Neuromorphic-Based Neuroprostheses for Brain Rewiring: State-of-the-Art and Perspectives in Neuroengineering
Abstract
1. Introduction
2. When Neuroscience Meets Engineering
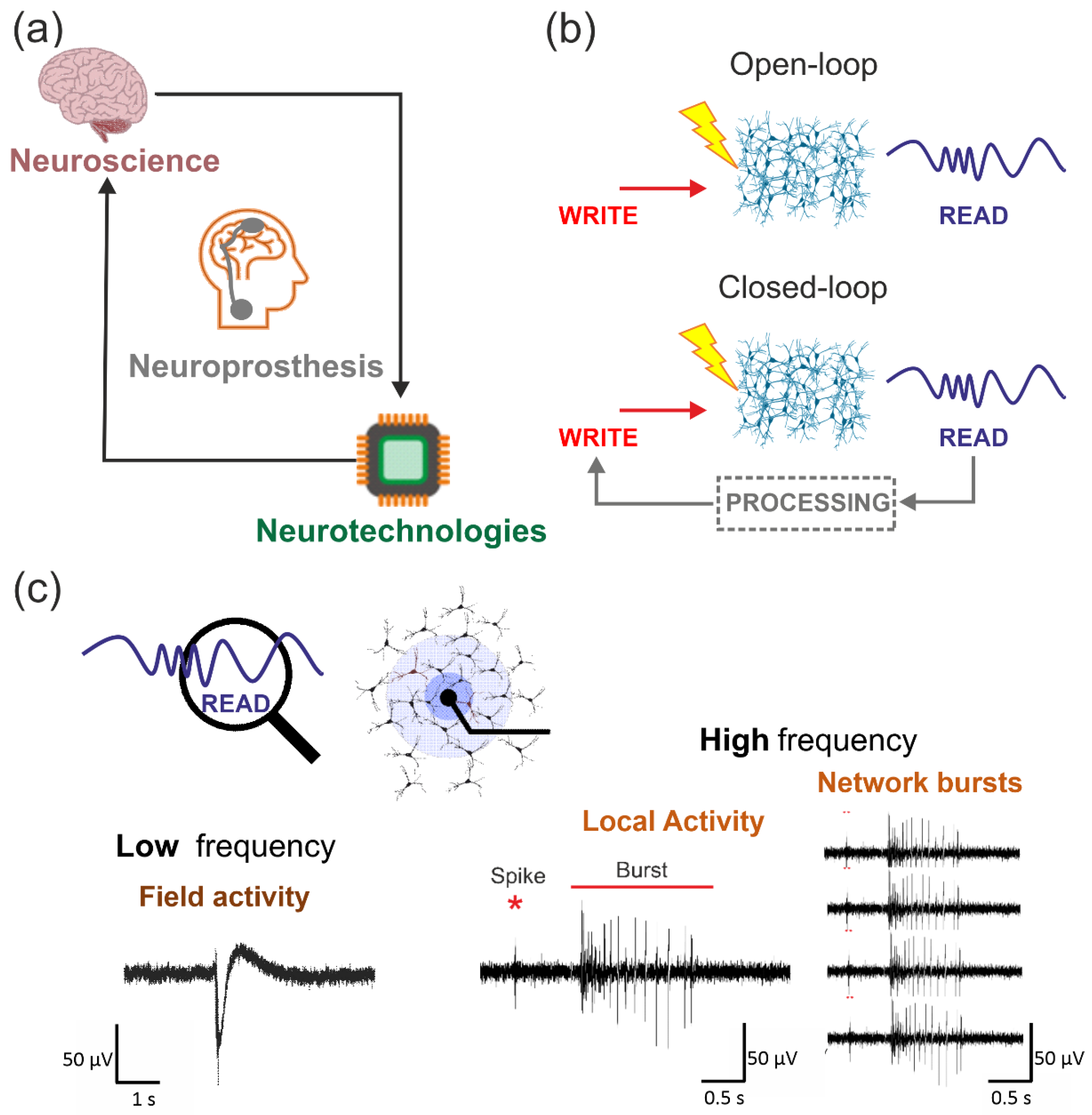
2.1. Neuroprosthetics: A Definition
2.2. Communication Modalities
2.3. Biohybrid Systems
3. Neuromorphic Neuroprostheses: State-of-the-Art and Perspectives
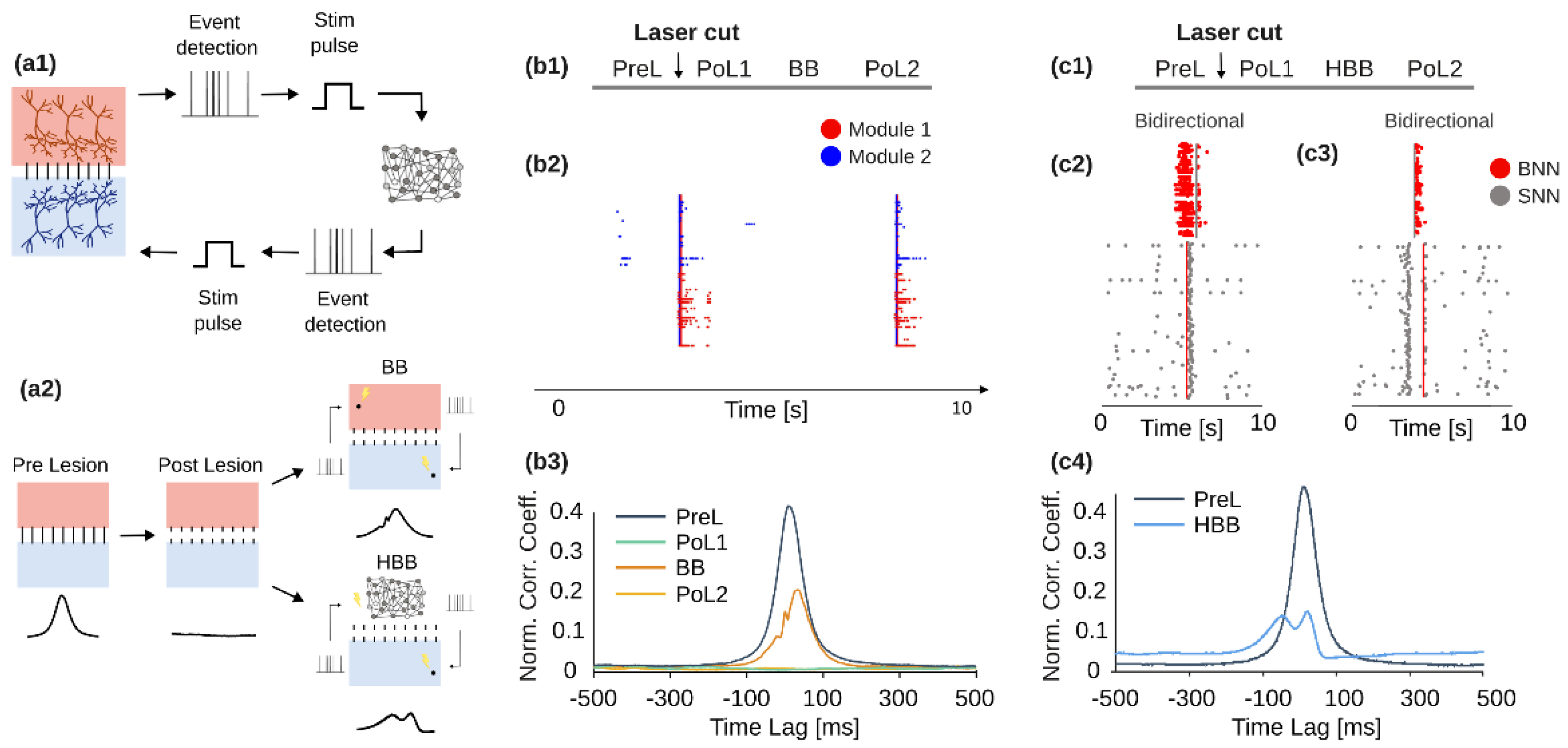
3.1. BrainBow, a Novel Bi-Directional Neuromorphic In Vitro Sytem for Brain Rewiring
3.2. Neuromorphic Neural Interfaces
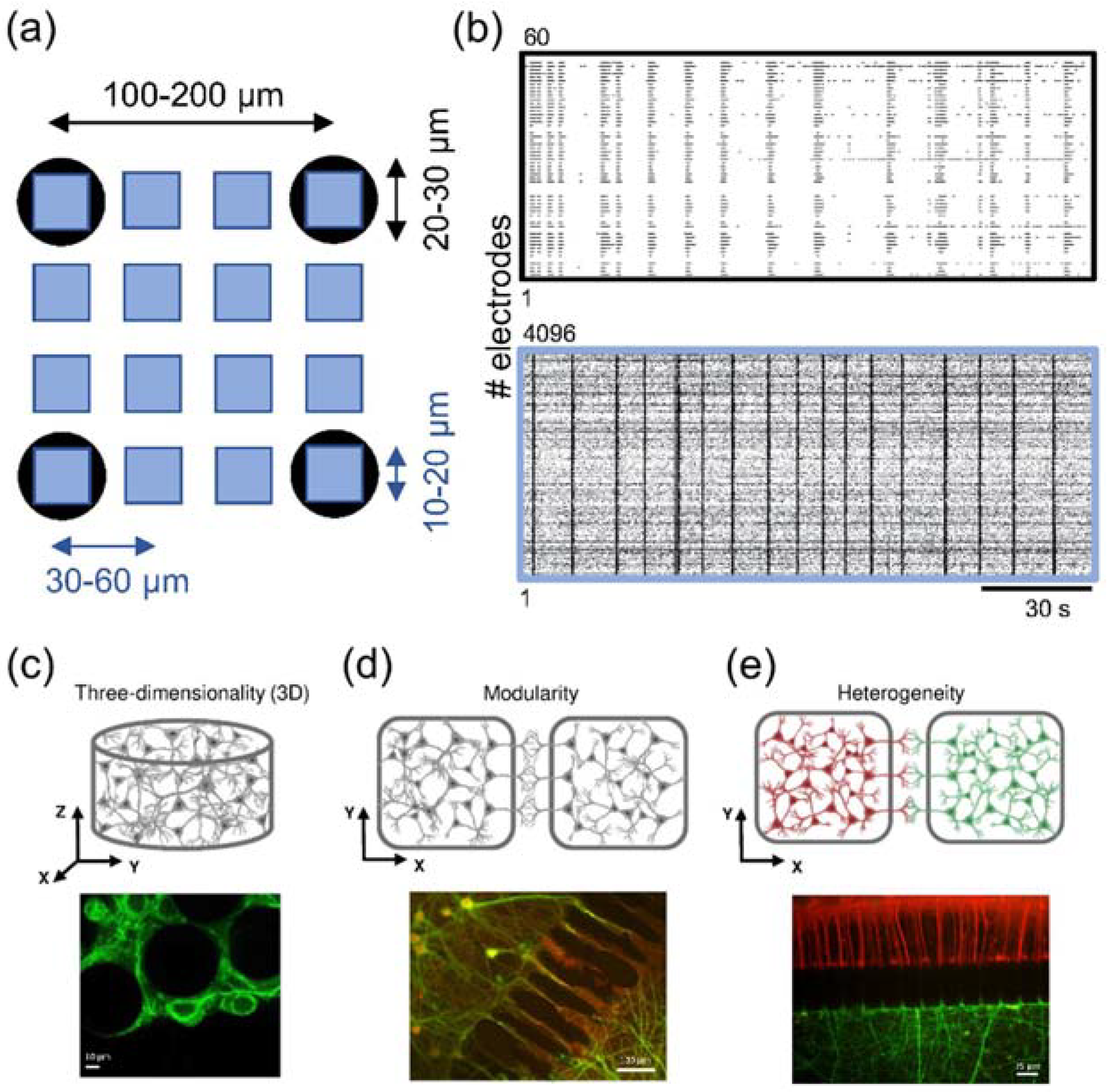
3.2.1. High-Density MEA-Based Recording and Stimulation
3.2.2. Optogenetics Recording and Stimulation
3.3. Neuromorphic Processing of Neural Signals
3.3.1. Detection and Classification
3.3.2. Mimicking Biological Dynamics
3.4. Towards Translation of Neuromorphic Prostheses
