An Experimental Study of Subliminal Self-Face Processing in Depersonalization–Derealization Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Settings
2.2. Ethics Approval
2.3. Clinical Assessment
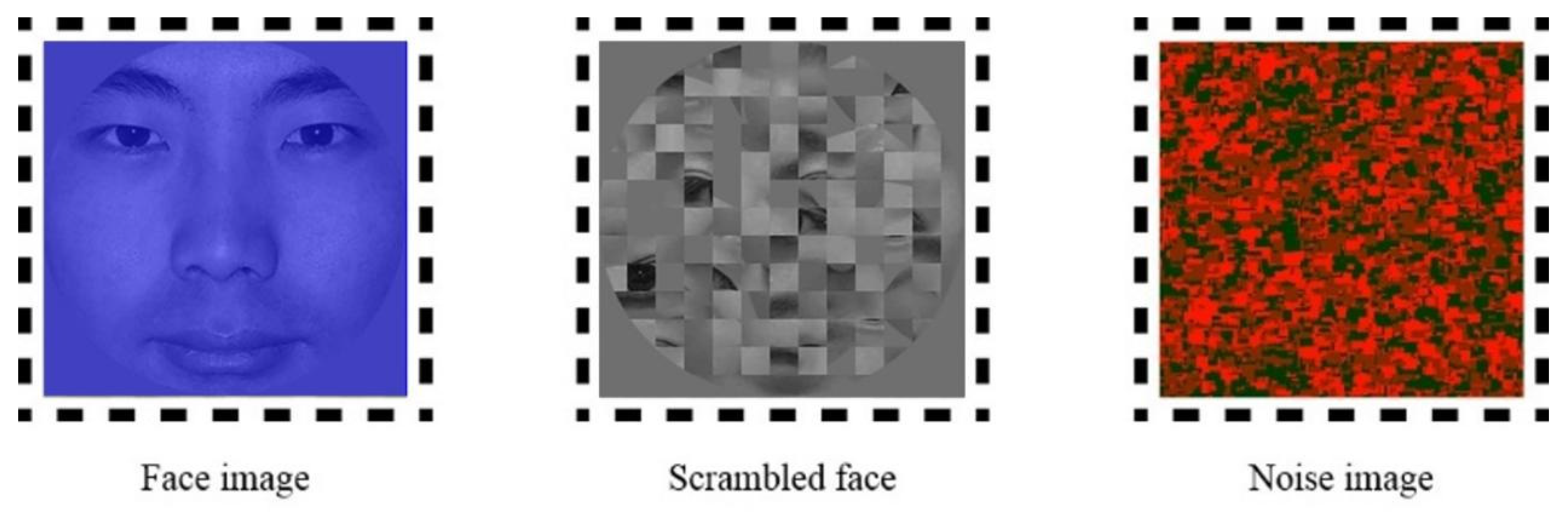
2.4. Experimental Stimuli
2.5. Experimental Procedure
2.6. Statistical Analysis
3. Results
3.1. Demographic Information and Clinical Data
3.2. The Comparison of Reaction Times between DPD and HC in Recognizing Different Faces
3.3. Correlation Analysis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spiegel, D.; Lewis-Fernández, R.; Lanius, R.; Vermetten, E.; Simeon, D.; Friedman, M. Dissociative disorders in DSM-5. Annu. Rev. Clin. Psychol. 2013, 9, 299–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millman, L.S.M.; Hunter, E.C.M.; Orgs, G.; David, A.S.; Terhune, D.B. Symptom variability in depersonalization-derealization disorder: A latent profile analysis. J. Clin. Psychol. 2022, 78, 637–655. [Google Scholar] [CrossRef]
- Heydrich, L.; Marillier, G.; Evans, N.; Seeck, M.; Blanke, O. Depersonalization- and derealization-like phenomena of epileptic origin. Ann. Clin. Transl. Neurol. 2019, 6, 1739–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adler, J.; Schabinger, N.; Michal, M.; Beutel, M.E.; Gillmeister, H. Is that me in the mirror? Depersonalisation modulates tactile mirroring mechanisms. Neuropsychologia 2016, 85, 148–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierra, M.; David, A.S. Depersonalization: A selective impairment of self-awareness. Conscious Cogn. 2011, 20, 99–108. [Google Scholar] [CrossRef]
- Turk, D.J.; Heatherton, T.F.; Kelley, W.M.; Funnell, M.G.; Gazzaniga, M.S.; Macrae, C.N. Mike or me? Self-recognition in a split-brain patient. Nat. Neurosci. 2002, 5, 841–842. [Google Scholar] [CrossRef]
- Rosa, C.; Lassonde, M.; Pinard, C.; Keenan, J.P.; Belin, P. Investigations of hemispheric specialization of self-voice recognition. Brain Cogn. 2008, 68, 204–214. [Google Scholar] [CrossRef]
- Medford, N.; Sierra, M.; Stringaris, A.; Giampietro, V.; Brammer, M.J.; David, A.S. Emotional Experience and Awareness of Self: Functional MRI Studies of Depersonalization Disorder. Front. Psychol. 2016, 7, 432. [Google Scholar] [CrossRef] [Green Version]
- Bortolon, C.; Raffard, S. Self-face advantage over familiar and unfamiliar faces: A three-level meta-analytic approach. Psychon. Bull. Rev. 2018, 25, 1287–1300. [Google Scholar] [CrossRef]
- Gallup, G.G., Jr. Self-awareness and the evolution of social intelligence. Behav. Processes 1998, 42, 239–247. [Google Scholar] [CrossRef]
- Tacikowski, P.; Jednoróg, K.; Marchewka, A.; Nowicka, A. How multiple repetitions influence the processing of self-, famous and unknown names and faces: An ERP study. Int. J. Psychophysiol. 2011, 79, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M. Three faces of self-face recognition: Potential for a multi-dimensional diagnostic tool. Neurosci. Res. 2015, 90, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Sass, L.; Pienkos, E.; Nelson, B.; Medford, N. Anomalous self-experience in depersonalization and schizophrenia: A comparative investigation. Conscious Cogn. 2013, 22, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Værnes, T.G.; Røssberg, J.I.; Møller, P. Anomalous Self-Experiences: Markers of Schizophrenia Vulnerability or Symptoms of Depersonalization Disorder? A Phenomenological Investigation of Two Cases. Psychopathology 2018, 51, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Millman, L.S.M.; David, A.S.; Hunter, E.C.M. The Prevalence of Depersonalization-Derealization Disorder: A Systematic Review. J. Trauma Dissociation 2022, 1–34. [Google Scholar] [CrossRef]
