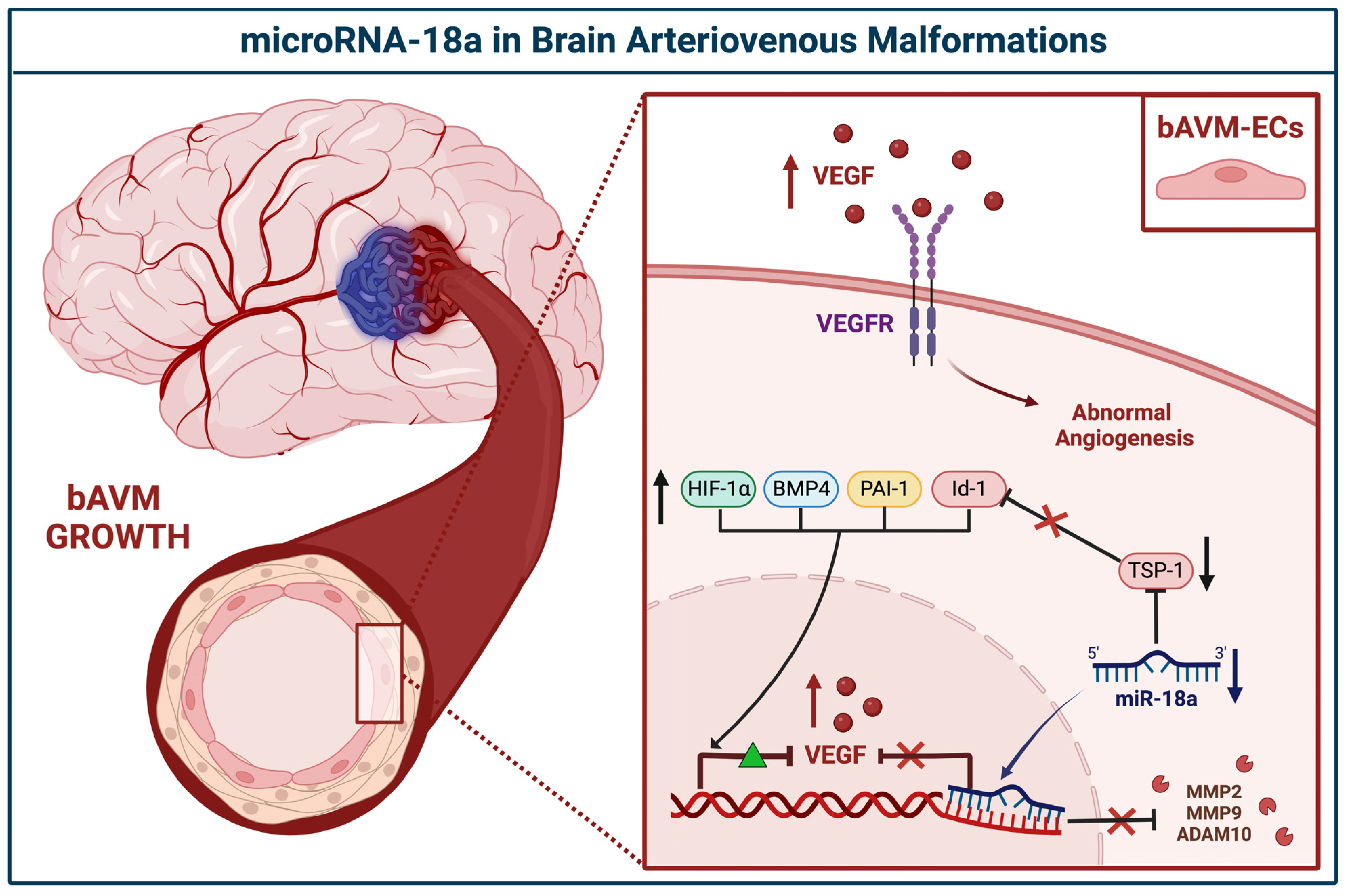
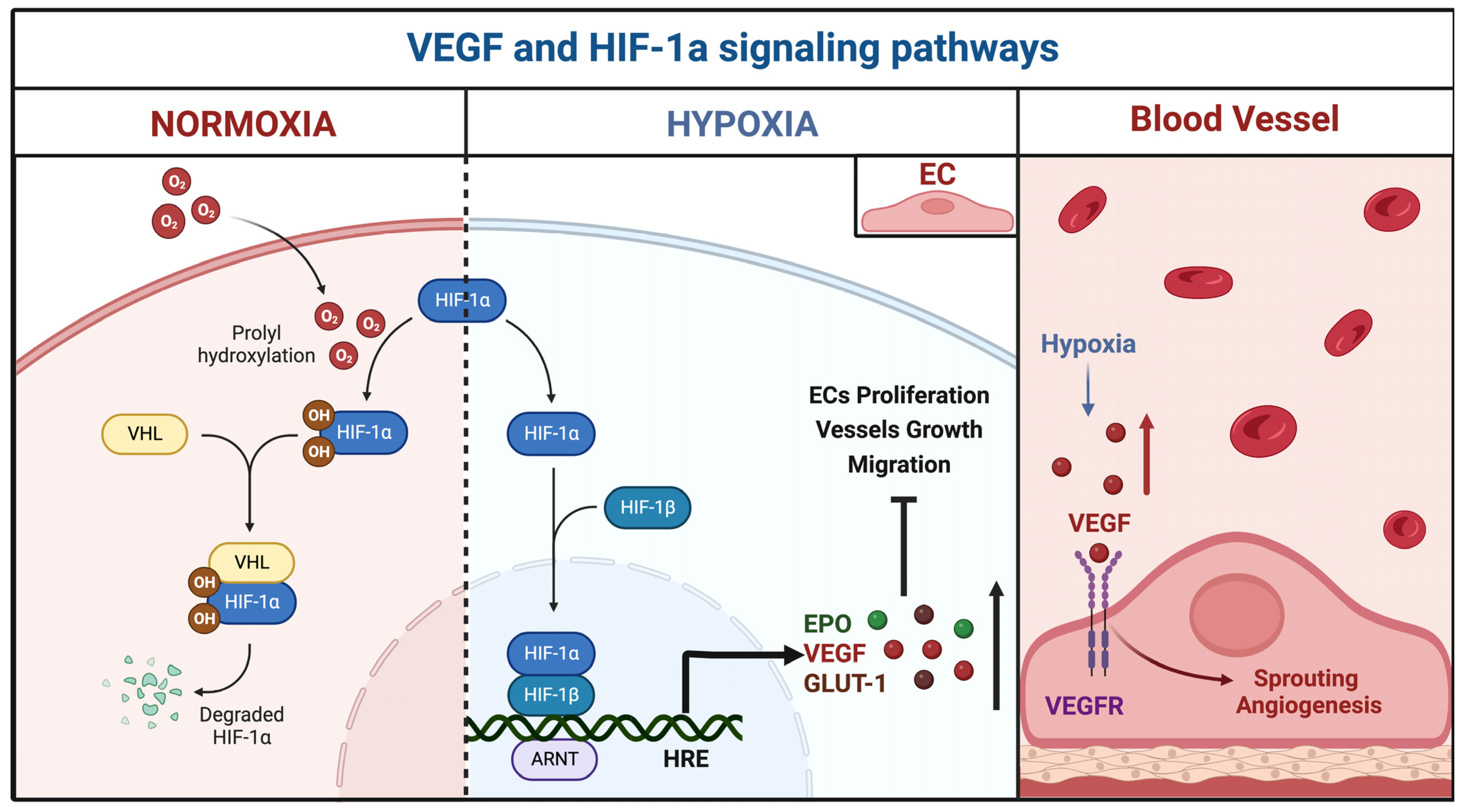
3.2.1. miR-18a
3.2.2. miR-137 and miR-195*
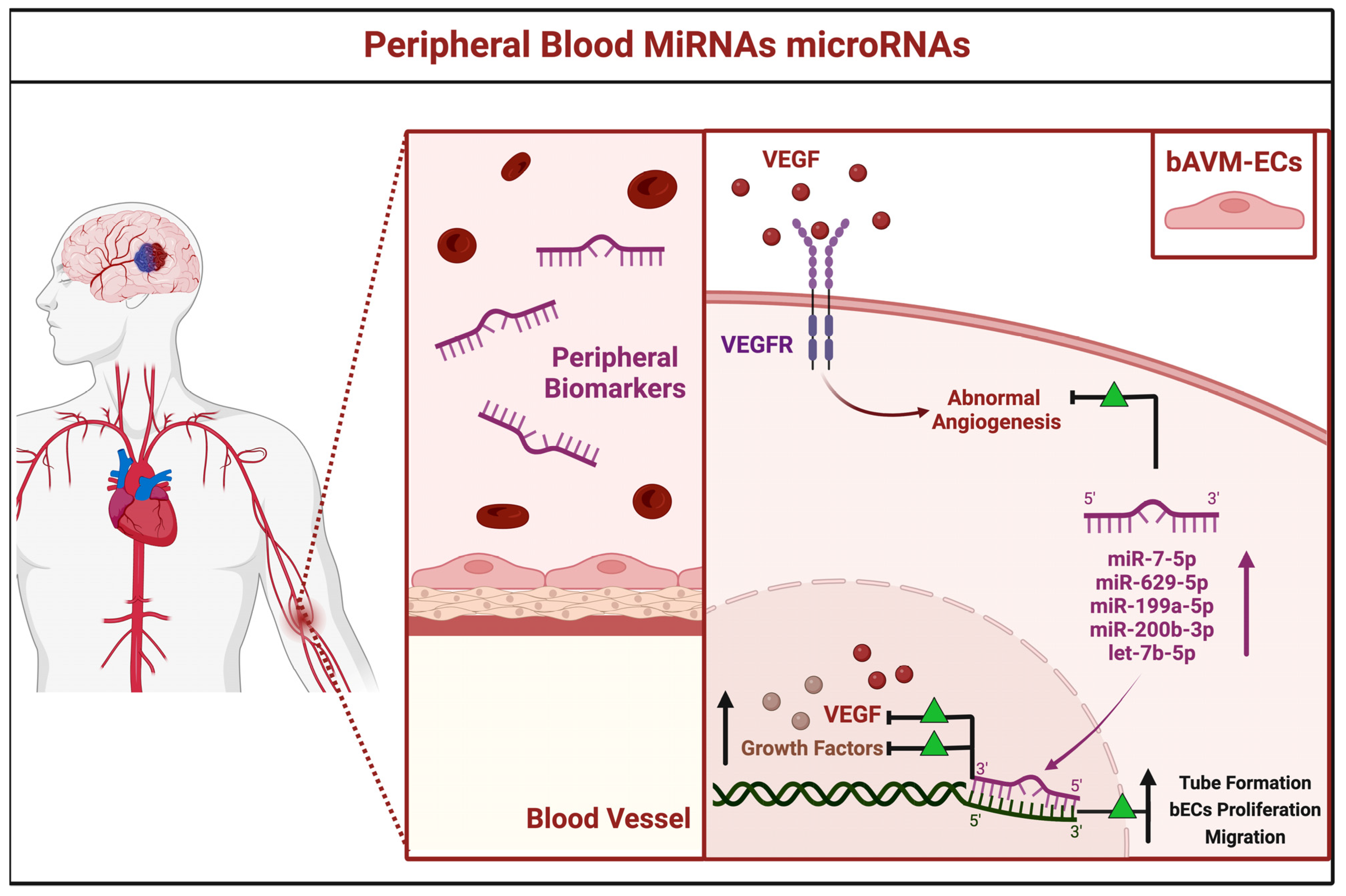
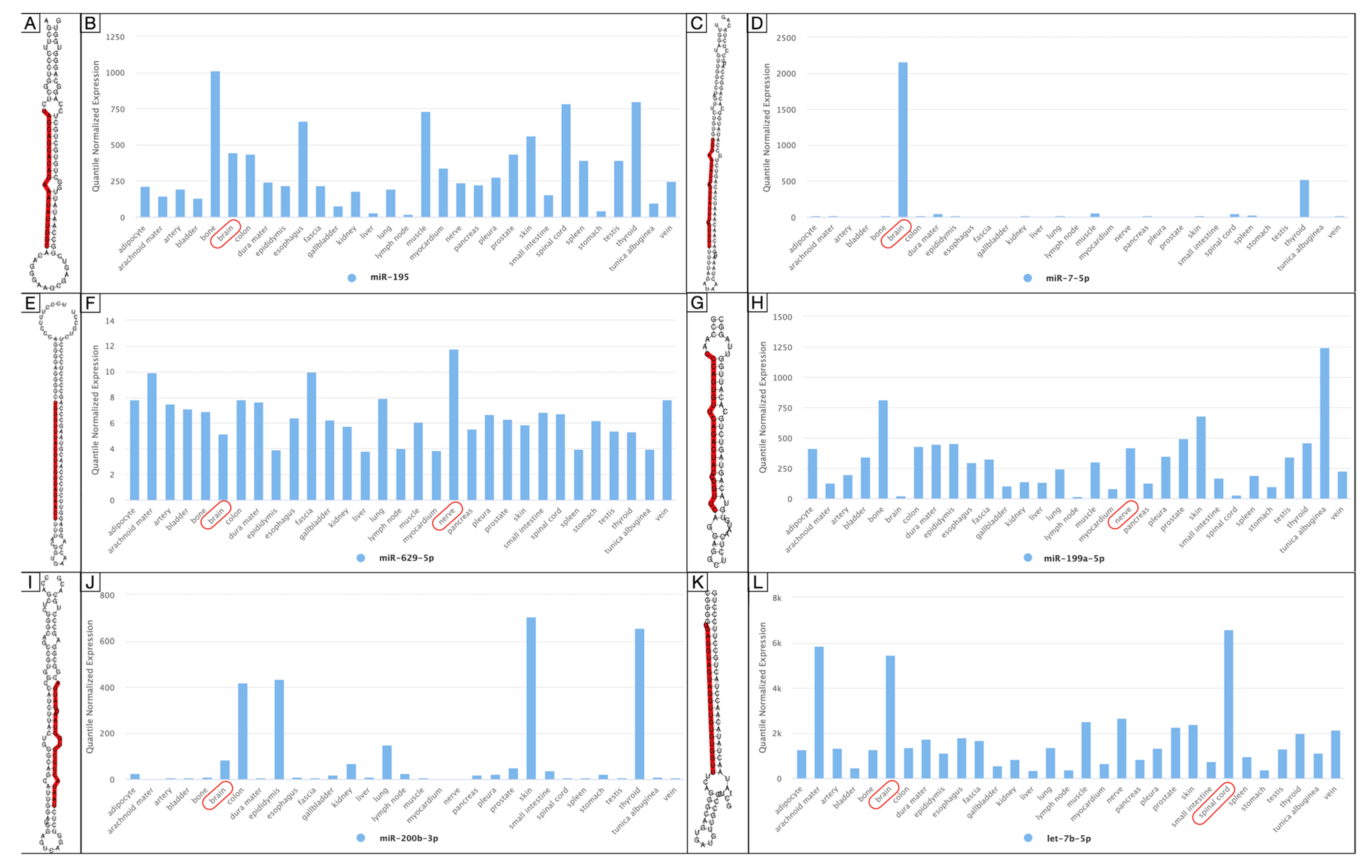
3.2.3. Peripheral Blood miRNAs
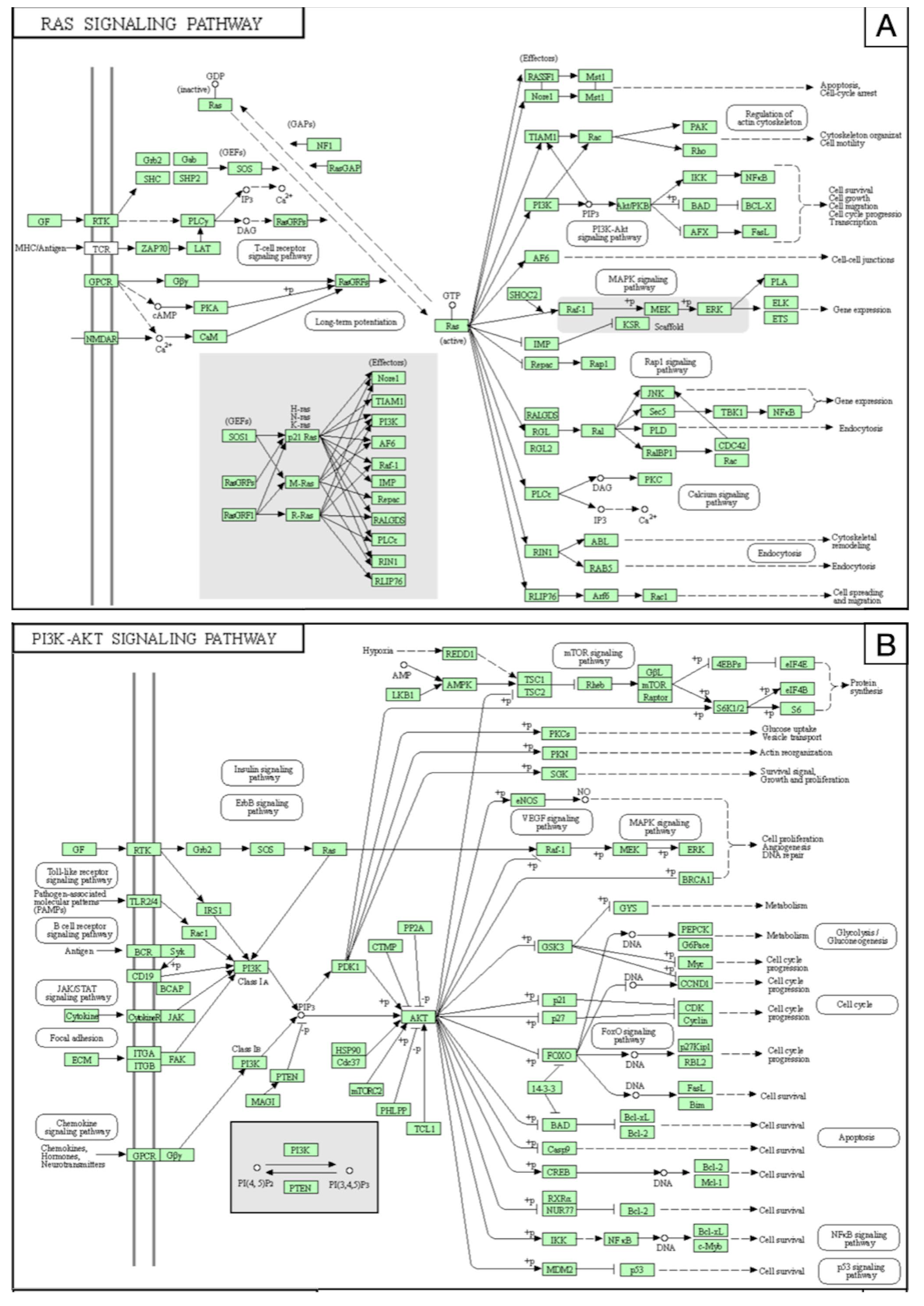
3.3. Prediction of miRNA-Related Targets
4. Discussion
4.1. Experimental Strategies and Future Perspectives for bAVMs
4.2. Emerging miRNA-Based Therapies
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crowley, R.W.; Ducruet, A.F.; McDougall, C.G.; Albuquerque, F.C. Endovascular advances for brain arteriovenous malformations. Neurosurgery 2014, 74 (Suppl. 1), S74–S82. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, R.L.; Lazzaro, M.A.; Castonguay, A.C.; Zaidat, O.O. The diagnosis and management of brain arteriovenous malformations. Neurol. Clin. 2013, 31, 749–763. [Google Scholar] [CrossRef] [PubMed]
- Stapf, C.; Mast, H.; Sciacca, R.R.; Berenstein, A.; Nelson, P.K.; Gobin, Y.P.; Pile-Spellman, J.; Mohr, J.P. The New York Islands AVM Study: Design, study progress, and initial results. Stroke 2003, 34, e29–e33. [Google Scholar] [CrossRef]
- Morris, Z.; Whiteley, W.N.; Longstreth, W.T., Jr.; Weber, F.; Lee, Y.C.; Tsushima, Y.; Alphs, H.; Ladd, S.C.; Warlow, C.; Wardlaw, J.M.; et al. Incidental findings on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ 2009, 339, b3016. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.T.; Rutledge, W.C.; Kim, H.; Stapf, C.; Whitehead, K.J.; Li, D.Y.; Krings, T.; terBrugge, K.; Kondziolka, D.; Morgan, M.K.; et al. Brain arteriovenous malformations. Nat. Rev. Dis. Prim. 2015, 1, 15008. [Google Scholar] [CrossRef]
- Rutledge, C.; Cooke, D.L.; Hetts, S.W.; Abla, A.A. Brain arteriovenous malformations. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 176, pp. 171–178. [Google Scholar] [CrossRef]
- Crawford, P.M.; West, C.R.; Chadwick, D.W.; Shaw, M.D. Arteriovenous malformations of the brain: Natural history in unoperated patients. J. Neurol. Neurosurg. Psychiatry 1986, 49, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.J.; Perret, G.E.; Torner, J.C. Bleeding from cerebral arteriovenous malformations as part of their natural history. J. Neurosurg. 1983, 58, 331–337. [Google Scholar] [CrossRef]
- Mast, H.; Young, W.L.; Koennecke, H.C.; Sciacca, R.R.; Osipov, A.; Pile-Spellman, J.; Hacein-Bey, L.; Duong, H.; Stein, B.M.; Mohr, J.P. Risk of spontaneous haemorrhage after diagnosis of cerebral arteriovenous malformation. Lancet 1997, 350, 1065–1068. [Google Scholar] [CrossRef]
- Gross, B.A.; Du, R. Diagnosis and treatment of vascular malformations of the brain. Curr. Treat. Options Neurol. 2014, 16, 279. [Google Scholar] [CrossRef]
- Kim, H.; Al-Shahi Salman, R.; McCulloch, C.E.; Stapf, C.; Young, W.L. Untreated brain arteriovenous malformation: Patient-level meta-analysis of hemorrhage predictors. Neurology 2014, 83, 590–597. [Google Scholar] [CrossRef]
- Stapf, C.; Mast, H.; Sciacca, R.R.; Choi, J.H.; Khaw, A.V.; Connolly, E.S.; Pile-Spellman, J.; Mohr, J.P. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology 2006, 66, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Castilla, L.; Russin, J.J.; Martinez-Del-Campo, E.; Soriano-Baron, H.; Spetzler, R.F.; Nakaji, P. Molecular and cellular biology of cerebral arteriovenous malformations: A review of current concepts and future trends in treatment. Neurosurg. Focus 2014, 37, E1. [Google Scholar] [CrossRef]
- Florian, I.A.; Timiș, T.L.; Ungureanu, G.; Florian, I.S.; Bălașa, A.; Berindan-Neagoe, I. Deciphering the vascular labyrinth: Role of microRNAs and candidate gene SNPs in brain AVM development—Literature review. Neurol. Res. 2020, 42, 1043–1054. [Google Scholar] [CrossRef]