3.4.1. In Vitro Experiments
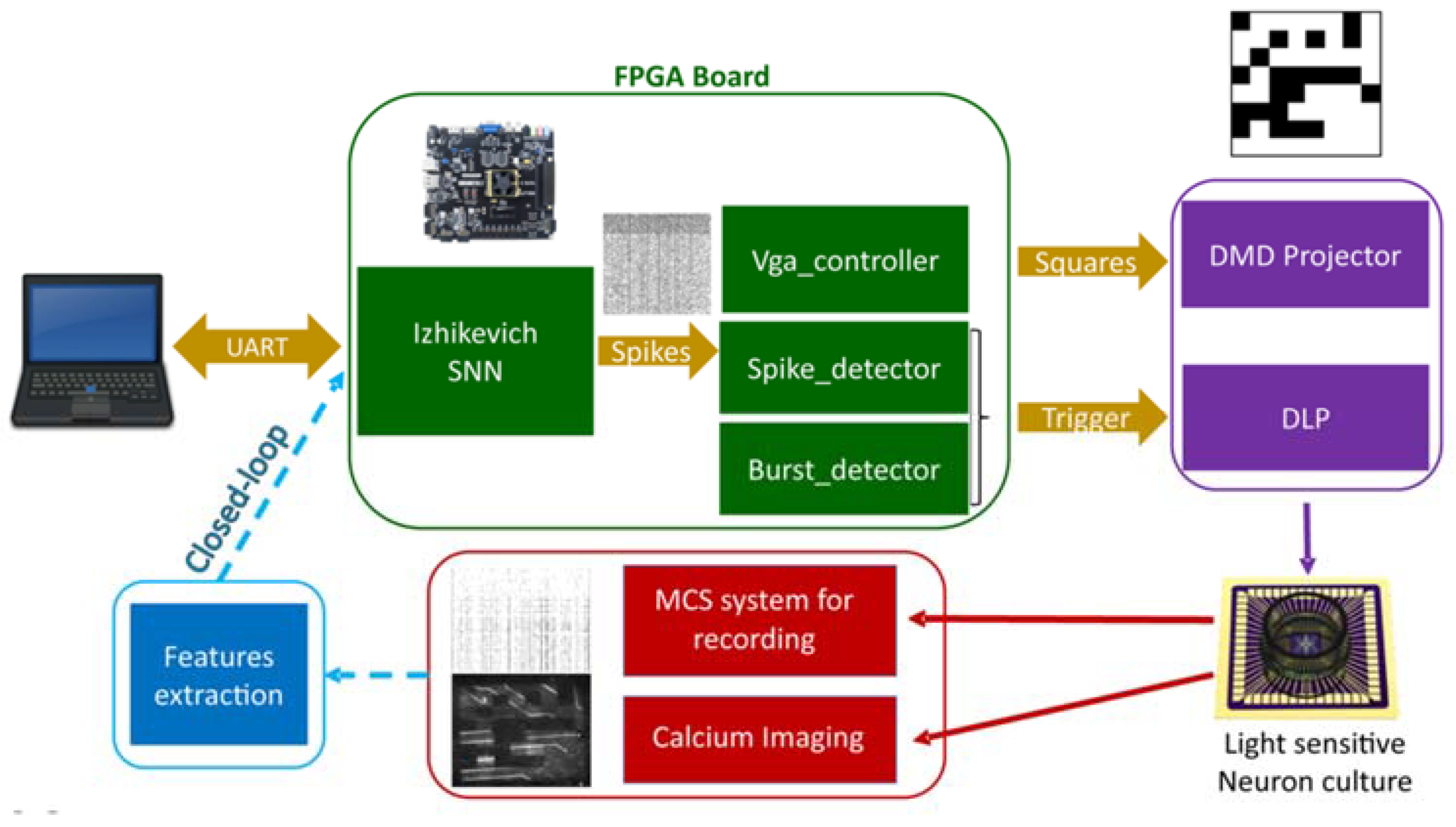
3.4.2. In Vivo Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panuccio, G.; Semprini, M.; Natale, L.; Buccelli, S.; Colombi, I.; Chiappalone, M. Progress in Neuroengineering for brain repair: New challenges and open issues. Brain Neurosci. Adv. 2018, 2, 2398212818776475. [Google Scholar] [CrossRef]
- Chin, J.H.; Vora, N. The global burden of neurologic diseases. Neurology 2014, 83, 349–351. [Google Scholar] [CrossRef] [PubMed]
- French, B.; Thomas, L.H.; Coupe, J.; McMahon, N.E.; Connell, L.; Harrison, J.; Sutton, C.J.; Tishkovskaya, S.; Watkins, C.L. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst. Rev. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- WHO, The Top 10 Causes of Death. World Health Oraganization, 9 December 2020.
