Effect of Virtual Reality on Cognitive Impairment and Clinical Symptoms among Patients with Schizophrenia in the Remission Stage: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Cognitive Function Assessment
2.3. Clinical Symptom Assessment
2.4. VRT Procedure
- The patients learned to wear the helmet in a comfortable way, to enter the virtual supermarket and to use the joysticks to manipulate items in the virtual supermarket.
- When a list of goods was presented on the screen, the patients read the list and closed it after memorizing the list.
- The patients collected the goods presented on the list and put them in the shopping cart in the virtual supermarket using joysticks.
- If the patients forgot the contents of the list, they could press the button on the joystick, and the list would be presented again.
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics and Baseline Data for the Two Groups
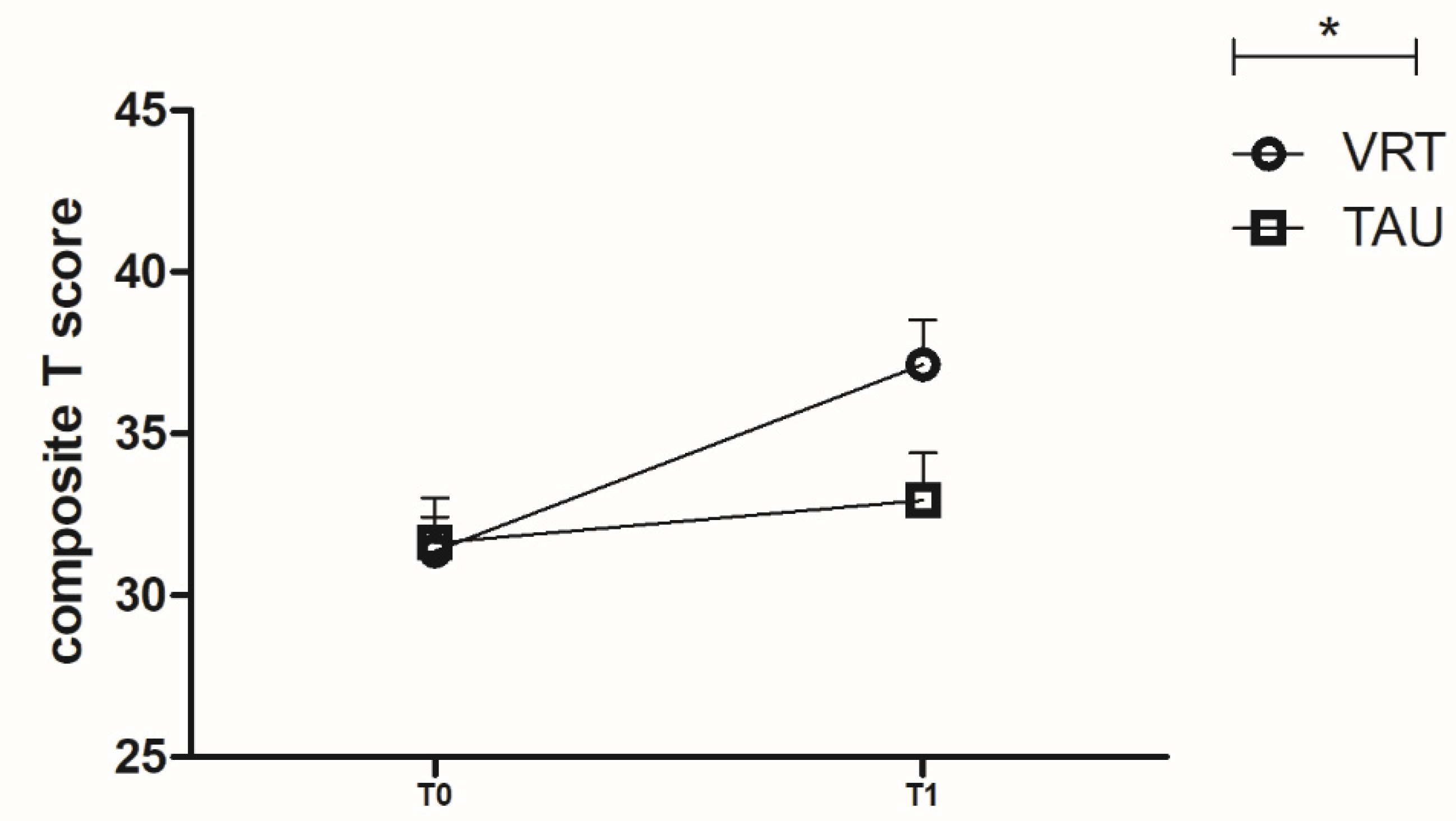
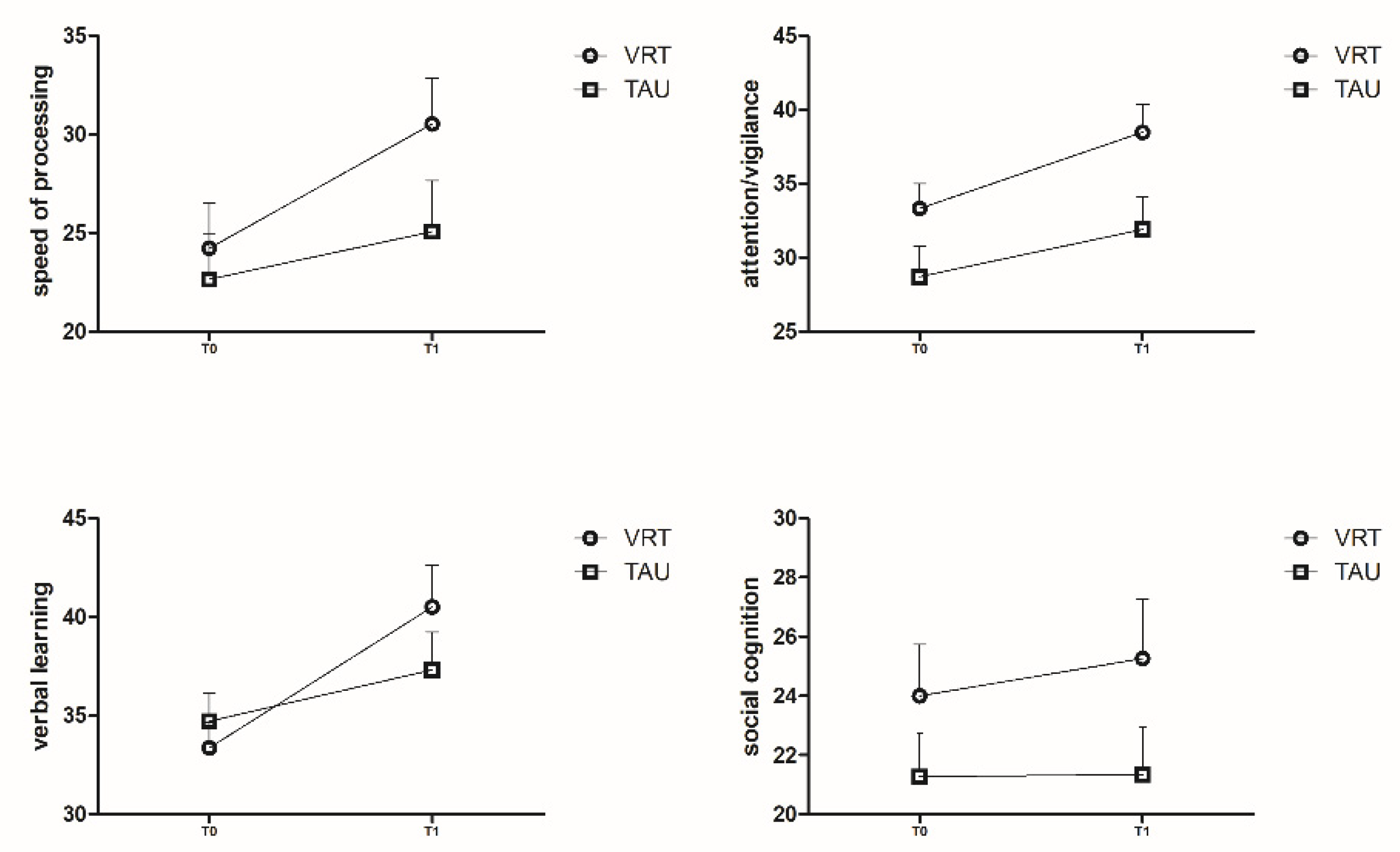
3.2. MCCB T Scores for the Two Groups
3.3. Clinical Symptoms Assessment in the Two Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef]
- Holder, S.D.; Wayhs, A. Schizophrenia. Am. Fam. Physician 2014, 90, 775–782. [Google Scholar] [PubMed]
- Edmunds, A.L. Psychotic and Bipolar Disorders: Schizophrenia. FP Essent. 2017, 455, 11–17. [Google Scholar] [PubMed]
- Kahn, R.S.; Sommer, I.E.; Murray, R.M.; Meyer-Lindenberg, A.; Weinberg, D.R.; Cannon, T.D.; Kane, J.M. Schizophrenia. Nat. Rev. Dis. Primers 2015, 1, 15067. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Y.; Ragland, J.D.; Carter, C.S. Memory and cognition in schizophrenia. Mol. Psychiatry 2018, 24, 633–642. [Google Scholar] [CrossRef]