- Thomas, J.M.; Surendran, S.; Abraham, M.; Rajavelu, A.; Kartha, C.C. Genetic and epigenetic mechanisms in the development of arteriovenous malformations in the brain. Clin. Epigenet. 2016, 8, 78. [Google Scholar] [CrossRef]
- Bameri, O.; Salarzaei, M.; Parooie, F. KRAS/BRAF mutations in brain arteriovenous malformations: A systematic review and meta-analysis. Interv. Neuroradiol. 2021, 27, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Schotland, H.; Denstaedt, S. Hereditary Hemorrhagic Telangiectasia. N. Engl. J. Med. 2019, 381, 2552. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Carter, M.T.; Latino, G.A.; Dirks, P.; Ratjen, F. Brain arteriovenous malformations in patients with hereditary hemorrhagic telangiectasia: Clinical presentation and anatomical distribution. Pediatr. Neurol. 2013, 49, 445–450. [Google Scholar] [CrossRef]
- Chen, W.; Choi, E.J.; McDougall, C.M.; Su, H. Brain arteriovenous malformation modeling, pathogenesis, and novel therapeutic targets. Transl. Stroke Res. 2014, 5, 316–329. [Google Scholar] [CrossRef]
- Ota, T.; Komiyama, M. Pathogenesis of non-hereditary brain arteriovenous malformation and therapeutic implications. Interv. Neuroradiol. 2020, 26, 244–253. [Google Scholar] [CrossRef]
- Araldi, E.; Suárez, Y. MicroRNAs as regulators of endothelial cell functions in cardiometabolic diseases. Biochim. Biophys. Acta 2016, 1861, 2094–2103. [Google Scholar] [CrossRef]
- Zammar, S.G.; El Tecle, N.E.; El Ahmadieh, T.Y.; Mcclendon, J., Jr.; Comair, Y.G.; Bendok, B.R. A Biological Approach to Treating Brain Arteriovenous Malformations. Neurosurgery 2014, 74, N15–N17. [Google Scholar] [CrossRef] [PubMed]
- Florian, I.A.; Buruiana, A.; Timis, T.L.; Susman, S.; Florian, I.S.; Balasa, A.; Berindan-Neagoe, I. An Insight into the microRNAs Associated with Arteriovenous and Cavernous Malformations of the Brain. Cells 2021, 10, 1373. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.J.; Searles, C.D. Development of MicroRNA-Based Therapeutics for Vascular Disease. Circ. Res. 2020, 127, 1179–1181. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Dweep, H.; Gretz, N.; Sticht, C. miRWalk database for miRNA-target interactions. Methods Mol. Biol. 2014, 1182, 289–305. [Google Scholar] [CrossRef]
- Huang, H.Y.; Lin, Y.C.; Li, J.; Huang, K.Y.; Shrestha, S.; Hong, H.C.; Tang, Y.; Chen, Y.G.; Jin, C.N.; Yu, Y.; et al. miRTarBase 2020: Updates to the experimentally validated microRNA-target interaction database. Nucleic Acids Res. 2020, 48, D148–D154. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Santos, T.; Amar, A.; Tahara, S.M.; Chen, T.C.; Giannotta, S.L.; Hofman, F.M. MicroRNA-18a improves human cerebral arteriovenous malformation endothelial cell function. Stroke 2014, 45, 293–297. [Google Scholar] [CrossRef]
- Marín-Ramos, N.I.; Thein, T.Z.; Ghaghada, K.B.; Chen, T.C.; Giannotta, S.L.; Hofman, F.M. miR-18a Inhibits BMP4 and HIF-1α Normalizing Brain Arteriovenous Malformations. Circ. Res. 2020, 127, e210–e231. [Google Scholar] [CrossRef]
- Huang, J.; Song, J.; Qu, M.; Wang, Y.; An, Q.; Song, Y.; Yan, W.; Wang, B.; Wang, X.; Zhang, S.; et al. MicroRNA-137 and microRNA-195* inhibit vasculogenesis in brain arteriovenous malformations. Ann. Neurol. 2017, 82, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Z.; Shi, Y.; Huang, G.; Chen, L.; Tan, H.; Wang, Z.; Yin, C.; Hu, J. Deep Sequencing of Small RNAs in Blood of Patients with Brain Arteriovenous Malformations. World Neurosurg. 2018, 115, e570–e579. [Google Scholar] [CrossRef]