- Catani, M.; Ffytche, D.H. The rises and falls of disconnection syndromes. Brain 2005, 128, 2224–2239. [Google Scholar] [CrossRef] [PubMed]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Kenzie, E.S.; Parks, E.L.; Bigler, E.D.; Lim, M.M.; Chesnutt, J.C.; Wakeland, W. Concussion as a multi-scale complex system: An interdisciplinary synthesis of current knowledge. Front. Neurol. 2017, 8, 513. [Google Scholar] [CrossRef]
- Kane, E.; Ward, N.S. Neurobiology of Stroke Recovery. In Clinical Pathways in Stroke Rehabilitation; Springer: Cham, Switzerland, 2021; pp. 1–13. [Google Scholar]
- Brewer, L.; Horgan, F.; Hickey, A.; Williams, D. Stroke rehabilitation: Recent advances and future therapies. QJM Int. J. Med. 2013, 106, 11–25. [Google Scholar] [CrossRef]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef]
- Rust, R.; Grönnert, L.; Gantner, C.; Enzler, A.; Mulders, G.; Weber, R.Z.; Siewert, A.; Limasale, Y.D.; Meinhardt, A.; Maurer, M.A. Nogo-A targeted therapy promotes vascular repair and functional recovery following stroke. Proc. Natl. Acad. Sci. USA 2019, 116, 14270–14279. [Google Scholar] [CrossRef]
- Semprini, M.; Laffranchi, M.; Sanguineti, V.; Avanzino, L.; De Icco, R.; De Michieli, L.; Chiappalone, M. Technological Approaches for Neurorehabilitation: From robotic Devices to Brain stimulation and Beyond. Front. Neurol. 2018, 9, 212. [Google Scholar] [CrossRef]
- Panuccio, G.; Semprini, M.; Chiappalone, M. Intelligent biohybrid systems for functional brain repair. New Horiz. Transl. Med. 2016, 3, 162–174. [Google Scholar] [CrossRef]
- Reardon, S. Electroceuticals spark interest. Nature 2014, 511, 18. [Google Scholar] [CrossRef]
- Famm, K.; Litt, B.; Tracey, K.J.; Boyden, E.S.; Slaoui, M. Drug discovery: A jump-start for electroceuticals. Nature 2013, 496, 159. [Google Scholar] [CrossRef] [PubMed]
- Vassanelli, S.; Mahmud, M. Trends and Challenges in neuroengineering: Toward “intelligent” neuroprostheses through brain-“brain inspired systems” communication. Front. Neurosci. 2016, 10, 438. [Google Scholar] [CrossRef]
- George, R.; Chiappalone, M.; Giugliano, M.; Levi, T.; Vassanelli, S.; Partzsch, J.; Mayr, C. Plasticity and Adaptation in Neuromorphic Biohybrid Systems. Iscience 2020, 23, 101589. [Google Scholar] [CrossRef]
- Broccard, F.D.; Joshi, S.; Wang, J.; Cauwenberghs, G. Neuromorphic neural interfaces: From neurophysiological inspiration to biohybrid coupling with nervous systems. J. Neural Eng. 2017, 14, 041002. [Google Scholar] [CrossRef] [PubMed]
- Sharifshazileh, M.; Burelo, K.; Sarnthein, J.; Indiveri, G. An electronic neuromorphic system for real-time detection of high frequency oscillations (HFO) in intracranial EEG. Nat. Commun. 2021, 12, 3095. [Google Scholar] [CrossRef]
- Corradi, F.; Indiveri, G. A neuromorphic event-based neural recording system for smart brain-machine-interfaces. IEEE Trans. Biomed. Circuits Syst. 2015, 9, 699–709. [Google Scholar] [CrossRef]
- Christensen, D.V.; Dittmann, R.; Linares-Barranco, B.; Sebastian, A.; Le Gallo, M.; Redaelli, A.; Slesazeck, S.; Mikolajick, T.; Spiga, S.; Menzel, S.; et al. 2022 roadmap on neuromorphic computing and engineering. Neuromorphic Comput. Eng. 2022, 2, 022501. [Google Scholar] [CrossRef]
- Betzel, R.F.; Bassett, D.S. Multi-scale brain networks. Neuroimage 2017, 160, 73–83. [Google Scholar] [CrossRef]
- Durand, D.M. Neural engineering. Methods Inf. Med. 2007, 46, 142–146. [Google Scholar] [CrossRef]
- Hetling, J. Comment on ‘What is neural engineering?’. J. Neural Eng. 2008, 5, 360. [Google Scholar] [CrossRef] [PubMed]
- Zrenner, C.; Belardinelli, P.; Müller-Dahlhaus, F.; Ziemann, U. Closed-loop neuroscience and non-invasive brain stimulation: A tale of two loops. Front. Cell. Neurosci. 2016, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.; Macefield, V.G.; van Schaik, A.; Tapson, J.C. A review of control strategies in closed-loop neuroprosthetic systems. Front. Neurosci. 2016, 10, 312. [Google Scholar] [CrossRef]
- Loeb, G.; Byers, C.; Rebscher, S.; Casey, D.; Fong, M.; Schindler, R.; Gray, R.; Merzenich, M. Design and fabrication of an experimental cochlear prosthesis. Med. Biol. Eng. Comput. 1983, 21, 241–254. [Google Scholar] [CrossRef]
- Humayun, M.S. Intraocular retinal prosthesis. Trans. Am. Ophthalmol. Soc. 2001, 99, 271. [Google Scholar] [PubMed]
- Farina, D.; Vujaklija, I.; Brånemark, R.; Bull, A.M.; Dietl, H.; Graimann, B.; Hargrove, L.J.; Hoffmann, K.-P.; Huang, H.H.; Ingvarsson, T. Toward higher-performance bionic limbs for wider clinical use. Nat. Biomed. Eng. 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bouton, C.E.; Shaikhouni, A.; Annetta, N.V.; Bockbrader, M.A.; Friedenberg, D.A.; Nielson, D.M.; Sharma, G.; Sederberg, P.B.; Glenn, B.C.; Mysiw, W.J. Restoring cortical control of functional movement in a human with quadriplegia. Nature 2016, 533, 247. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Fifer, M.; Bickel, S.; Osborn, L.; Herrero, J.; Christie, B.; Xu, J.; Murphy, R.K.; Singh, S.; Glasser, M.F. Historical perspectives, challenges, and future directions of implantable brain-computer interfaces for sensorimotor applications. Bioelectron. Med. 2021, 7, 14. [Google Scholar] [CrossRef]
- Bouton, C.E.; Annetta, N.; Friedenberg, D.A.; Sharma, G. Systems and Methods for Neural Bridging of the Nervous System. U.S. Patent 10,857,358, 8 December 2020. [Google Scholar]
- Berger, T.W.; Hampson, R.E.; Song, D.; Goonawardena, A.; Marmarelis, V.Z.; Deadwyler, S.A. A cortical neural prosthesis for restoring and enhancing memory. J. Neural Eng. 2011, 8, 046017. [Google Scholar] [CrossRef]
