Hydrogen-Rich Saline Attenuates Chronic Allodynia after Bone Fractures via Reducing Spinal CXCL1/CXCR2-Mediated Iron Accumulation in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgical Procedure
2.3. Preparation of Hydrogen-Rich Saline
2.4. Drug and Administration
2.5. Behavioral Tests
2.6. ELISA Analysis
2.7. Western Blot
2.8. Iron Content Assay
2.9. Statistical Analysis
3. Results
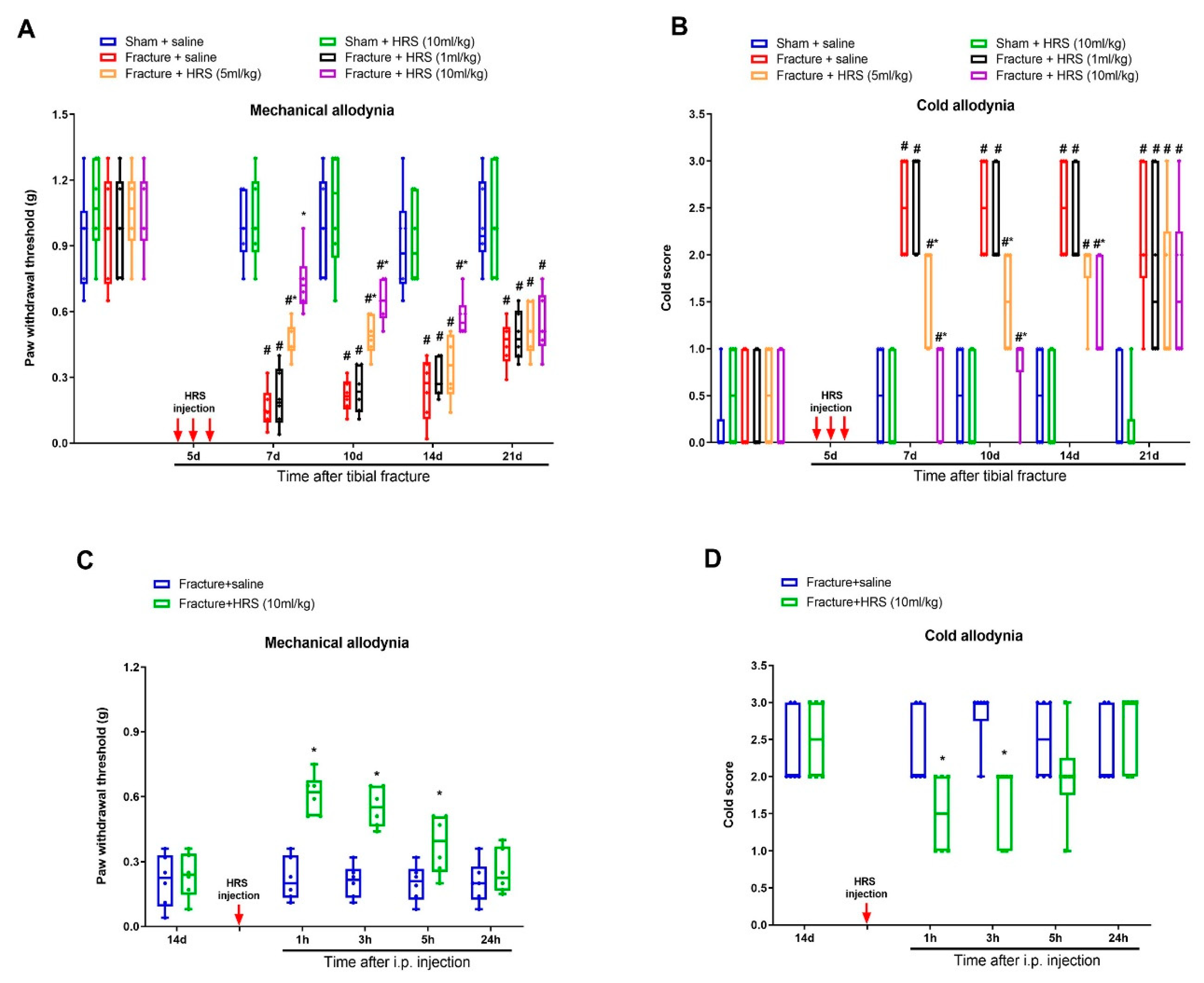
3.1. Hydrogen-Rich Saline Reduces the Generation and Maintenance of Mechanical Allodynia and Cold Allodynia Following Tibial Fracture and Orthopedic Surgeries
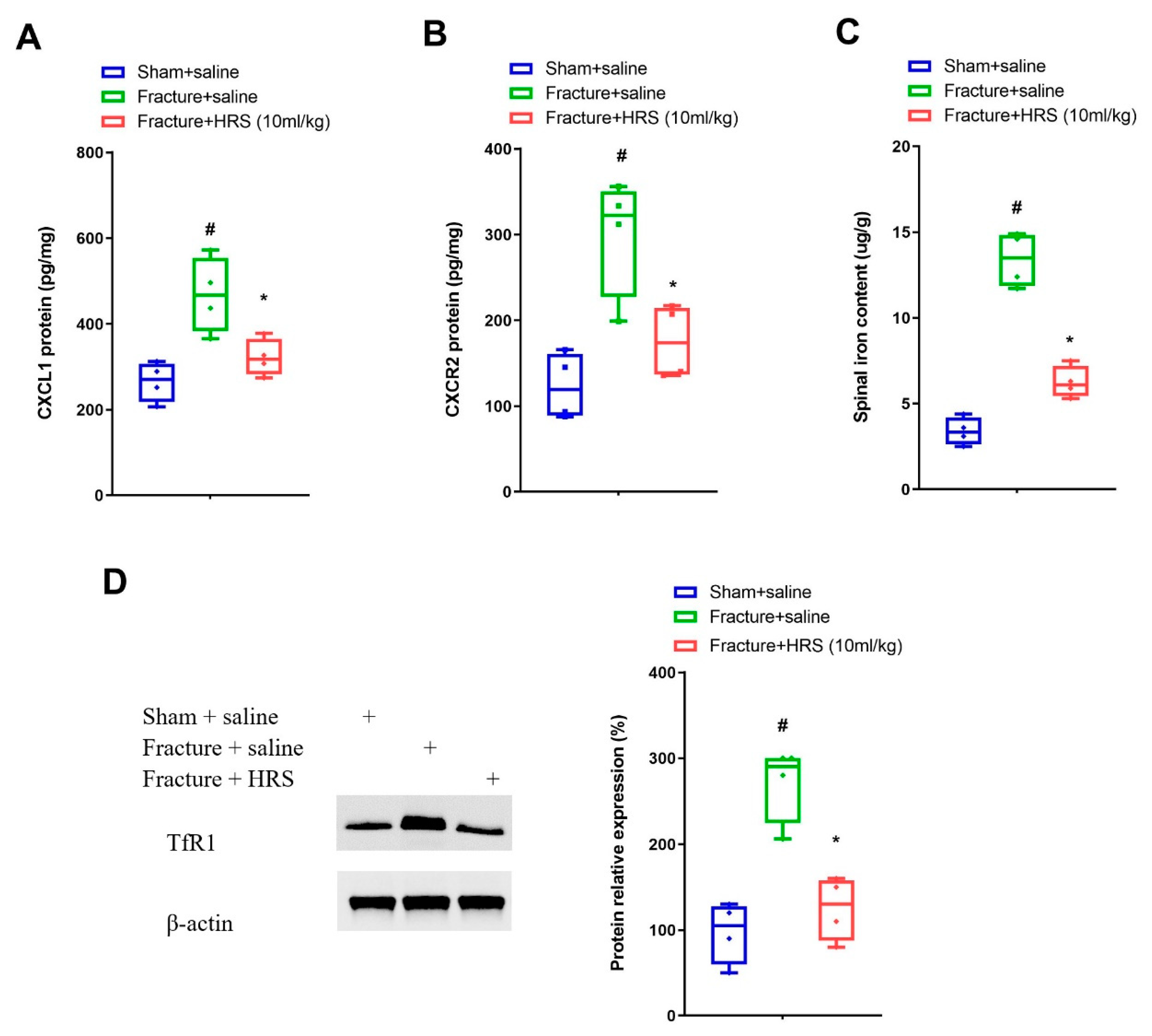
3.2. Hydrogen-Rich Saline Reduces the Spinal CXCL1/CXCR2 Expressions and Tfr1-Dependent Iron Accumulation upon Tibial Fracture Procedures in Mice
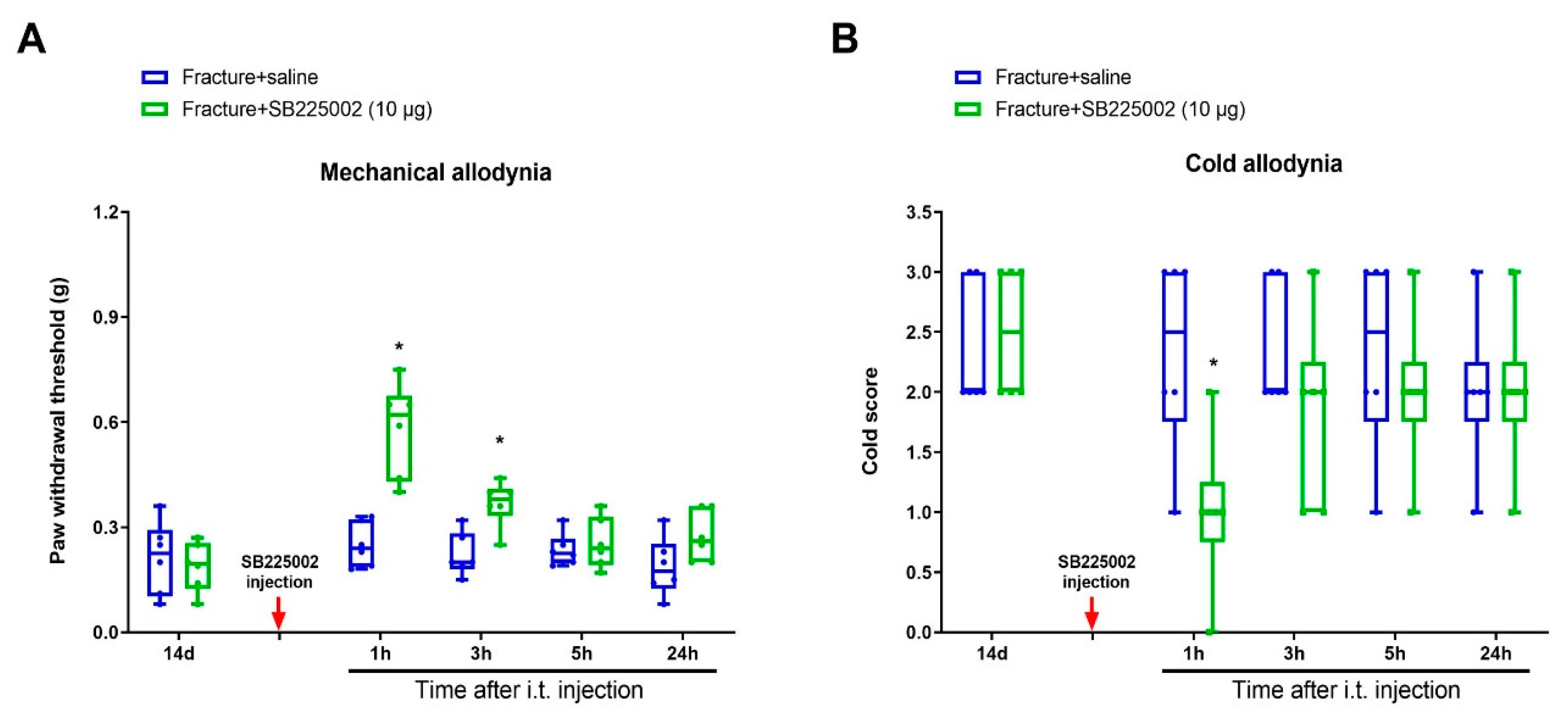
3.3. CXCR2 Antagonism Reduces Chronic Allodynia Behaviors and Spinal Tfr1-Dependent Iron Overload after Fracture Procedures
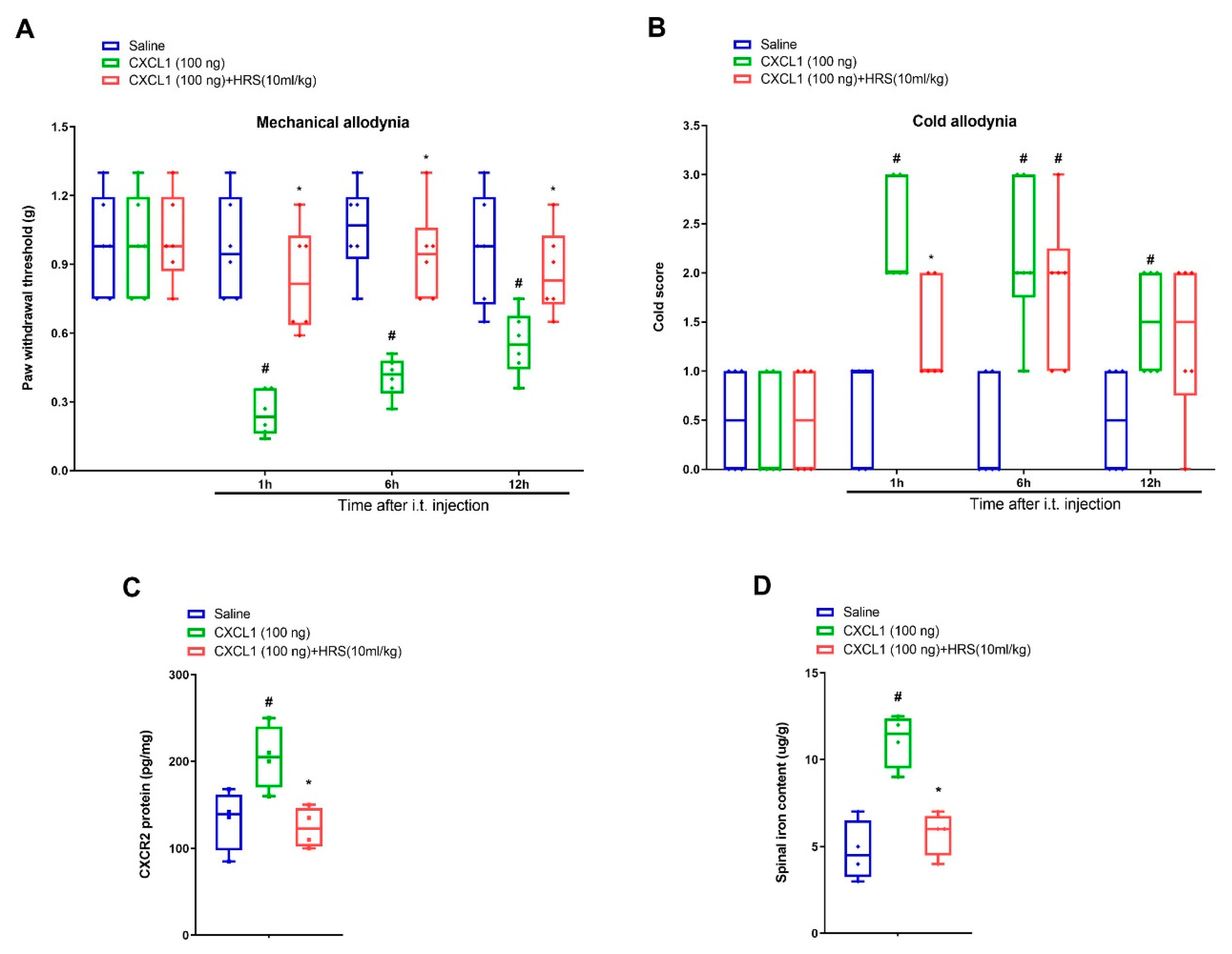
3.4. Hydrogen-Rich Saline Impairs Exogenous CXCL1-Elicited Acute Allodynia Behaviors and Spinal Iron Overload
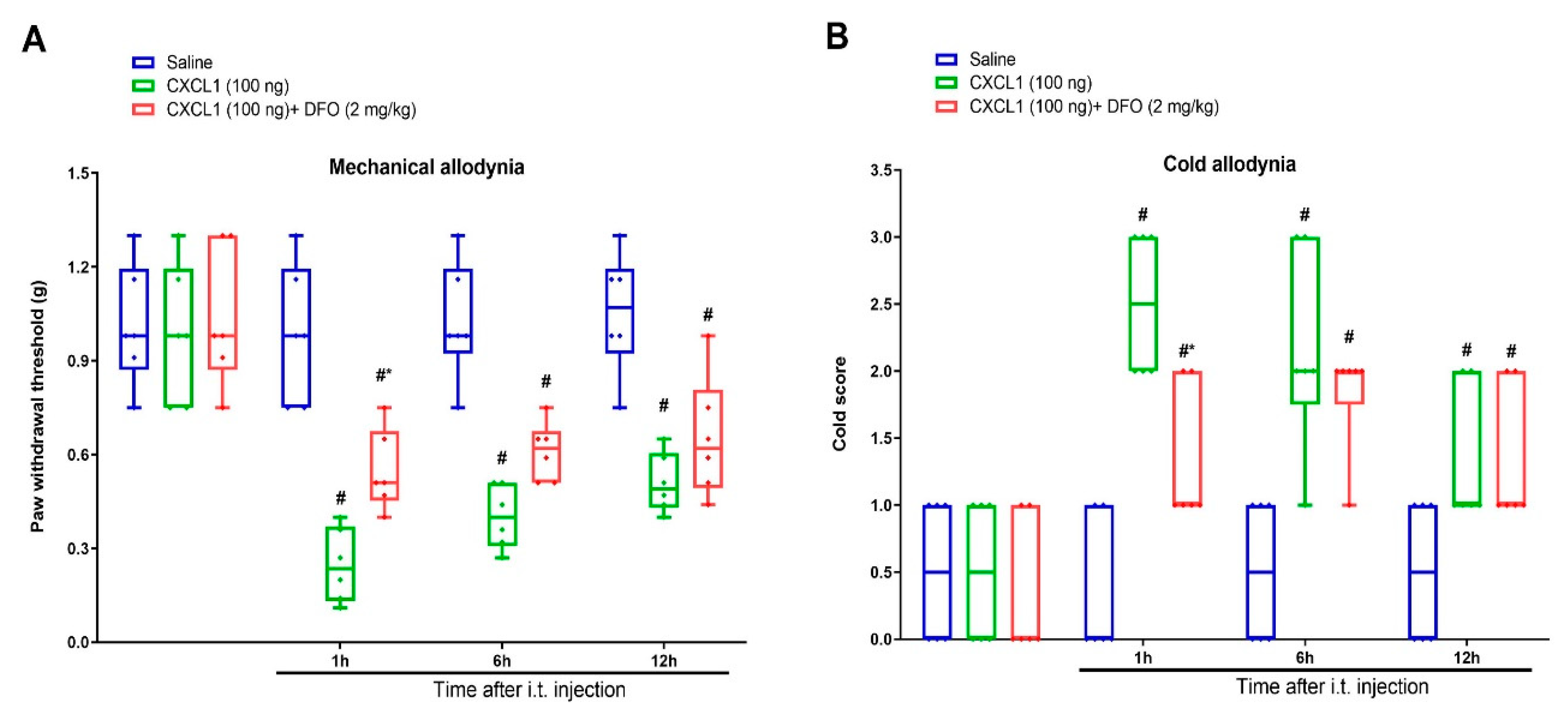
3.5. Exogenous CXCL1-Elicited Acute Allodynia Behaviors Are Reversed by Iron Chelation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.; Lv, H.; Liu, S.; Liu, B.; Zhu, Y.; Chen, X.; Yang, G.; Liu, L.; Zhang, T.; Wang, H.; et al. National incidence of traumatic fractures in China: A retrospective survey of 512187 individuals. Lancet Glob. Health 2017, 5, e807-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, J.S.; Devereaux, P.J.; LeManach, Y.; Busse, J.W. Patient coping and expectations about recovery predict the development of chronic post-surgical pain after traumatic tibial fracture repair. Br. J. Anaesth. 2016, 117, 365–370. [Google Scholar] [CrossRef] [Green Version]