- Xu, M.; Xu, H.; Qin, Z.; Zhang, J.; Yang, X.; Xu, F. Increased expression of angiogenic factors in cultured human brain arteriovenous malformation endothelial cells. Cell Biochem. Biophys. 2014, 70, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, D.; Wang, Z.; Li, X.; Xia, W.; Han, Y.; Su, L.; Fan, X. MiR-18a-5p acts as a novel serum biomarker for venous malformation and promotes angiogenesis by regulating the thrombospondin-1/P53 signaling axis. Am. J. Transl. Res. 2021, 13, 11271–11286. [Google Scholar] [PubMed]
- Dogar, A.M.; Semplicio, G.; Guennewig, B.; Hall, J. Multiple microRNAs derived from chemically synthesized precursors regulate thrombospondin 1 expression. Nucleic Acid Ther. 2014, 24, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Gao, Y.; Li, F.; Pan, Q.; Liu, Z.; Lu, X.; Song, C.; Diao, X. MicroRNA-18a regulates invasive meningiomas via hypoxia-inducible factor-1α. Exp. Ther. Med. 2015, 10, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Uranishi, R.; Baev, N.I.; Kim, J.H.; Awad, I.A. Vascular smooth muscle cell differentiation in human cerebral vascular malformations. Neurosurgery 2001, 49, 671–679; discussion 679–680. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Ding, D.; Derdeyn, C.P.; Lanzino, G.; Friedlander, R.M.; Southerland, A.M.; Lawton, M.T.; Sheehan, J.P. Brain arteriovenous malformations: A review of natural history, pathobiology, and interventions. Neurology 2020, 95, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Hafez, A.; Koroknay-Pál, P.; Oulasvirta, E.; Elseoud, A.A.; Lawton, M.T.; Niemelä, M.; Laakso, A. The Application of the Novel Grading Scale (Lawton-Young Grading System) to Predict the Outcome of Brain Arteriovenous Malformation. Neurosurgery 2019, 84, 529–536. [Google Scholar] [CrossRef]
- Rutledge, W.C.; Ko, N.U.; Lawton, M.T.; Kim, H. Hemorrhage rates and risk factors in the natural history course of brain arteriovenous malformations. Transl. Stroke Res. 2014, 5, 538–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, R.D., Jr.; Wiebers, D.O.; Forbes, G.; O’Fallon, W.M.; Piepgras, D.G.; Marsh, W.R.; Maciunas, R.J. The natural history of unruptured intracranial arteriovenous malformations. J. Neurosurg. 1988, 68, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Bayrak-Toydemir, P.; Mao, R.; Lewin, S.; McDonald, J. Hereditary hemorrhagic telangiectasia: An overview of diagnosis and management in the molecular era for clinicians. Genet. Med. 2004, 6, 175–191. [Google Scholar] [CrossRef]
- Berg, J.N.; Gallione, C.J.; Stenzel, T.T.; Johnson, D.W.; Allen, W.P.; Schwartz, C.E.; Jackson, C.E.; Porteous, M.E.; Marchuk, D.A. The activin receptor-like kinase 1 gene: Genomic structure and mutations in hereditary hemorrhagic telangiectasia type 2. Am. J. Hum. Genet. 1997, 61, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Nishino, K.; Ito, Y.; Sorimachi, T.; Shimbo, J.; Fujii, Y. Sturge-Weber syndrome associated with arteriovenous malformation in a patient presenting with progressive brain edema and cyst formation. J. Neurosurg. Pediatr. 2010, 5, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, S.; Del Maestro, M.; Elbabaa, S.K.; Galzio, R. Letter to the Editor Regarding “One and Done: Multimodal Treatment of Pediatric Cerebral Arteriovenous Malformations in a Single Anesthesia Event”. World Neurosurg. 2020, 134, 660. [Google Scholar] [CrossRef] [PubMed]
- Brinjikji, W.; Iyer, V.N.; Wood, C.P.; Lanzino, G. Prevalence and characteristics of brain arteriovenous malformations in hereditary hemorrhagic telangiectasia: A systematic review and meta-analysis. J. Neurosurg. 2017, 127, 302–310. [Google Scholar] [CrossRef] [PubMed]
- McAllister, K.A.; Grogg, K.M.; Johnson, D.W.; Gallione, C.J.; Baldwin, M.A.; Jackson, C.E.; Helmbold, E.A.; Markel, D.S.; McKinnon, W.C.; Murrel, J.; et al. Endoglin, a TGF-β binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat. Genet. 1994, 8, 345–351. [Google Scholar] [CrossRef]