- Hampson, R.E.; Gerhardt, G.A.; Marmarelis, V.; Song, D.; Opris, I.; Santos, L.; Berger, T.W.; Deadwyler, S.A. Facilitation and restoration of cognitive function in primate prefrontal cortex by a neuroprosthesis that utilizes minicolumn-specific neural firing. J. Neural Eng. 2012, 9, 056012. [Google Scholar] [CrossRef]
- Hampson, R.E.; Song, D.; Robinson, B.S.; Fetterhoff, D.; Dakos, A.S.; Roeder, B.M.; She, X.; Wicks, R.T.; Witcher, M.R.; Couture, D.E. Developing a hippocampal neural prosthetic to facilitate human memory encoding and recall. J. Neural Eng. 2018, 15, 036014. [Google Scholar] [CrossRef]
- Greenwald, E.; Masters, M.R.; Thakor, N.V. Implantable neurotechnologies: Bidirectional neural interfaces—Applications and VLSI circuit implementations. Med. Biol. Eng. Comput. 2016, 54, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zanos, S. Closed-loop neuromodulation in physiological and translational research. Cold Spring Harb. Perspect. Med. 2019, 9, a034314. [Google Scholar] [CrossRef]
- Jackson, A.; Mavoori, J.; Fetz, E.E. Long-term motor cortex plasticity induced by an electronic neural implant. Nature 2006, 444, 56. [Google Scholar] [CrossRef]
- Guggenmos, D.J.; Azin, M.; Barbay, S.; Mahnken, J.D.; Dunham, C.; Mohseni, P.; Nudo, R.J. Restoration of function after brain damage using a neural prosthesis. Proc. Natl. Acad. Sci. USA 2013, 110, 21177–21182. [Google Scholar] [CrossRef] [PubMed]
- Fetz, E.E.; Finocchio, D.V. Operant conditioning of specific patterns of neural and muscular activity. Science 1971, 174, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Kohler, F.; Gkogkidis, C.A.; Bentler, C.; Wang, X.; Gierthmuehlen, M.; Fischer, J.; Stolle, C.; Reindl, L.M.; Rickert, J.; Stieglitz, T.; et al. Closed-loop interaction with the cerebral cortex: A review of wireless implant technology. Brain-Comput. Interfaces 2017, 4, 146–154. [Google Scholar] [CrossRef]
- Le Masson, G.; Le Masson, S.; Moulins, M. From conductances to neural network properties: Analysis of simple circuits using the hybrid network method. Prog. Biophys. Mol. Biol. 1995, 64, 201–220. [Google Scholar] [CrossRef]
- Joucla, S.; Ambroise, M.; Levi, T.; Lafon, T.; Chauvet, P.; Saïghi, S.; Bornat, Y.; Lewis, N.; Renaud, S.; Yvert, B. Generation of locomotor-like activity in the isolated rat spinal cord using intraspinal electrical microstimulation driven by a digital neuromorphic CPG. Front. Neurosci. 2016, 10, 67. [Google Scholar] [CrossRef]
- Bisio, M.; Pimashkin, A.; Buccelli, S.; Tessadori, J.; Semprini, M.; Levi, T.; Colombi, I.; Gladkov, A.; Mukhina, I.; Averna, A. Closed-Loop Systems and In Vitro Neuronal Cultures: Overview and Applications. In Vitro. Neuronal. Netw. 2019, 22, 351–387. [Google Scholar]
- Orlandi, J.G.; Soriano, J.; Alvarez-Lacalle, E.; Teller, S.; Casademunt, J. Noise focusing and the emergence of coherent activity in neuronal cultures. Nat. Phys. 2013, 9, 582. [Google Scholar] [CrossRef]
- Potter, S.M. Closing the loop between neurons and neurotechnology. Front. Neurosci. 2010, 4, 15. [Google Scholar] [CrossRef]
- Buccelli, S.; Bornat, Y.; Colombi, I.; Ambroise, M.; Martines, L.; Pasquale, V.; Bisio, M.; Tessadori, J.; Nowak, P.; Grassia, F. A neuromorphic prosthesis to restore communication in neuronal networks. iScience 2019, 19, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Bonifazi, P.; Difato, F.; Massobrio, P.; Breschi, G.L.; Pasquale, V.; Levi, T.; Goldin, M.; Bornat, Y.; Tedesco, M.; Bisio, M.; et al. In Vitro large-scale experimental and theoretical studies for the realization of bi-directional brain-prostheses. Front. Neural Circuits 2013, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Sharp, A.A.; O’Neil, M.B.; Abbott, L.; Marder, E. The dynamic clamp: Artificial conductances in biological neurons. Trends Neurosci. 1993, 16, 389–394. [Google Scholar] [CrossRef]
- Sharp, A.A.; O’Neil, M.B.; Abbott, L.F.; Marder, E. Dynamic clamp: Computer-generated conductances in real neurons. J. Neurophysiol. 1993, 69, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Bisio, M.; Bosca, A.; Pasquale, V.; Berdondini, L.; Chiappalone, M. Emergence of bursting activity in connected neuronal sub-populations. PLoS ONE 2014, 9, e107400. [Google Scholar] [CrossRef]
- Soloperto, A.; Bisio, M.; Palazzolo, G.; Chiappalone, M.; Bonifazi, P.; Difato, F. Modulation of neural network activity through single cell ablation: An in vitro model of minimally invasive neurosurgery. Molecules 2016, 21, 1018. [Google Scholar] [CrossRef]
- Pasquale, V.; Martinoia, S.; Chiappalone, M. A self-adapting approach for the detection of bursts and network bursts in neuronal cultures. J. Comput. Neurosci. 2010, 29, 213–229. [Google Scholar] [CrossRef]
- Pasquale, V.; Martinoia, S.; Chiappalone, M. Stimulation triggers endogenous activity patterns in cultured cortical networks. Sci. Rep. 2017, 7, 9080. [Google Scholar] [CrossRef] [PubMed]
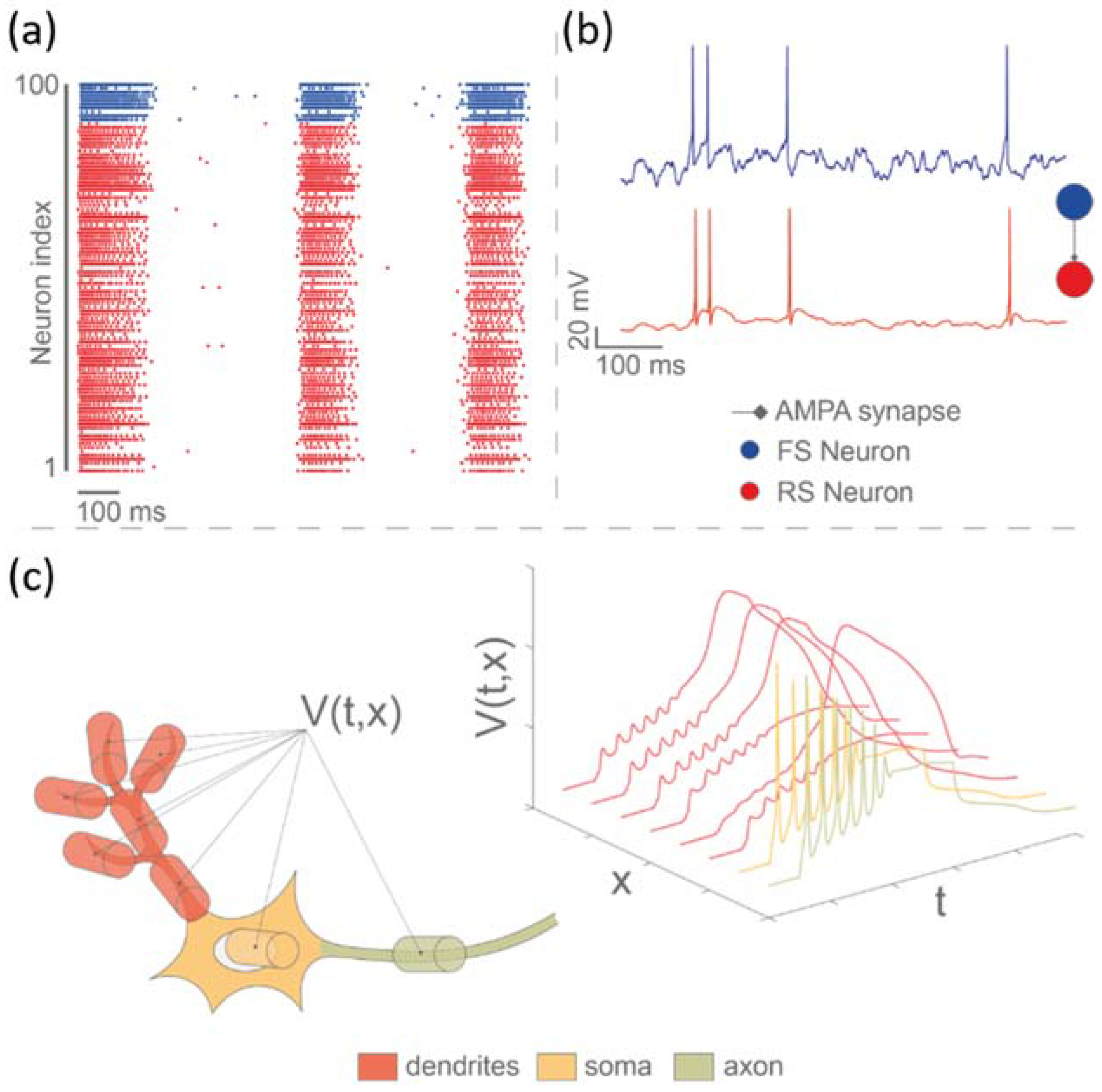
- Beaubois, R.; Khoyratee, F.; Branchereau, P.; Ikeuchi, Y.; Levi, T. From real-time single to multicompartmental Hodgkin-Huxley neurons on FPGA for bio-hybrid systems. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glassgow, UK, 11–15 July 2022; pp. 1602–1606. [Google Scholar]
- Yang, S.; Wang, J.; Hao, X.; Li, H.; Wei, X.; Deng, B.; Loparo, K.A. BiCoSS: Toward large-scale cognition brain with multigranular neuromorphic architecture. IEEE Trans. Neural Netw. Learn. Syst. 2021, 33, 2801–2815. [Google Scholar] [CrossRef]
- Mikhaylov, A.; Pimashkin, A.; Pigareva, Y.; Gerasimova, S.; Gryaznov, E.; Shchanikov, S.; Zuev, A.; Talanov, M.; Lavrov, I.; Demin, V. Neurohybrid memristive CMOS-integrated systems for biosensors and neuroprosthetics. Front. Neurosci. 2020, 14, 358. [Google Scholar] [CrossRef]