- Smits, E.S.; Nijhuis, T.H.; Huygen, F.J.; Selles, R.W.; Hovius, S.E.; Niehof, S.P. Rewarming patterns in hand fracture patients with and without cold intolerance. J. Hand Surg. Am. 2011, 36, 670–676. [Google Scholar] [CrossRef] [PubMed]
- McVeigh, L.G.; Perugini, A.J.; Fehrenbacher, J.C.; White, F.A.; Kacena, M.A. Assessment, quantification, and management of fracture pain: From animals to the clinic. Curr. Osteoporos. Rep. 2020, 18, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Baral, P.; Udit, S.; Chiu, I.M. Pain and immunity: Implications for host defence. Nat. Rev. Immunol. 2019, 19, 433–447. [Google Scholar] [CrossRef]
- Inoue, K.; Tsuda, M. Microglia in neuropathic pain: Cellular and molecular mechanisms and therapeutic potential. Nat. Rev. Neurosci. 2018, 19, 138–152. [Google Scholar] [CrossRef]
- Pan, T.T.; Pan, F.; Gao, W.; Hu, S.S.; Wang, D. Involvement of macrophages and spinal microglia in osteoarthritis pain. Curr. Rheumatol. Rep. 2021, 23, 29. [Google Scholar] [CrossRef]
- Montague, K.; Malcangio, M. The therapeutic potential of targeting chemokine signalling in the treatment of chronic pain. J. Neurochem. 2017, 141, 520–531. [Google Scholar] [CrossRef] [Green Version]
- Hirth, M.; Gandla, J.; Höper, C.; Gaida, M.M.; Agarwal, N.; Simonetti, M.; Demir, A.; Xie, Y.; Weiss, C.; Michalski, C.W.; et al. CXCL10 and CCL21 promote migration of pancreatic cancer cells toward sensory neurons and neural remodeling in tumors in mice, associated with pain in patients. Gastroenterology 2020, 159, 665–681.e13. [Google Scholar] [CrossRef]