- Johnson, D.W.; Berg, J.N.; Baldwin, M.A.; Gallione, C.J.; Marondel, I.; Yoon, S.J.; Stenzel, T.T.; Speer, M.; Pericak-Vance, M.A.; Diamond, A.; et al. Mutations in the activin receptor–like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat. Genet. 1996, 13, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Jiang, W.; Lin, W.; Wang, Y.; Chen, H.; Zou, H.; Huang, S.; Zhu, N.; Han, S. A novel BMPR2 mutation in a patient with heritable pulmonary arterial hypertension and suspected hereditary hemorrhagic telangiectasia: A case report. Medicine 2020, 99, e21342. [Google Scholar] [CrossRef] [PubMed]
- Gallione, C.J.; Repetto, G.M.; Legius, E.; Rustgi, A.K.; Schelley, S.L.; Tejpar, S.; Mitchell, G.; Drouin, E.; Westermann, C.J.; Marchuk, D.A. A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4). Lancet 2004, 363, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Li, Y.-P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [PubMed]
- Burks, T.N.; Cohn, R.D. Role of TGF-β signaling in inherited and acquired myopathies. Skelet. Muscle 2011, 1, 19. [Google Scholar] [CrossRef] [PubMed]
- Alsina-Sanchís, E.; García-Ibáñez, Y.; Figueiredo, A.M.; Riera-Domingo, C.; Figueras, A.; Matias-Guiu, X.; Casanovas, O.; Botella, L.M.; Pujana, M.A.; Riera-Mestre, A.; et al. ALK1 Loss Results in Vascular Hyperplasia in Mice and Humans Through PI3K Activation. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1216–1229. [Google Scholar] [CrossRef]
- Ola, R.; Dubrac, A.; Han, J.; Zhang, F.; Fang, J.S.; Larrivée, B.; Lee, M.; Urarte, A.A.; Kraehling, J.R.; Genet, G.; et al. PI3 kinase inhibition improves vascular malformations in mouse models of hereditary haemorrhagic telangiectasia. Nat. Commun. 2016, 7, 13650. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, F.; Grossman, S.R.; Takahashi, Y.; Rokas, M.V.; Nakamura, N.; Sellers, W.R. Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex. J. Biol. Chem. 2001, 276, 48627–48630. [Google Scholar] [CrossRef] [PubMed]
- Bourdeau, A.; Dumont, D.J.; Letarte, M. A murine model of hereditary hemorrhagic telangiectasia. J. Clin. Investig. 1999, 104, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Torsney, E.; Charlton, R.; Diamond, A.G.; Burn, J.; Soames, J.V.; Arthur, H.M. Mouse model for hereditary hemorrhagic telangiectasia has a generalized vascular abnormality. Circulation 2003, 107, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Hanes, M.A.; Dickens, T.; Porteous, M.E.M.; Oh, S.P.; Hale, L.P.; Marchuk, D.A. A mouse model for hereditary hemorrhagic telangiectasia (HHT) type 2. Hum. Mol. Genet. 2003, 12, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Sturiale, C.L.; Puca, A.; Sebastiani, P.; Gatto, I.; Albanese, A.; Di Rocco, C.; Maira, G.; Pola, R. Single nucleotide polymorphisms associated with sporadic brain arteriovenous malformations: Where do we stand? Brain 2013, 136, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska, L.; Tran, M.N.; Achrol, A.S.; McCulloch, C.E.; Ha, C.; Lind, D.L.; Hashimoto, T.; Zaroff, J.; Lawton, M.T.; Marchuk, D.A.; et al. Polymorphisms in genes involved in inflammatory and angiogenic pathways and the risk of hemorrhagic presentation of brain arteriovenous malformations. Stroke 2004, 35, 2294–2300. [Google Scholar] [CrossRef]