- Shchanikov, S.; Zuev, A.; Bordanov, I.; Danilin, S.; Lukoyanov, V.; Korolev, D.; Belov, A.; Pigareva, Y.; Gladkov, A.; Pimashkin, A. Designing a bidirectional, adaptive neural interface incorporating machine learning capabilities and memristor-enhanced hardware. Chaos Solitons Fractals 2021, 142, 110504. [Google Scholar] [CrossRef]
- Wei, H.; Shi, R.; Sun, L.; Yu, H.; Gong, J.; Liu, C.; Xu, Z.; Ni, Y.; Xu, J.; Xu, W. Mimicking efferent nerves using a graphdiyne-based artificial synapse with multiple ion diffusion dynamics. Nat. Commun. 2021, 12, 1068. [Google Scholar] [CrossRef]
- Chow, S.Y.A.; Hu, H.; Osaki, T.; Levi, T.; Ikeuchi, Y. Advances in construction and modeling of functional neural circuits in vitro. Neurochem. Res. 2022, 47, 2529–2544. [Google Scholar] [CrossRef]
- Pastore, V.P.; Massobrio, P.; Godjoski, A.; Martinoia, S. Identification of excitatory-inhibitory links and network topology in large-scale neuronal assemblies from multi-electrode recordings. PLoS Comput. Biol. 2018, 14, e1006381. [Google Scholar] [CrossRef]
- Brofiga, M.; Pisano, M.; Tedesco, M.; Raiteri, R.; Massobrio, P. Three-dimensionality shapes the dynamics of cortical interconnected to hippocampal networks. J. Neural Eng. 2020, 17, 056044. [Google Scholar] [CrossRef] [PubMed]
- Berdondini, L.; Imfeld, K.; Maccione, A.; Tedesco, M.; Neukom, S.; Koudelka-Hep, M.; Martinoia, S. Active pixel sensor array for high spatio-temporal resolution electrophysiological recordings from single cell to large scale neuronal networks. Lab Chip 2009, 9, 2644–2651. [Google Scholar] [CrossRef] [PubMed]
- Frey, U.; Egert, U.; Heer, F.; Hafizovic, S.; Hierlemann, A. Microelectronic system for high-resolution mapping of extracellular electric fields applied to brain slices. Biosens. Bioelectron. 2009, 24, 2191–2198. [Google Scholar] [CrossRef]
- Lam, D.; Fischer, N.O.; Enright, H.A. Probing function in 3D neuronal cultures: A survey of 3D multielectrode array advances. Curr. Opin. Pharmacol. 2021, 60, 255–260. [Google Scholar] [CrossRef]
- Jun, J.J.; Steinmetz, N.A.; Siegle, J.H.; Denman, D.J.; Bauza, M.; Barbarits, B.; Lee, A.K.; Anastassiou, C.A.; Andrei, A.; Aydin, C.; et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 2017, 551, 232–236. [Google Scholar] [CrossRef]
- Angotzi, G.N.; Boi, F.; Lecomte, A.; Miele, E.; Malerba, M.; Zucca, S.; Casile, A.; Berdondini, L. SiNAPS: An implantable active pixel sensor CMOS-probe for simultaneous large-scale neural recordings. Biosens. Bioelectron. 2019, 126, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Fiáth, R.; Raducanu, B.C.; Musa, S.; Andrei, A.; Lopez, C.M.; van Hoof, C.; Ruther, P.; Aarts, A.; Horváth, D.; Ulbert, I. A silicon-based neural probe with densely-packed low-impedance titanium nitride microelectrodes for ultrahigh-resolution in vivo recordings. Biosens. Bioelectron. 2018, 106, 86–92. [Google Scholar] [CrossRef]
- Steinmetz, N.A.; Aydin, C.; Lebedeva, A.; Okun, M.; Pachitariu, M.; Bauza, M.; Beau, M.; Bhagat, J.; Böhm, C.; Broux, M. Neuropixels 2.0: A miniaturized high-density probe for stable, long-term brain recordings. Science 2021, 372, eabf4588. [Google Scholar] [CrossRef] [PubMed]
- Paulk, A.C.; Kfir, Y.; Khanna, A.; Mustroph, M.; Trautmann, E.M.; Soper, D.J.; Stavisky, S.D.; Welkenhuysen, M.; Dutta, B.; Shenoy, K.V. Large-scale neural recordings with single-cell resolution in human cortex using high-density Neuropixels probes. bioRxiv 2021, 25, 252–263. [Google Scholar] [CrossRef]
- Obaid, A.; Hanna, M.-E.; Wu, Y.-W.; Kollo, M.; Racz, R.; Angle, M.R.; Müller, J.; Brackbill, N.; Wray, W.; Franke, F. Massively parallel microwire arrays integrated with CMOS chips for neural recording. Sci. Adv. 2020, 6, eaay2789. [Google Scholar] [CrossRef]
- Kollo, M.; Racz, R.; Hanna, M.-E.; Obaid, A.; Angle, M.R.; Wray, W.; Kong, Y.; Müller, J.; Hierlemann, A.; Melosh, N.A. CHIME: CMOS-hosted in vivo microelectrodes for massively scalable neuronal recordings. Front. Neurosci. 2020, 14, 834. [Google Scholar] [CrossRef]
- Sahasrabuddhe, K.; Khan, A.A.; Singh, A.P.; Stern, T.M.; Ng, Y.; Tadić, A.; Orel, P.; LaReau, C.; Pouzzner, D.; Nishimura, K. The Argo: A high channel count recording system for neural recording in vivo. J. Neural Eng. 2021, 18, 015002. [Google Scholar] [CrossRef]
- Fenno, L.; Yizhar, O.; Deisseroth, K. The development and application of optogenetics. Annu. Rev. Neurosci. 2011, 34, 389–412. [Google Scholar] [CrossRef] [PubMed]
- Warden, M.R.; Cardin, J.A.; Deisseroth, K. Optical neural interfaces. Annu. Rev. Biomed. Eng. 2014, 16, 103–129. [Google Scholar] [CrossRef]
- Ronzitti, E.; Ventalon, C.; Canepari, M.; Forget, B.C.; Papagiakoumou, E.; Emiliani, V. Recent advances in patterned photostimulation for optogenetics. J. Opt. 2017, 19, 113001. [Google Scholar] [CrossRef]
- Dieter, A.; Duque-Afonso, C.J.; Rankovic, V.; Jeschke, M.; Moser, T. Near physiological spectral selectivity of cochlear optogenetics. Nat. Commun. 2019, 10, 1962. [Google Scholar] [CrossRef] [PubMed]
- Mosbacher, Y.; Khoyratee, F.; Goldin, M.; Kanner, S.; Malakai, Y.; Silva, M.; Grassia, F.; Simon, Y.B.; Cortes, J.; Barzilai, A. Toward neuroprosthetic real-time communication from in silico to biological neuronal network via patterned optogenetic stimulation. Sci. Rep. 2020, 10, 7512. [Google Scholar] [CrossRef]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Bi, A.; Cui, J.; Ma, Y.-P.; Olshevskaya, E.; Pu, M.; Dizhoor, A.M.; Pan, Z.-H. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron 2006, 50, 23–33. [Google Scholar] [CrossRef]