- Lee, K.M.; Jarnicki, A.; Achuthan, A.; Fleetwood, A.J.; Anderson, G.P.; Ellson, C.; Feeney, M.; Modis, L.K.; Smith, J.E.; Hamilton, J.A.; et al. CCL17 in inflammation and pain. J. Immunol. 2020, 205, 213–222. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Cao, D.L.; Zhang, X.; Ji, R.R.; Gao, Y.J. Chemokine contribution to neuropathic pain: Respective induction of CXCL1 and CXCR2 in spinal cord astrocytes and neurons. Pain 2013, 154, 2185–2197. [Google Scholar] [CrossRef] [Green Version]
- Cao, D.L.; Qian, B.; Zhang, Z.J.; Gao, Y.J.; Wu, X.B. Chemokine receptor CXCR2 in dorsal root ganglion contributes to the maintenance of inflammatory pain. Brain Res. Bull. 2016, 127, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Wang, Y.; An, K.; Liu, Q.; Xu, L.; Zhu, C.; Deng, H.; He, Q.; Wang, T.; Xu, M.; et al. Crosstalk between NFκB-dependent astrocytic CXCL1 and neuron CXCR2 plays a role in descending pain facilitation. J. Neuroinflamm. 2019, 16, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.H.; Xu, G.M.; Wang, Y. Up-regulation of CXCL1 and CXCR2 contributes to remifentanil-induced hypernociception via modulating spinal NMDA receptor expression and phosphorylation in rats. Neurosci. Lett. 2016, 626, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Shu, R.; Zhang, L.; Zhang, H.; Li, Y.; Wang, C.; Su, L.; Zhao, H.; Wang, G. NMDA receptor modulates spinal iron accumulation via activating DMT1(-) IRE in remifentanil-induced hyperalgesia. J. Pain 2021, 22, 32–47. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Song, C.; Liu, H.; Li, Y.; Li, J.; Yu, Y.; Wang, G.; Cui, W. Spinal NR2B phosphorylation at Tyr1472 regulates IRE (-) DMT1-mediated iron accumulation and spine morphogenesis via kalirin-7 in tibial fracture-associated postoperative pain after orthopedic surgery in female mice. Reg. Anesth. Pain Med. 2021, 46, 363–373. [Google Scholar] [CrossRef]
- Xu, W.; Liu, W.; Yu, W. The involvement of iron responsive element (-) divalent metal transporter 1-mediated the spinal iron overload via CXCL10/CXCR3 pathway in neuropathic pain in rats. Neurosci. Lett. 2019, 694, 154–160. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.T.; Qin, S.C. Neuroprotective effects of molecular hydrogen: A critical review. Neurosci. Bull. 2021, 37, 389–404. [Google Scholar] [CrossRef]
- Shu, R.; Zhang, L.; Wang, C.; Li, N.; Wang, H.; Xie, K.; Yu, Y.; Wang, G. Spinal peroxynitrite contributes to remifentanil-induced postoperative hyperalgesia via enhancement of divalent metal transporter 1 without iron-responsive element-mediated iron accumulation in rats. Anesthesiology 2015, 122, 908–920. [Google Scholar] [CrossRef]
- Lian, N.; Shen, M.; Zhang, K.; Pan, J.; Jiang, Y.; Yu, Y.; Yu, Y. Drinking hydrogen-rich water alleviates chemotherapy-induced neuropathic pain through the regulation of gut microbiota. J. Pain Res. 2021, 14, 681–691. [Google Scholar] [CrossRef]