- Nikolaev, S.I.; Vetiska, S.; Bonilla, X.; Boudreau, E.; Jauhiainen, S.; Rezai Jahromi, B.; Khyzha, N.; DiStefano, P.V.; Suutarinen, S.; Kiehl, T.R.; et al. Somatic Activating KRAS Mutations in Arteriovenous Malformations of the Brain. N. Engl. J. Med. 2018, 378, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Oka, M.; Kushamae, M.; Aoki, T.; Yamaguchi, T.; Kitazato, K.; Abekura, Y.; Kawamata, T.; Mizutani, T.; Miyamoto, S.; Takagi, Y. KRAS G12D or G12V Mutation in Human Brain Arteriovenous Malformations. World Neurosurg. 2019, 126, e1365–e1373. [Google Scholar] [CrossRef] [PubMed]
- Al-Olabi, L.; Polubothu, S.; Dowsett, K.; Andrews, K.A.; Stadnik, P.; Joseph, A.P.; Knox, R.; Pittman, A.; Clark, G.; Baird, W.; et al. Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J. Clin. Investig. 2018, 128, 1496–1508. [Google Scholar] [CrossRef]
- Eerola, I.; Boon, L.M.; Mulliken, J.B.; Burrows, P.E.; Dompmartin, A.; Watanabe, S.; Vanwijck, R.; Vikkula, M. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am. J. Hum. Genet. 2003, 73, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Limaye, N.; Kangas, J.; Mendola, A.; Godfraind, C.; Schlögel, M.J.; Helaers, R.; Eklund, L.; Boon, L.M.; Vikkula, M. Somatic Activating PIK3CA Mutations Cause Venous Malformation. Am. J. Hum. Genet. 2015, 97, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Priemer, D.S.; Vortmeyer, A.O.; Zhang, S.; Chang, H.Y.; Curless, K.L.; Cheng, L. Activating KRAS mutations in arteriovenous malformations of the brain: Frequency and clinicopathologic correlation. Hum. Pathol. 2019, 89, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Simanshu, D.K.; Nissley, D.V.; McCormick, F. RAS Proteins and Their Regulators in Human Disease. Cell 2017, 170, 17–33. [Google Scholar] [CrossRef]
- Jin, Y.; Muhl, L.; Burmakin, M.; Wang, Y.; Duchez, A.C.; Betsholtz, C.; Arthur, H.M.; Jakobsson, L. Endoglin prevents vascular malformation by regulating flow-induced cell migration and specification through VEGFR2 signalling. Nat. Cell Biol. 2017, 19, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Ola, R.; Künzel, S.H.; Zhang, F.; Genet, G.; Chakraborty, R.; Pibouin-Fragner, L.; Martin, K.; Sessa, W.; Dubrac, A.; Eichmann, A. SMAD4 Prevents Flow Induced Arteriovenous Malformations by Inhibiting Casein Kinase 2. Circulation 2018, 138, 2379–2394. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, M.; Zhao, S.; Xie, Z.; Zhang, Y.; Liu, J.; Zhang, Y.; Yang, X.; Wu, N. Mutational spectrum of syndromic genes in sporadic brain arteriovenous malformation. Chin. Neurosurg. J. 2022, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.; Fish, J.E.; D’Abreo, C.; Lin, S.; Robb, G.B.; Teichert, A.M.; Karantzoulis-Fegaras, F.; Keightley, A.; Steer, B.M.; Marsden, P.A. The cell-specific expression of endothelial nitric-oxide synthase: A role for DNA methylation. J. Biol. Chem. 2004, 279, 35087–35100. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Wei, S.Y.; Chiu, J.J. Mechanical regulation of epigenetics in vascular biology and pathobiology. J. Cell. Mol. Med. 2013, 17, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.J.; Chien, S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol. Rev. 2011, 91, 327–387. [Google Scholar] [CrossRef]
- Illi, B.; Nanni, S.; Scopece, A.; Farsetti, A.; Biglioli, P.; Capogrossi, M.C.; Gaetano, C. Shear stress-mediated chromatin remodeling provides molecular basis for flow-dependent regulation of gene expression. Circ. Res. 2003, 93, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Balta, S. Endothelial Dysfunction and Inflammatory Markers of Vascular Disease. Curr. Vasc. Pharmacol. 2021, 19, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Godo, S.; Shimokawa, H. Endothelial Functions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef]