- Ketay, S.; Hamilton, H.K.; Haas, B.W.; Simeon, D. Face processing in depersonalization: An fMRI study of the unfamiliar self. Psychiatry Res. 2014, 222, 107–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farmer, H.; Cataldo, A.; Adel, N.; Wignall, E.; Gallese, V.; Deroy, O.; Hamilton, A.; Ciaunica, A. The Detached Self: Investigating the Effect of Depersonalisation on Self-Bias in the Visual Remapping of Touch. Multisens. Res. 2020, 34, 365–386. [Google Scholar] [CrossRef]
- Ma, Y.; Han, S. Why we respond faster to the self than to others? An implicit positive association theory of self-advantage during implicit face recognition. J. Exp. Psychol. Hum. Percept. Perform. 2010, 36, 619–633. [Google Scholar] [CrossRef] [Green Version]
- Gayet, S.; Stein, T. Between-Subject Variability in the Breaking Continuous Flash Suppression Paradigm: Potential Causes, Consequences, and Solutions. Front. Psychol. 2017, 8, 437. [Google Scholar] [CrossRef] [Green Version]
- Stein, T.; Siebold, A.; van Zoest, W. Testing the idea of privileged awareness of self-relevant information. J. Exp. Psychol. Hum. Percept. Perform. 2016, 42, 303–307. [Google Scholar] [CrossRef]
- Stein, T.; Hebart, M.N.; Sterzer, P. Breaking Continuous Flash Suppression: A New Measure of Unconscious Processing during Interocular Suppression? Front. Hum. Neurosci. 2011, 5, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Xu, Y.; Wang, N.; Zhang, S.; Geng, H.; Jia, H. Deficits of subliminal self-face processing in schizophrenia. Conscious Cogn. 2020, 79, 102896. [Google Scholar] [CrossRef] [PubMed]
- Sierra, M.; Gomez, J.; Molina, J.J.; Luque, R.; Muñoz, J.F.; David, A.S. Depersonalization in psychiatric patients: A transcultural study. J. Nerv. Ment. Dis. 2006, 194, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yuan, Y.; Gong, W.; Zhang, J.; Liu, X.; Zhu, P.; Takashi, E.; Kitayama, A.; Wan, X.; Jiao, J. Reliability and validity of the Chinese version of Oldenburg Burnout Inventory for Chinese nurses. Nurs. Open 2022, 9, 320–328. [Google Scholar] [CrossRef]
- Cheng, S.-T.; Hamid, P.N. An error in the use of translated scales: The Rosenberg Self-Esteem Scale for Chinese. Percept. Mot. Ski. 1995, 81, 431–434. [Google Scholar] [CrossRef]
- Cui, C.; Wang, L.; Wang, X. Effects of Self-Esteem on the Associations Between Infertility-Related Stress and Psychological Distress Among Infertile Chinese Women: A Cross-Sectional Study. Psychol. Res. Behav. Manag. 2021, 14, 1245–1255. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, S.; Geng, H. Gaze-induced joint attention persists under high perceptual load and does not depend on awareness. Vision Res. 2011, 51, 2048–2056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchiya, N.; Koch, C. Continuous flash suppression reduces negative afterimages. Nat. Neurosci. 