- Andreasen, N.C.; Carpenter, W.T., Jr.; Kane, J.M.; Lasser, R.A.; Marder, S.R.; Weinberger, D.R. Remission in schizophrenia: Proposed criteria and rationale for consensus. Am. J. Psychiatry 2005, 162, 441–449. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiao, W.; Chen, K.; Zhan, Q.; Ye, F.; Tang, X.; Zhang, X. Neurocognition and social cognition in remitted first-episode schizophrenia: Correlation with VEGF serum levels. BMC Psychiatry 2019, 19, 403. [Google Scholar] [CrossRef]
- Insel, T.R. Rethinking schizophrenia. Nature 2010, 468, 187–193. [Google Scholar] [CrossRef]
- Hasan, A.; Falkai, P.; Wobrock, T.; Lieberman, J.; Glenthoj, B.; Gattaz, W.F.; Thibaut, F.; Möller, H.-J.; World Federation of Societies of Biological Psychiatry (WFSBP) Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, Part 1: Update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J. Biol. Psychiatry 2012, 13, 318–378. [Google Scholar] [CrossRef]
- Hasan, A.; Falkai, P.; Wobrock, T.; Lieberman, J.; Glenthøj, B.; Gattaz, W.F.; Thibaut, F.; Möller, H.-J.; World Federation of Societies of Biological Psychiatry (WFSBP) Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia Part 3: Update 2015 Management of special circumstances: Depression, Suicidality, substance use disorders and pregnancy and lactation. World J. Biol. Psychiatry 2015, 16, 142–170. [Google Scholar] [CrossRef]
- Mojtabai, R.; Lavelle, J.; Gibson, P.J.; Sohler, N.L.; Craig, T.J.; Carlson, G.A.; Bromet, E.J. Gaps in Use of Antipsychotics After Discharge by First-Admission Patients with Schizophrenia, 1989 to 1996. Psychiatr. Serv. 2002, 53, 337–339. [Google Scholar] [CrossRef]