- Giotta Lucifero, A.; Baldoncini, M.; Bruno, N.; Galzio, R.; Hernesniemi, J.; Luzzi, S. Shedding the Light on the Natural History of Intracranial Aneurysms: An Updated Overview. Medicina 2021, 57, 742. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hao, Q.; Zhao, Y.; Guo, Y.; Ge, W. Dysregulation of cell-cell interactions in brain arteriovenous malformations: A quantitative proteomic study. Proteom. Clin. Appl. 2017, 11, 1600093. [Google Scholar] [CrossRef] [PubMed]
- Spetzler, R.F.; Martin, N.A. A proposed grading system for arteriovenous malformations. J. Neurosurg. 1986, 65, 476–483. [Google Scholar] [CrossRef] [Green Version]
- Spetzler, R.F.; Ponce, F.A. A 3-tier classification of cerebral arteriovenous malformations. Clinical article. J. Neurosurg. 2011, 114, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Giotta Lucifero, A.; Luzzi, S. Against the Resilience of High-Grade Gliomas: The Immunotherapeutic Approach (Part I). Brain Sci. 2021, 11, 386. [Google Scholar] [CrossRef]
- Giotta Lucifero, A.; Luzzi, S.; Brambilla, I.; Guarracino, C.; Mosconi, M.; Foiadelli, T.; Savasta, S. Gene therapies for high-grade gliomas: From the bench to the bedside. Acta Biomed. 2020, 91, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Giotta Lucifero, A.; Luzzi, S.; Brambilla, I.; Schena, L.; Mosconi, M.; Foiadelli, T.; Savasta, S. Potential roads for reaching the summit: An overview on target therapies for high-grade gliomas. Acta Biomed. 2020, 91, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Giotta Lucifero, A.; Luzzi, S.; Brambilla, I.; Trabatti, C.; Mosconi, M.; Savasta, S.; Foiadelli, T. Innovative therapies for malignant brain tumors: The road to a tailored cure. Acta Biomed. 2020, 91, 5–17. [Google Scholar] [CrossRef]
- Luzzi, S.; Crovace, A.M.; Del Maestro, M.; Giotta Lucifero, A.; Elbabaa, S.K.; Cinque, B.; Palumbo, P.; Lombardi, F.; Cimini, A.; Cifone, M.G.; et al. The cell-based approach in neurosurgery: Ongoing trends and future perspectives. Heliyon 2019, 5, e02818. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, S.; Giotta Lucifero, A.; Brambilla, I.; Magistrali, M.; Mosconi, M.; Savasta, S.; Foiadelli, T. Adoptive immunotherapies in neuro-oncology: Classification, recent advances, and translational challenges. Acta Biomed. 2020, 91, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, S.; Giotta Lucifero, A.; Brambilla, I.; Trabatti, C.; Mosconi, M.; Savasta, S.; Foiadelli, T. The impact of stem cells in neuro-oncology: Applications, evidence, limitations and challenges. Acta Biomed. 2020, 91, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Giotta Lucifero, A.; Luzzi, S. Against the Resilience of High-Grade Gliomas: Gene Therapies (Part II). Brain Sci. 2021, 11, 976. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, S.; Del Maestro, M.; Galzio, R. Letter to the Editor. Preoperative embolization of brain arteriovenous malformations. J. Neurosurg. 2019, 132, 2014–2016. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, S.; Crovace, A.M.; Lacitignola, L.; Valentini, V.; Francioso, E.; Rossi, G.; Invernici, G.; Galzio, R.J.; Crovace, A. Engraftment, neuroglial transdifferentiation and behavioral recovery after complete spinal cord transection in rats. Surg. Neurol. Int. 2018, 9, 19. [Google Scholar] [CrossRef]
- Luzzi, S.; Giotta Lucifero, A.; Del Maestro, M.; Marfia, G.; Navone, S.E.; Baldoncini, M.; Nuñez, M.; Campero, A.; Elbabaa, S.K.; Galzio, R. Anterolateral Approach for Retrostyloid Superior Parapharyngeal Space Schwannomas Involving the Jugular Foramen Area: A 20-Year Experience. World Neurosurg. 2019, 132, e40–e52. [Google Scholar] [CrossRef] [PubMed]