- Lima, S.Q.; Miesenböck, G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell 2005, 121, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Adhikari, A.; Deisseroth, K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat. Rev. Neurosci. 2017, 18, 222–235. [Google Scholar] [CrossRef]
- Shemesh, O.A.; Tanese, D.; Zampini, V.; Linghu, C.; Piatkevich, K.; Ronzitti, E.; Papagiakoumou, E.; Boyden, E.S.; Emiliani, V. Temporally precise single-cell-resolution optogenetics. Nat. Neurosci. 2017, 20, 1796–1806. [Google Scholar] [CrossRef]
- Knöpfel, T. Genetically encoded optical indicators for the analysis of neuronal circuits. Nat. Rev. Neurosci. 2012, 13, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Bando, Y.; Grimm, C.; Cornejo, V.H.; Yuste, R. Genetic voltage indicators. BMC Biol. 2019, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Grienberger, C.; Konnerth, A. Imaging calcium in neurons. Neuron 2012, 73, 862–885. [Google Scholar] [CrossRef] [PubMed]
- Grewe, B.F.; Langer, D.; Kasper, H.; Kampa, B.M.; Helmchen, F. High-speed in vivo calcium imaging reveals neuronal network activity with near-millisecond precision. Nat. Methods 2010, 7, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Lin, B.-J.; Chen, T.-W.; Daie, K.; Svoboda, K.; Druckmann, S. A comparison of neuronal population dynamics measured with calcium imaging and electrophysiology. PLoS Comput. Biol. 2020, 16, e1008198. [Google Scholar] [CrossRef]
- Ali, F.; Kwan, A.C. Interpreting in vivo calcium signals from neuronal cell bodies, axons, and dendrites: A review. Neurophotonics 2019, 7, 011402. [Google Scholar] [CrossRef]
- Knöpfel, T.; Song, C. Optical voltage imaging in neurons: Moving from technology development to practical tool. Nat. Rev. Neurosci. 2019, 20, 719–727. [Google Scholar] [CrossRef]
- Yang, W.; Carrillo-Reid, L.; Bando, Y.; Peterka, D.S.; Yuste, R. Simultaneous two-photon imaging and two-photon optogenetics of cortical circuits in three dimensions. Elife 2018, 7, e32671. [Google Scholar] [CrossRef]
- Ozbay, B.N.; Futia, G.L.; Ma, M.; Bright, V.M.; Gopinath, J.T.; Hughes, E.G.; Restrepo, D.; Gibson, E.A. Three dimensional two-photon brain imaging in freely moving mice using a miniature fiber coupled microscope with active axial-scanning. Sci. Rep. 2018, 8, 8108. [Google Scholar] [CrossRef]
- Stamatakis, A.M.; Schachter, M.J.; Gulati, S.; Zitelli, K.T.; Malanowski, S.; Tajik, A.; Fritz, C.; Trulson, M.; Otte, S.L. Simultaneous optogenetics and cellular resolution calcium imaging during active behavior using a miniaturized microscope. Front. Neurosci. 2018, 12, 496. [Google Scholar] [CrossRef]
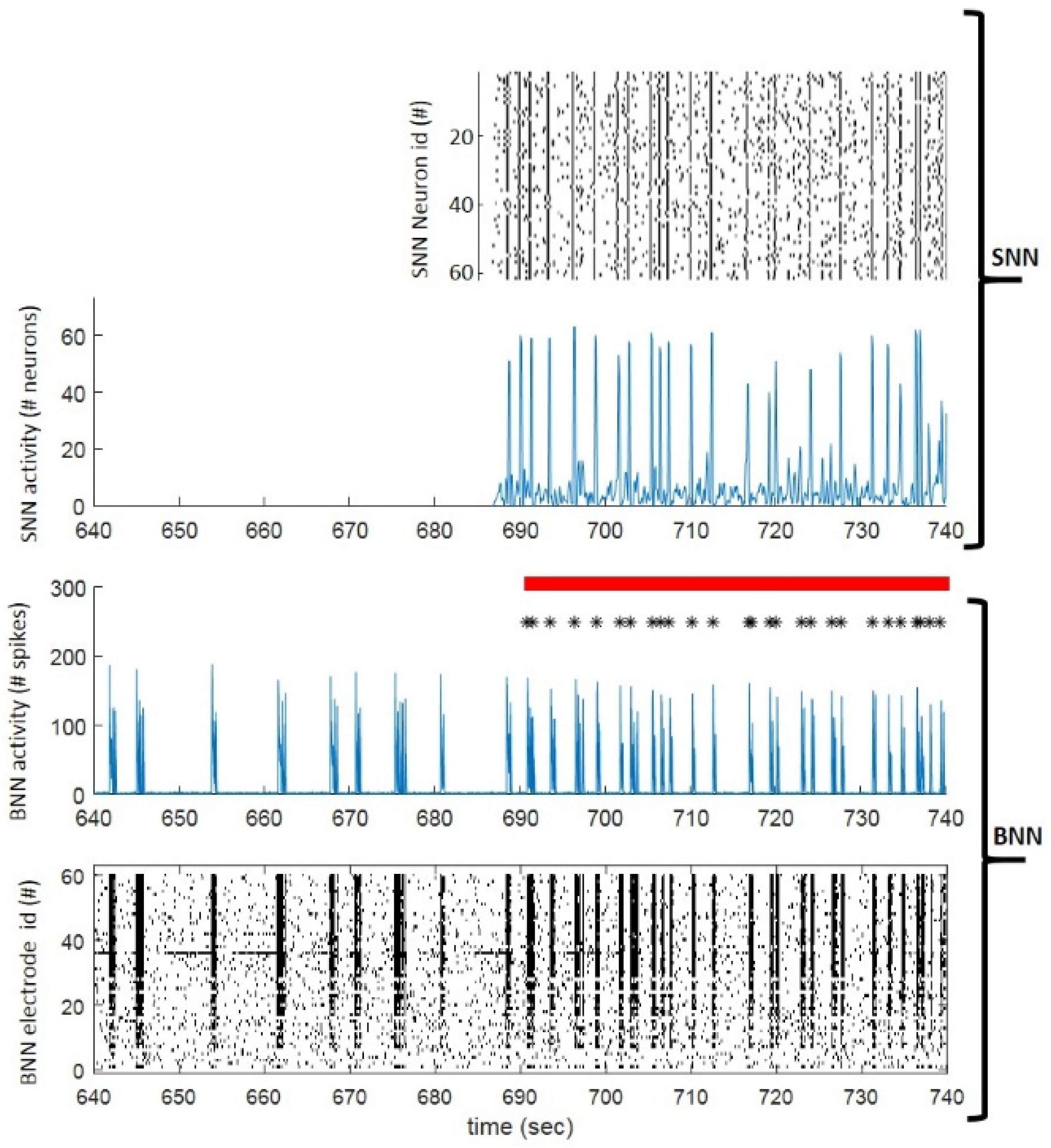
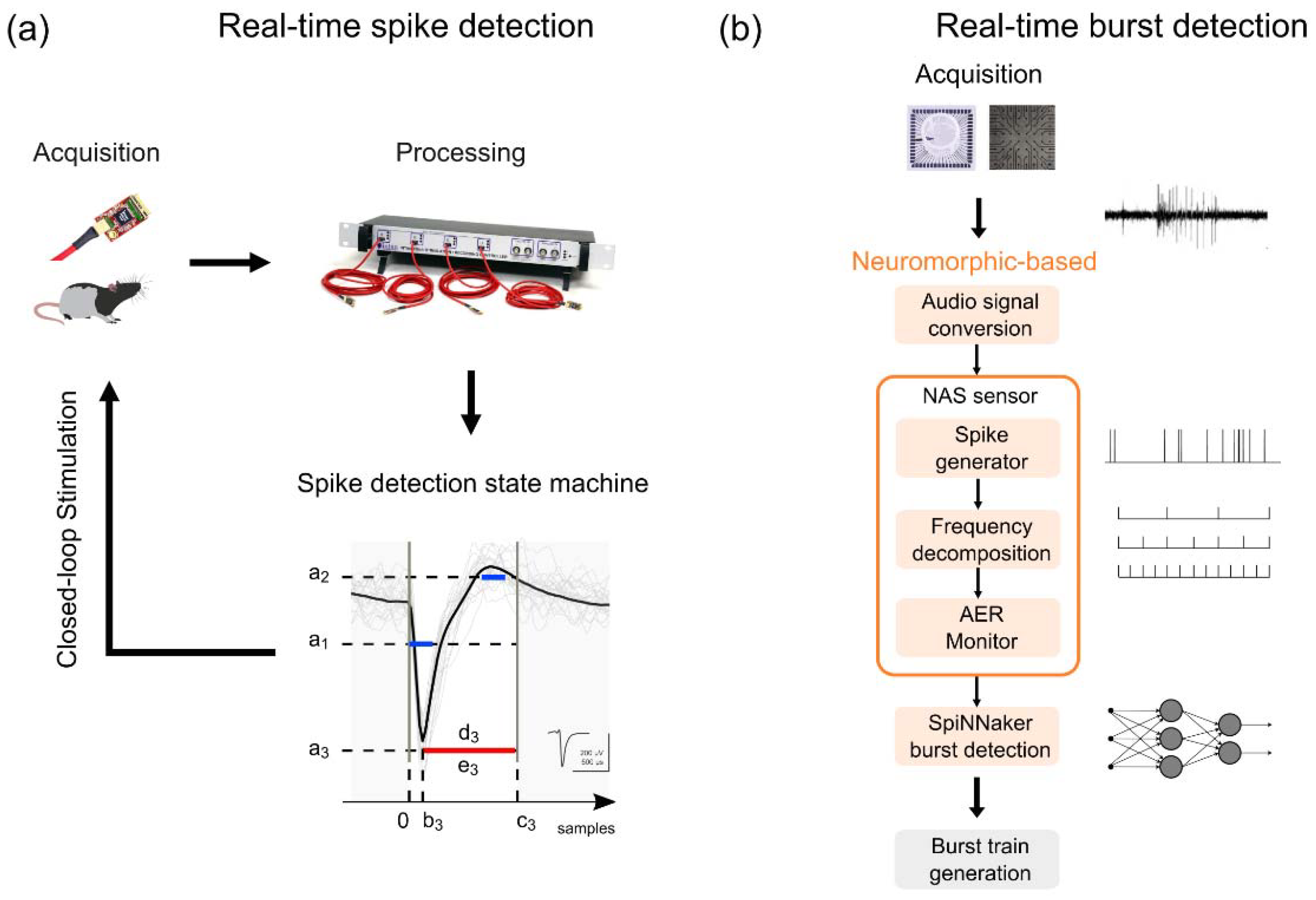
- Dominguez-Morales, J.P.; Buccelli, S.; Gutierrez-Galan, D.; Colombi, I.; Jimenez-Fernandez, A.; Chiappalone, M. Real-time detection of bursts in neuronal cultures using a Neuromorphic Auditory Sensor and Spiking Neural Networks. Neurocomputing 2021, 449, 422–434. [Google Scholar] [CrossRef]
- Murphy, M.; Buccelli, S.; Bornat, Y.; Bundy, D.; Nudo, R.; Guggenmos, D.; Chiappalone, M. Improving an open-source commercial system to reliably perform activity-dependent stimulation. J. Neural Eng. 2019, 16, 066022. [Google Scholar] [CrossRef] [PubMed]
- Cheney, P.D.; Fetz, E.E. Comparable patterns of muscle facilitation evoked by individual corticomotoneuronal (CM) cells and by single intracortical microstimuli in primates: Evidence for functional groups of CM cells. J. Neurophysiol. 1985, 53, 786–804. [Google Scholar] [CrossRef]
- Biffi, E.; Ghezzi, D.; Pedrocchi, A.; Ferrigno, G. Development and validation of a spike detection and classification algorithm aimed at implementation on hardware devices. Comput. Intell. Neurosci. 2010, 2010, 659050. [Google Scholar] [CrossRef][Green Version]
- Gibson, S.; Judy, J.W.; Marković, D. An FPGA-based platform for accelerated offline spike sorting. J. Neurosci. Methods 2013, 215, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vallicelli, E.A.; De Matteis, M.; Baschirotto, A.; Rescati, M.; Reato, M.; Maschietto, M.; Vassanelli, S.; Guarrera, D.; Collazuol, G.; Zeiter, R. Neural spikes digital detector/sorting on FPGA. In Proceedings of the 2017 IEEE Biomedical Circuits and Systems Conference (BioCAS), Turin, Italy, 19–21 October 2017; pp. 1–4. [Google Scholar]
- Park, J.; Kim, G.; Jung, S.-D. A 128-channel FPGA-based real-time spike-sorting bidirectional closed-loop neural interface system. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 2227–2238. [Google Scholar] [CrossRef] [PubMed]
- Donati, E.; Payvand, M.; Risi, N.; Krause, R.; Indiveri, G. Discrimination of EMG signals using a neuromorphic implementation of a spiking neural network. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 795–803. [Google Scholar] [CrossRef]
- Bernert, M.; Yvert, B. An attention-based spiking neural network for unsupervised spike-sorting. Int. J. Neural Syst. 2019, 29, 1850059. [Google Scholar] [CrossRef]
- Gal, A.; Marom, S. Entrainment of the intrinsic dynamics of single isolated neurons by natural-like input. J. Neurosci. 2013, 33, 7912–7918. [Google Scholar] [CrossRef]
- Scarsi, F.; Tessadori, J.; Chiappalone, M.; Pasquale, V. Investigating the impact of electrical stimulation temporal distribution on cortical network responses. BMC Neurosci. 2017, 18, 49. [Google Scholar] [CrossRef]
- Cota, V.R.; de Oliveira, J.C.; Damázio, L.C.M.; Moraes, M.F.D. Nonperiodic stimulation for the treatment of refractory epilepsy: Applications, mechanisms, and novel insights. Epilepsy Behav. 2021, 121, 106609. [Google Scholar] [CrossRef]