- Cui, W.; Li, Y.; Wang, Z.; Song, C.; Yu, Y.; Wang, G.; Li, J.; Wang, C.; Zhang, L. Spinal caspase-6 regulates AMPA receptor trafficking and dendritic spine plasticity through netrin-1 in postoperative pain after orthopedic surgery for tibial fracture in mice. Pain 2021, 162, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shu, R.; Wang, H.; Yu, Y.; Wang, C.; Yang, M.; Wang, M.; Wang, G. Hydrogen-rich saline prevents remifentanil-induced hyperalgesia and inhibits MnSOD nitration via regulation of NR2B-containing NMDA receptor in rats. Neuroscience 2014, 280, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.R.; Jiang, C.; Andriessen, A.S.; Wang, K.; Wang, Z.; Ding, H.; Zhao, J.; Luo, X.; Lee, M.S.; Lei, Y.L.; et al. STING controls nociception via type I interferon signalling in sensory neurons. Nature 2021, 591, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, N.; Zhang, H.; Wang, Y.; Gao, T.; Zhao, Y.; Wang, G.; Yu, Y.; Wang, C.; Li, Y. Artesunate therapy alleviates fracture-associated chronic pain after orthopedic surgery by suppressing CCL21-dependent TREM2/DAP12 inflammatory signaling in mice. Front. Pharmacol. 2022, 13, 894963. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, R.; Wang, X.; Li, Q.; Li, Y.; Jiao, Y.; Zhao, Q.; Guo, S.; Su, L.; Yu, Y.; et al. Spinal CCL1/CCR8 regulates phosphorylation of GluA1-containing AMPA receptor in postoperative pain after tibial fracture and orthopedic surgery in mice. Neurosci. Res. 2020, 154, 20–26. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, H.; Li, N.; Li, J.; Zhang, L. Chronic pain after bone fracture: Current insights into molecular mechanisms and therapeutic strategies. Brain Sci. 2022, 12, 1056. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, H.; Ji, Z. Downregulating lncRNA PVT1 relieves astrocyte overactivation induced neuropathic pain through targeting miR-186-5p/CXCL13/CXCR5 axis. Neurochem. Res. 2021, 46, 1457–1469. [Google Scholar] [CrossRef]