- Bellantoni, G.; Guerrini, F.; Del Maestro, M.; Galzio, R.; Luzzi, S. Simple schwannomatosis or an incomplete Coffin-Siris? Report of a particular case. eNeurologicalSci 2019, 14, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Campanella, R.; Guarnaccia, L.; Cordiglieri, C.; Trombetta, E.; Caroli, M.; Carrabba, G.; La Verde, N.; Rampini, P.; Gaudino, C.; Costa, A.; et al. Tumor-Educated Platelets and Angiogenesis in Glioblastoma: Another Brick in the Wall for Novel Prognostic and Targetable Biomarkers, Changing the Vision from a Localized Tumor to a Systemic Pathology. Cells 2020, 9, 294. [Google Scholar] [CrossRef]
- Walker, E.J.; Su, H.; Shen, F.; Choi, E.J.; Oh, S.P.; Chen, G.; Lawton, M.T.; Kim, H.; Chen, Y.; Chen, W.; et al. Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann. Neurol. 2011, 69, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Choe, S.W.; Kim, Y.H.; Acharya, A.P.; Keselowsky, B.G.; Sorg, B.S.; Lee, Y.J.; Oh, S.P. VEGF neutralization can prevent and normalize arteriovenous malformations in an animal model for hereditary hemorrhagic telangiectasia 2. Angiogenesis 2014, 17, 823–830. [Google Scholar] [CrossRef]
- Kim, H.; Pawlikowska, L.; Chen, Y.; Su, H.; Yang, G.Y.; Young, W.L. Brain arteriovenous malformation biology relevant to hemorrhage and implication for therapeutic development. Stroke 2009, 40, S95–S97. [Google Scholar] [CrossRef]
- Merrill, M.J.; Oldfield, E.H. A reassessment of vascular endothelial growth factor in central nervous system pathology. J. Neurosurg. 2005, 103, 853–868. [Google Scholar] [CrossRef]
- Raper, D.M.S.; Winkler, E.A.; Rutledge, W.C.; Cooke, D.L.; Abla, A.A. An Update on Medications for Brain Arteriovenous Malformations. Neurosurgery 2020, 87, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Z.; Young, W.L. Management of brain arteriovenous malformations. Curr. Opin. Anaesthesiol. 2005, 18, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Liu, M.; Wang, Y.; Chen, X.; Xu, J.; Sun, Y.; Zhao, L.; Qu, H.; Fan, Y.; Wu, C. Antagonism of miR-21 reverses epithelial-mesenchymal transition and cancer stem cell phenotype through AKT/ERK1/2 inactivation by targeting PTEN. PLoS ONE 2012, 7, e39520. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Z.; Li, C.; Chen, Q.; Jing, Y.; Carpenter, R.; Jiang, Y.; Kung, H.F.; Lai, L.; Jiang, B.H. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1α expression. PLoS ONE 2011, 6, e19139. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, J.F.; Ruffino, L.; Kelnar, K.; Omotola, M.; Patrawala, L.; Brown, D.; Bader, A.G. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 2010, 70, 5923–5930. [Google Scholar] [CrossRef] [PubMed]
- Tivnan, A.; Orr, W.S.; Gubala, V.; Nooney, R.; Williams, D.E.; McDonagh, C.; Prenter, S.; Harvey, H.; Domingo-Fernández, R.; Bray, I.M.; et al. Inhibition of neuroblastoma tumor growth by targeted delivery of microRNA-34a using anti-disialoganglioside GD2 coated nanoparticles. PLoS ONE 2012, 7, e38129. [Google Scholar] [CrossRef]
- Wong, M.Y.; Yu, Y.; Walsh, W.R.; Yang, J.L. microRNA-34 family and treatment of cancers with mutant or wild-type p53 (Review). Int. J. Oncol. 2011, 38, 1189–1195. [Google Scholar] [CrossRef]
- Jansson, M.D.; Lund, A.H. MicroRNA and cancer. Mol. Oncol. 2012, 6, 590–610. [Google Scholar] [CrossRef] [PubMed]
- Jopling, C.L. Targeting microRNA-122 to Treat Hepatitis C Virus Infection. Viruses 2010, 2, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Jangra, R.K.; Yi, M.; Lemon, S.M. Regulation of hepatitis C virus translation and infectious virus production by the MicroRNA miR-122. J. Virol. 2010, 84, 6615–6625. [Google Scholar] [CrossRef]
- Lanford, R.E.; Hildebrandt-Eriksen, E.S.; Petri, A.; Persson, R.; Lindow, M.; Munk, M.E.; Kauppinen, S.; Ørum, H. Therapeutic Silencing of MicroRNA-122 in Primates with Chronic Hepatitis C Virus Infection. Science 2010, 327, 198–201. [Google Scholar] [CrossRef]