- Réboli, L.A.; Maciel, R.M.; de Oliveira, J.C.; Moraes, M.F.D.; Tilelli, C.Q.; Cota, V.R. Persistence of neural function in animals submitted to seizure-suppressing scale-free nonperiodic electrical stimulation applied to the amygdala. Behav. Brain Res. 2022, 426, 113843. [Google Scholar] [CrossRef] [PubMed]
- Khaledi-Nasab, A.; Kromer, J.A.; Tass, P.A. Long-Lasting Desynchronization of Plastic Neuronal Networks by Double-Random Coordinated Reset Stimulation. Front. Netw. Physiol. 2022, 22, 864859. [Google Scholar] [CrossRef]
- Hauptmann, C.; Popovych, O.; Tass, P. Multisite coordinated delayed feedback for an effective desynchronization of neuronal networks. Stoch. Dyn. 2005, 5, 307–319. [Google Scholar] [CrossRef]
- Tass, P.A.; Hauptmann, C. Therapeutic modulation of synaptic connectivity with desynchronizing brain stimulation. Int. J. Psychophysiol. 2007, 64, 53–61. [Google Scholar] [CrossRef]
- Tass, P.A.; Qin, L.; Hauptmann, C.; Dovero, S.; Bezard, E.; Boraud, T.; Meissner, W.G. Coordinated reset has sustained aftereffects in Parkinsonian monkeys. Ann. Neurol. 2012, 72, 816–820. [Google Scholar] [CrossRef]
- Okun, M.; Hickey, P.; Machado, A.; Kuncel, A.; Grill, W. Temporally optimized patterned stimulation (TOPS®) as a therapy to personalize deep brain stimulation treatment of Parkinson’s disease. Front. Hum. Neurosci. 2022, 16, 929509. [Google Scholar] [CrossRef]
- Cottone, C.; Cancelli, A.; Pasqualetti, P.; Porcaro, C.; Salustri, C.; Tecchio, F. A new, high-efficacy, noninvasive transcranial electric stimulation tuned to local neurodynamics. J. Neurosci. 2018, 38, 586–594. [Google Scholar] [CrossRef]
- Birdno, M.J.; Kuncel, A.M.; Dorval, A.D.; Turner, D.A.; Gross, R.E.; Grill, W.M. Stimulus features underlying reduced tremor suppression with temporally patterned deep brain stimulation. J. Neurophysiol. 2012, 107, 364–383. [Google Scholar] [CrossRef]
- Grill, W.M. Temporal pattern of electrical stimulation is a new dimension of therapeutic innovation. Curr. Opin. Biomed. Eng. 2018, 8, 1–6. [Google Scholar] [CrossRef]
- Izhikevich, E.M. Simple model of spiking neurons. IEEE Trans. Neural Netw. 2003, 14, 1569–1572. [Google Scholar] [CrossRef]
- Painkras, E.; Plana, L.A.; Garside, J.; Temple, S.; Galluppi, F.; Patterson, C.; Lester, D.R.; Brown, A.D.; Furber, S.B. SpiNNaker: A 1-W 18-core system-on-chip for massively-parallel neural network simulation. IEEE J. Solid-State Circuits 2013, 48, 1943–1953. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, T.-W. Organic synapses for neuromorphic electronics: From brain-inspired computing to sensorimotor nervetronics. Acc. Chem. Res. 2019, 52, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Keene, S.T.; Lubrano, C.; Kazemzadeh, S.; Melianas, A.; Tuchman, Y.; Polino, G.; Scognamiglio, P.; Cinà, L.; Salleo, A.; van de Burgt, Y. A biohybrid synapse with neurotransmitter-mediated plasticity. Nat. Mater. 2020, 19, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Serb, A.; Corna, A.; George, R.; Khiat, A.; Rocchi, F.; Reato, M.; Maschietto, M.; Mayr, C.; Indiveri, G.; Vassanelli, S. Memristive synapses connect brain and silicon spiking neurons. Sci. Rep. 2020, 10, 2590. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.E. Modularity and community structure in networks. Proc. Natl. Acad. Sci. USA 2006, 103, 8577–8582. [Google Scholar] [CrossRef] [PubMed]
- Virlogeux, A.; Moutaux, E.; Christaller, W.; Genoux, A.; Bruyere, J.; Fino, E.; Charlot, B.; Cazorla, M.; Saudou, F. Reconstituting corticostriatal network on-a-chip reveals the contribution of the presynaptic compartment to Huntington’s disease. Cell Rep. 2018, 22, 110–122. [Google Scholar] [CrossRef]
- Kanagasabapathi, T.T.; Massobrio, P.; Barone, R.A.; Tedesco, M.; Martinoia, S.; Wadman, W.J.; Decré, M.M. Functional connectivity and dynamics of cortical–thalamic networks co-cultured in a dual compartment device. J. Neural Eng. 2012, 9, 036010. [Google Scholar] [CrossRef]
- Brofiga, M.; Pisano, M.; Callegari, F.; Massobrio, P. Exploring the Contribution of Thalamic and Hippocampal Input on Cortical Dynamics in a Brain-on-a-Chip Model. IEEE Trans. Med. Robot. Bionics 2021, 3, 315–327. [Google Scholar] [CrossRef]
- Brofiga, M.; Pisano, M.; Tedesco, M.; Boccaccio, A.; Massobrio, P. Functional inhibitory connections modulate the electrophysiological activity patterns of cortical-hippocampal ensembles. Cereb. Cortex 2022, 32, 1866–1881. [Google Scholar] [CrossRef]
- Callegari, F.; Brofiga, M.; Poggio, F.; Massobrio, P. Stimulus-Evoked Activity Modulation of In Vitro Engineered Cortical and Hippocampal Networks. Micromachines 2022, 13, 1212. [Google Scholar] [CrossRef]
- Dauth, S.; Maoz, B.M.; Sheehy, S.P.; Hemphill, M.A.; Murty, T.; Macedonia, M.K.; Greer, A.M.; Budnik, B.; Parker, K.K. Neurons derived from different brain regions are inherently different in vitro: A novel multiregional brain-on-a-chip. J. Neurophysiol. 2017, 117, 1320–1341. [Google Scholar] [CrossRef] [PubMed]
- Smalley, K.S.; Lioni, M.; Herlyn, M. Life ins’t flat: Taking cancer biology to the next dimension. In Vitro Cell. Dev. Biol.-Anim. 2006, 42, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Pautot, S.; Wyart, C.; Isacoff, E.Y. Colloid-guided assembly of oriented 3D neuronal networks. Nat. Methods 2008, 5, 735–740. [Google Scholar] [CrossRef]
- Pan, L.; Ren, Y.; Cui, F.; Xu, Q. Viability and differentiation of neural precursors on hyaluronic acid hydrogel scaffold. J. Neurosci. Res. 2009, 87, 3207–3220. [Google Scholar] [CrossRef]