- Li, T.; Wang, H.; Wang, J.; Chen, Y.; Yang, C.; Zhao, M.; Wang, G.; Yang, Z. Annexin 1 inhibits remifentanil-induced hyperalgesia and NMDA receptor phosphorylation via regulating spinal CXCL12/CXCR4 in rats. Neurosci. Res. 2019, 144, 48–55. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, S.B.; Gao, Y.J.; Xing, J.L.; Xian, H.; Li, Z.Z.; Shen, S.N.; Wu, S.X.; Luo, C.; Xie, R.G. Spinal CCL2 promotes pain sensitization by rapid enhancement of NMDA-induced currents through the ERK-GluN2B pathway in mouse Lamina II neurons. Neurosci. Bull. 2020, 36, 1344–1354. [Google Scholar] [CrossRef]
- Cheah, J.H.; Kim, S.F.; Hester, L.D.; Clancy, K.W.; Patterson, S.E.; Papadopoulos, V.; Snyder, S.H. NMDA receptor-nitric oxide transmission mediates neuronal iron homeostasis via the GTPase Dexras1. Neuron 2006, 51, 431–440. [Google Scholar] [CrossRef]
- De La Fuente-Ortega, E.; Plaza-Briceño, W.; Vargas-Robert, S.; Haeger, P. Prenatal ethonal exposure misregulates genes involved in iron homeostasis promoting a maladaptation of iron dependent hippocampal synaptic transmission and plasticity. Front. Pharmacol. 2019, 10, 1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, R.J.; Dexter, D.T.; Crichton, R.R. Iron, Neuroinflammation and neurodegeneration. Int. J. Mol. Sci. 2022, 23, 7267. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Min, L.; Chen, H.; Deng, W.; Tan, M.; Liu, H.; Hou, J. Iron overload in the motor cortex induces neuronal ferroptosis following spinal cord injury. Redox Biol. 2021, 43, 101984. [Google Scholar] [CrossRef]