- Cuesta, M.J.; Peralta, V.; Zarzuela, A. Effects of olanzapine and other antipsychotics on cognitive function in chronic schizophrenia: A longitudinal study. Schizophr. Res. 2001, 48, 17–28. [Google Scholar] [CrossRef]
- Hori, H.; Noguchi, H.; Hashimoto, R.; Nakabayashi, T.; Omori, M.; Takahashi, S.; Kunugi, H. Antipsychotic medication and cognitive function in schizophrenia. Schizophr. Res. 2006, 86, 138–146. [Google Scholar] [CrossRef]
- Moustafa, A.A.; Garami, J.K.; Mahlberg, J. Cognitive function in schizophrenia: Conflicting findings and future directions. Rev. Neurosci. 2016, 27, 435–448. [Google Scholar] [CrossRef]
- Jauhar, S.; McKenna, P.J.; Radua, J.; Fung, E.; Salvador, R.; Laws, K.R. Cognitive–behavioural therapy for the symptoms of schizophrenia: Systematic review and meta-analysis with examination of potential bias. Br. J. Psychiatry 2014, 204, 20–29. [Google Scholar] [CrossRef]
- Mcgurk, S.R.; Twamley, E.W.; Sitzer, D.I.; McHugo, G.J.; Mueser, K.T. A meta-analysis of cognitive remediation in schizophrenia. Am. J. Psychiatry 2007, 164, 1791–1802. [Google Scholar] [CrossRef]
- Eack, S.M.; Hogarty, G.E.; Cho, R.Y.; Prasad, K.M.; Greenwald, D.P.; Hogarty, S.S.; Keshavan, M.S. Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: Results from a 2-year randomized controlled trial. Arch. Gen. Psychiatry 2010, 67, 674–682. [Google Scholar] [CrossRef]
- Freeman, D.; Reeve, S.; Robinson, A.; Ehlers, A.; Clark, D.; Spanlang, B.; Slater, M. Virtual reality in the assessment, understanding, and treatment of mental health disorders. Psychol. Med. 2017, 47, 2393–2400. [Google Scholar] [CrossRef]