2005, 8, 1096–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bola, M.; Paź, M.; Doradzińska, Ł.; Nowicka, A. The self-face captures attention without consciousness: Evidence from the N2pc ERP component analysis. Psychophysiology 2021, 58, e13759. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, H.; Xu, M.; Jia, H.; Liu, J. Selective impairment in recognizing the familiarity of self faces in schizophrenia. Chin. Sci. Bull. 2012, 57, 6. [Google Scholar] [CrossRef]
- Buhrmester, M.D.; Blanton, H.; Swann, W.B., Jr. Implicit self-esteem: Nature, measurement, and a new way forward. J. Pers. Soc. Psychol. 2011, 100, 365–385. [Google Scholar] [CrossRef] [Green Version]
- Michal, M.; Kaufhold, J.; Overbeck, G.; Grabhorn, R. Narcissistic regulation of the self and interpersonal problems in depersonalized patients. Psychopathology 2006, 39, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, A.N.; Berlin, H.A. Implicit self-esteem in borderline personality and depersonalization disorder. Front. Psychol. 2012, 3, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, H.; Zhang, S.; Li, Q.; Tao, R.; Xu, S. Dissociations of subliminal and supraliminal self-face from other-face processing: Behavioral and ERP evidence. Neuropsychologia 2012, 50, 2933–2942. [Google Scholar] [CrossRef] [PubMed]
- Ota, C.; Nakano, T. Self-Face Activates the Dopamine Reward Pathway without Awareness. Cereb. Cortex 2021, 31, 4420–4426. [Google Scholar] [CrossRef]
- Tsakiris, M. Looking for myself: Current multisensory input alters self-face recognition. PLoS ONE 2008, 3, e4040. [Google Scholar] [CrossRef]
- Bortolon, C.; Capdevielle, D.; Salesse, R.N.; Raffard, S. Further insight into self-face recognition in schizophrenia patients: Why ambiguity matters. J. Behav. Ther. Exp. Psychiatry 2016, 50, 215–222. [Google Scholar] [CrossRef]
- Michal, M.; Reuchlein, B.; Adler, J.; Reiner, I.; Beutel, M.E.; Vögele, C.; Schächinger, H.; Schulz, A. Striking discrepancy of anomalous body experiences with normal interoceptive accuracy in depersonalization-derealization disorder. PLoS ONE 2014, 9, e89823. [Google Scholar] [CrossRef]
- Sedeño, L.; Couto, B.; Melloni, M.; Canales-Johnson, A.; Yoris, A.; Baez, S.; Esteves, S.; Velásquez, M.; Barttfeld, P.; Sigman, M.; et al. How do you feel when you can't feel your body? Interoception, functional connectivity and emotional processing in depersonalization-derealization disorder. PLoS ONE 2014, 9, e98769. [Google Scholar] [CrossRef]
- Simeon, D. Depersonalisation disorder: A contemporary overview. CNS Drugs 2004, 18, 343–354. [Google Scholar] [CrossRef]
- Sierk, A.; Daniels, J.K.; Manthey, A.; Kok, J.G.; Leemans, A.; Gaebler, M.; Lamke, J.P.; Kruschwitz, J.; Walter, H. White matter network alterations in patients with depersonalization/derealization disorder. J. Psychiatry Neurosci. 2018, 43, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Sierra, M.; Berrios, G.E. Depersonalization: Neurobiological perspectives. Biol. Psychiatry 1998, 44, 898–908. [Google Scholar] [CrossRef]