- Dillon, G.P.; Yu, X.; Bellamkonda, R.V. The polarity and magnitude of ambient charge influences three-dimensional neurite extension from DRGs. J. Biomed. Mater. Res. 2000, 51, 510–519. [Google Scholar] [CrossRef]
- Tang-Schomer, M.D.; White, J.D.; Tien, L.W.; Schmitt, L.I.; Valentin, T.M.; Graziano, D.J.; Hopkins, A.M.; Omenetto, F.G.; Haydon, P.G.; Kaplan, D.L. Bioengineered functional brain-like cortical tissue. Proc. Natl. Acad. Sci. USA 2014, 111, 13811–13816. [Google Scholar] [CrossRef]
- Bosi, S.; Rauti, R.; Laishram, J.; Turco, A.; Lonardoni, D.; Nieus, T.; Prato, M.; Scaini, D.; Ballerini, L. From 2D to 3D: Novel nanostructured scaffolds to investigate signalling in reconstructed neuronal networks. Sci. Rep. 2015, 5, srep09562. [Google Scholar] [CrossRef]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef]
- Brofiga, M.; Massobrio, P. Brain-on-a-Chip: Dream or Reality? Front. Neurosci. 2022, 16, 837623. [Google Scholar] [CrossRef]
- Averna, A.; Hayley, P.; Murphy, M.D.; Barban, F.; Nguyen, J.; Buccelli, S.; Nudo, R.J.; Chiappalone, M.; Guggenmos, D.J. Entrainment of network activity by closed-loop microstimulation in healthy ambulatory rats. Cereb. Cortex 2021, 31, 5042–5055. [Google Scholar] [CrossRef]
- Averna, A.; Pasquale, V.; Murphy, M.D.; Rogantin, M.P.; Van Acker, G.M.; Nudo, R.J.; Chiappalone, M.; Guggenmos, D.J. Differential effects of open-and closed-loop intracortical microstimulation on firing patterns of neurons in distant cortical areas. Cereb. Cortex 2020, 30, 2879–2896. [Google Scholar] [CrossRef] [PubMed]
- Kühn, A.A.; Kupsch, A.; Schneider, G.H.; Brown, P. Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur. J. Neurosci. 2006, 23, 1956–1960. [Google Scholar] [CrossRef] [PubMed]
- Kühn, A.A.; Tsui, A.; Aziz, T.; Ray, N.; Brücke, C.; Kupsch, A.; Schneider, G.-H.; Brown, P. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp. Neurol. 2009, 215, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Priori, A.; Foffani, G.; Pesenti, A.; Tamma, F.; Bianchi, A.M.; Pellegrini, M.; Locatelli, M.; Moxon, K.; Villani, R. Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson’s disease. Exp. Neurol. 2004, 189, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Little, S.; Pogosyan, A.; Neal, S.; Zavala, B.; Zrinzo, L.; Hariz, M.; Foltynie, T.; Limousin, P.; Ashkan, K.; FitzGerald, J. Adaptive deep brain stimulation in advanced Parkinson disease. Ann. Neurol. 2013, 74, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Priori, A.; Foffani, G.; Rossi, L.; Marceglia, S. Adaptive deep brain stimulation (aDBS) controlled by local field potential oscillations. Exp. Neurol. 2013, 245, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.; Arlotti, M.; Ardolino, G.; Cogiamanian, F.; Marceglia, S.; Di Fonzo, A.; Cortese, F.; Rampini, P.M.; Priori, A. Adaptive deep brain stimulation in a freely moving Parkinsonian patient. Mov. Disord. 2015, 30, 1003–1005. [Google Scholar] [CrossRef]
- Vázquez-Guardado, A.; Yang, Y.; Bandodkar, A.J.; Rogers, J.A. Recent advances in neurotechnologies with broad potential for neuroscience research. Nat. Neurosci. 2020, 23, 1522–1536. [Google Scholar] [CrossRef]
- Grassia, F.; Levi, T.; Doukkali, E.; Kohno, T. Spike pattern recognition using artificial neuron and spike-timing-dependent plasticity implemented on a multi-core embedded platform. Artif. Life Robot. 2018, 23, 200–204. [Google Scholar] [CrossRef]
- Pani, D.; Meloni, P.; Tuveri, G.; Palumbo, F.; Massobrio, P.; Raffo, L. An FPGA platform for real-time simulation of spiking neuronal networks. Front. Neurosci. 2017, 11, 90. [Google Scholar] [CrossRef]
- Khoyratee, F.; Grassia, F.; Saïghi, S.; Levi, T. Optimized real-time biomimetic neural network on FPGA for bio-hybridization. Front. Neurosci. 2019, 13, 377. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.; Billaudelle, S.; Müller, E.; Tetzlaff, C.; Schemmel, J.; Schmitt, S. Emulating Dendritic Computing Paradigms on Analog Neuromorphic Hardware. Neuroscience 2021, 489, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Beniaguev, D.; Segev, I.; London, M. Single cortical neurons as deep artificial neural networks. Neuron 2021, 109, 2727–2739. e2723. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiappalone, M.; Cota, V.R.; Carè, M.; Di Florio, M.; Beaubois, R.; Buccelli, S.; Barban, F.; Brofiga, M.; Averna, A.; Bonacini, F.; et al. Neuromorphic-Based Neuroprostheses for Brain Rewiring: State-of-the-Art and Perspectives in Neuroengineering. Brain Sci. 2022, 12, 1578. https://doi.org/10.3390/brainsci12111578
Chiappalone M, Cota VR, Carè M, Di Florio M, Beaubois R, Buccelli S, Barban F, Brofiga M, Averna A, Bonacini F, et al. Neuromorphic-Based Neuroprostheses for Brain Rewiring: State-of-the-Art and Perspectives in Neuroengineering. Brain Sciences. 2022; 12(11):1578. https://doi.org/10.3390/brainsci12111578
Chicago/Turabian StyleChiappalone, Michela, Vinicius R. Cota, Marta Carè, Mattia Di Florio, Romain Beaubois, Stefano Buccelli, Federico Barban, Martina Brofiga, Alberto Averna, Francesco Bonacini, and et al. 2022. "Neuromorphic-Based Neuroprostheses for Brain Rewiring: State-of-the-Art and Perspectives in Neuroengineering" Brain Sciences 12, no. 11: 1578. https://doi.org/10.3390/brainsci12111578
APA StyleChiappalone, M., Cota, V. R., Carè, M., Di Florio, M., Beaubois, R., Buccelli, S., Barban, F., Brofiga, M., Averna, A., Bonacini, F., Guggenmos, D. J., Bornat, Y., Massobrio, P., Bonifazi, P., & Levi, T. (2022). Neuromorphic-Based Neuroprostheses for Brain Rewiring: State-of-the-Art and Perspectives in Neuroengineering. Brain Sciences, 12(11), 1578. https://doi.org/10.3390/brainsci12111578