- Hildebrandt-Eriksen, E.S.; Aarup, V.; Persson, R.; Hansen, H.F.; Munk, M.E.; Ørum, H. A Locked Nucleic Acid Oligonucleotide Targeting MicroRNA 122 Is Well-Tolerated in Cynomolgus Monkeys. Nucleic Acid Ther. 2012, 22, 152–161. [Google Scholar] [CrossRef]
- Janssen, H.L.A.; Reesink, H.W.; Lawitz, E.J.; Zeuzem, S.; Rodriguez-Torres, M.; Patel, K.; van der Meer, A.J.; Patick, A.K.; Chen, A.; Zhou, Y.; et al. Treatment of HCV Infection by Targeting MicroRNA. N. Engl. J. Med. 2013, 368, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Esau, C.; Davis, S.; Murray, S.F.; Yu, X.X.; Pandey, S.K.; Pear, M.; Watts, L.; Booten, S.L.; Graham, M.; McKay, R.; et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006, 3, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Elmén, J.; Lindow, M.; Schütz, S.; Lawrence, M.; Petri, A.; Obad, S.; Lindholm, M.; Hedtjärn, M.; Hansen, H.F.; Berger, U.; et al. LNA-mediated microRNA silencing in non-human primates. Nature 2008, 452, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Rayner, K.J.; Sheedy, F.J.; Esau, C.C.; Hussain, F.N.; Temel, R.E.; Parathath, S.; Van Gils, J.M.; Rayner, A.J.; Chang, A.N.; Suarez, Y.; et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J. Clin. Investig. 2011, 121, 2921–2931. [Google Scholar] [CrossRef] [PubMed]
- Rayner, K.J.; Esau, C.C.; Hussain, F.N.; McDaniel, A.L.; Marshall, S.M.; van Gils, J.M.; Ray, T.D.; Sheedy, F.J.; Goedeke, L.; Liu, X.; et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature 2011, 478, 404–407. [Google Scholar] [CrossRef]
- Chau, B.N.; Xin, C.; Hartner, J.; Ren, S.; Castano, A.P.; Linn, G.; Li, J.; Tran, P.T.; Kaimal, V.; Huang, X.; et al. MicroRNA-21 Promotes Fibrosis of the Kidney by Silencing Metabolic Pathways. Sci. Transl. Med. 2012, 4, 121ra118. [Google Scholar] [CrossRef] [PubMed]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Bhandari, S.; Rath, G.; Goyal, A.K. Current strategies for targeted delivery of bio-active drug molecules in the treatment of brain tumor. J. Drug Target. 2015, 23, 865–887. [Google Scholar] [CrossRef]
- Simion, V.; Nadim, W.D.; Benedetti, H.; Pichon, C.; Morisset-Lopez, S.; Baril, P. Pharmacomodulation of microRNA Expression in Neurocognitive Diseases: Obstacles and Future Opportunities. Curr. Neuropharmacol. 2017, 15, 276–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| miRNA | Site | Status | Level of Gene Expression | Author, Year | Country | |
|---|---|---|---|---|---|---|
| High | Low | |||||
| miR-18a | BECs | Downregulated | TSP-1, VEGF-A | Id-1, VEGF-D | Ferreira et al., 2014 [29] | USA |
| TSP-1 | VEGF, PAI-1, BMP4, HIF-1α MMP2, MMP9, ADAM10 | Marín-Ramos et al., 2020 [30] | USA | |||
| miR-137 | VSMCs | Downregulated | NA | VEGF, PI3K/Akt, MAPK/ERK, P38, NFkB | Huang et al., 2017 [31] | China |
| miR-195* | ||||||
| miR-7-5p | PB | Over-expressed | VEGF | NA | Chen et al., 2018 [32] | China |
| miR-629-5p | ||||||
| miR-199a-5p | ||||||
| miR-200b-3p | ||||||
| let-7b-5p | NA | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giotta Lucifero, A.; Luzzi, S. Brain AVMs-Related microRNAs: Machine Learning Algorithm for Expression Profiles of Target Genes. Brain Sci. 2022, 12, 1628. https://doi.org/10.3390/brainsci12121628
Giotta Lucifero A, Luzzi S. Brain AVMs-Related microRNAs: Machine Learning Algorithm for Expression Profiles of Target Genes. Brain Sciences. 2022; 12(12):1628. https://doi.org/10.3390/brainsci12121628
Chicago/Turabian StyleGiotta Lucifero, Alice, and Sabino Luzzi. 2022. "Brain AVMs-Related microRNAs: Machine Learning Algorithm for Expression Profiles of Target Genes" Brain Sciences 12, no. 12: 1628. https://doi.org/10.3390/brainsci12121628