- Colvin, L.A.; Bull, F.; Hales, T.G. Perioperative opioid analgesia-when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet 2019, 393, 1558–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martyn, J.A.J.; Mao, J.; Bittner, E.A. Opioid tolerance in critical illness. N. Engl. J. Med. 2019, 380, 365–378. [Google Scholar] [CrossRef]
- Imam, M.Z.; Kuo, A.; Ghassabian, S.; Smith, M.T. Progress in understanding mechanisms of opioid-induced gastrointestinal adverse effects and respiratory depression. Neuropharmacology 2018, 131, 238–255. [Google Scholar] [CrossRef]
- Ishitsuka, Y.; Kondo, Y.; Kadowaki, D. Toxicological property of acetaminophen: The dark side of a safe antipyretic/analgesic drug? Biol. Pharm. Bull. 2020, 43, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Wu, G.; Chen, Z.; Wang, P.; Zhao, M.; Fujino, M.; Zhang, C.; Zhou, W.; Hirano, S.I.; Li, X.K.; Zhao, L. Hydrogen inhalation protects hypoxic-ischemic brain damage by attenuating inflammation and apoptosis in neonatal rats. Exp. Biol. Med. 2019, 244, 1017–1027. [Google Scholar] [CrossRef]
- Zhuang, X.; Yu, Y.; Jiang, Y.; Zhao, S.; Wang, Y.; Su, L.; Xie, K.; Yu, Y.; Lu, Y.; Lv, G. Molecular hydrogen attenuates sepsis-induced neuroinflammation through regulation of microglia polarization through an mTOR-autophagy-dependent pathway. Int. Immunopharmacol. 2020, 81, 106287. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, C.; Xie, K.; Meng, X.; Wang, Y.; Yu, Y. Hydrogen-rich saline alleviated the hyperpathia and microglia activation via autophagy mediated inflammasome inactivation in neuropathic pain rats. Neuroscience 2019, 421, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, H.; Jia, Z.; Zhang, L.; Li, Y.; Xu, R.; Wang, C.; Yu, Y. Hydrogen enriched saline alleviates morphine tolerance via inhibiting neuroinflammation, GLT-1, GS nitration and NMDA receptor trafficking and functioning in the spinal cord of rats. Neurosci. Lett. 2021, 755, 135847. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, P.; Liu, C.; Chen, W.; Wang, P.; Jiang, L. Hydrogen-Rich Saline Attenuates Chronic Allodynia after Bone Fractures via Reducing Spinal CXCL1/CXCR2-Mediated Iron Accumulation in Mice. Brain Sci. 2022, 12, 1610. https://doi.org/10.3390/brainsci12121610
Wang Y, Wang P, Liu C, Chen W, Wang P, Jiang L. Hydrogen-Rich Saline Attenuates Chronic Allodynia after Bone Fractures via Reducing Spinal CXCL1/CXCR2-Mediated Iron Accumulation in Mice. Brain Sciences. 2022; 12(12):1610. https://doi.org/10.3390/brainsci12121610
Chicago/Turabian StyleWang, Yanting, Pei Wang, Cuicui Liu, Wei Chen, Pingping Wang, and Lili Jiang. 2022. "Hydrogen-Rich Saline Attenuates Chronic Allodynia after Bone Fractures via Reducing Spinal CXCL1/CXCR2-Mediated Iron Accumulation in Mice" Brain Sciences 12, no. 12: 1610. https://doi.org/10.3390/brainsci12121610
APA StyleWang, Y., Wang, P., Liu, C., Chen, W., Wang, P., & Jiang, L. (2022). Hydrogen-Rich Saline Attenuates Chronic Allodynia after Bone Fractures via Reducing Spinal CXCL1/CXCR2-Mediated Iron Accumulation in Mice. Brain Sciences, 12(12), 1610. https://doi.org/10.3390/brainsci12121610