- Daniels, J.K.; Gaebler, M.; Lamke, J.P.; Walter, H. Grey matter alterations in patients with depersonalization disorder: A voxel-based morphometry study. J. Psychiatry Neurosci. 2015, 40, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, A.A.; Densmore, M.; Frewen, P.A.; Théberge, J.; Neufeld, R.W.; McKinnon, M.C.; Lanius, R.A. The Dissociative Subtype of Posttraumatic Stress Disorder: Unique Resting-State Functional Connectivity of Basolateral and Centromedial Amygdala Complexes. Neuropsychopharmacology 2015, 40, 2317–2326. [Google Scholar] [CrossRef] [Green Version]
- Tsakiris, M. My body in the brain: A neurocognitive model of body-ownership. Neuropsychologia 2010, 48, 703–712. [Google Scholar] [CrossRef]
- Farrer, C.; Frith, C.D. Experiencing oneself vs. another person as being the cause of an action: The neural correlates of the experience of agency. Neuroimage 2002, 15, 596–603. [Google Scholar] [CrossRef] [Green Version]
- Craig, A.D. How do you feel—Now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef]
- Baier, B.; Karnath, H.O. Tight link between our sense of limb ownership and self-awareness of actions. Stroke 2008, 39, 486–488. [Google Scholar] [CrossRef]



| Variables | DPD (n = 23) | HC (n = 23) | t/χ2 | p |
|---|---|---|---|---|
| Gender (Male/Female) | 14/9 | 8/15 | 3.136 | 0.139 |
| Age (years) | 25.87 ± 7.24 | 25.52 ± 2.50 | −0.218 | 0.829 |
| Education (years) | 14.39 ± 2.21 | 15.30 ± 1.55 | 1.622 | 0.113 |
| Duration of DPD (months) | 7.43 ± 7.84 | N/A | N/A | N/A |
| CDS | 154.61 ± 50.52 | N/A | N/A | N/A |
| SES | 27.73 ± 3.11 | 31.43 ± 3.65 | 3.695 | 0.001 * |
| Task (Face Stimuli) | Block | DPD | HC |
|---|---|---|---|
| Self-face | Experimental condition | 1.76 ± 0.66 | 1.28 ± 0.32 |
| Control condition | 1.38 ± 0.35 | 1.27 ± 0.50 | |
| Famous face | Experimental condition | 1.74 ± 0.67 | 1.41 ± 0.33 |
| Control condition | 1.43 ± 0.53 | 1.27 ± 0.40 | |
| Stranger's face | Experimental condition | 1.88 ± 0.54 | 1.56 ± 0.47 |
| Control condition | 1.72 ± 0.52 | 1.50 ± 0.64 |
| Self-Face | ||||
|---|---|---|---|---|
| Control Condition | Experimental Condition | |||
| r | p | r | p | |
| Age | 0.07 | 0.77 | 0.11 | 0.63 |
| Duration of DPD | −0.16 | 0.46 | −0.04 | 0.85 |
| CDS score | 0.08 | 0.71 | 0.04 | 0.87 |
| SES score | 0.14 | 0.54 | 0.23 | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Jia, Y.; Zheng, S.; Feng, S.; Zhu, H.; Wang, R.; Jia, H. An Experimental Study of Subliminal Self-Face Processing in Depersonalization–Derealization Disorder. Brain Sci. 2022, 12, 1598. https://doi.org/10.3390/brainsci12121598
Liu S, Jia Y, Zheng S, Feng S, Zhu H, Wang R, Jia H. An Experimental Study of Subliminal Self-Face Processing in Depersonalization–Derealization Disorder. Brain Sciences. 2022; 12(12):1598. https://doi.org/10.3390/brainsci12121598
Chicago/Turabian StyleLiu, Shanshan, Yuan Jia, Sisi Zheng, Sitong Feng, Hong Zhu, Rui Wang, and Hongxiao Jia. 2022. "An Experimental Study of Subliminal Self-Face Processing in Depersonalization–Derealization Disorder" Brain Sciences 12, no. 12: 1598. https://doi.org/10.3390/brainsci12121598
APA StyleLiu, S., Jia, Y., Zheng, S., Feng, S., Zhu, H., Wang, R., & Jia, H. (2022). An Experimental Study of Subliminal Self-Face Processing in Depersonalization–Derealization Disorder. Brain Sciences, 12(12), 1598. https://doi.org/10.3390/brainsci12121598