- Rus-Calafell, M.; Gutiérrez-Maldonado, J.; Ribas-Sabaté, J. A virtual reality-integrated program for improving social skills in patients with schizophrenia: A pilot study. J. Behav. Ther. Exp. Psychiatry 2014, 45, 81–89. [Google Scholar] [CrossRef]
- Pot-Kolder, R.; Geraets CN, W.; Veling, W.; van Beilen, M.; Staring, A.B.; Gijsman, H.J.; van der Gaag, M. Virtual-reality-based cognitive behavioural therapy versus waiting list control for paranoid ideation and social avoidance in patients with psychotic disorders: A single-blind randomised controlled trial. Lancet Psychiatry 2018, 5, 217–226. [Google Scholar] [CrossRef]
- Freeman, D.; Bradley, J.; Antley, A.; Bourke, E.; DeWeever, N.; Evans, N.; Clark, D.M. Virtual reality in the treatment of persecutory delusions: Randomised controlled experimental study testing how to reduce delusional conviction. Br. J. Psychiatry 2016, 209, 62–67. [Google Scholar] [CrossRef]
- Liang, N.; Li, X.; Guo, X.; Liu, S.; Liu, Y.; Zhao, W.; Wen, Y.; Li, Y.; Li, J.; Li, F.; et al. Visual P300 as a neurophysiological correlate of symptomatic improvement by a virtual reality-based computer AT system in patients with auditory verbal hallucinations: A Pilot study. J. Psychiatr. Res. 2022, 151, 261–271. [Google Scholar] [CrossRef]
- Faria, A.L.; Andrade, A.; Soares, L.; Badia, S.B. Benefits of virtual reality based cognitive rehabilitation through simulated activities of daily living: A randomized controlled trial with stroke patients. J. Neuroeng. Rehabil. 2016, 13, 96. [Google Scholar] [CrossRef]
- Russo, M.; De Luca, R.; Naro, A.; Sciarrone, F.; Aragona, B.; Silvestri, G.; Calabrò, R.S. Does body shadow improve the efficacy of virtual reality-based training with BTS NIRVANA?: A pilot study. Medicine 2017, 96, e8096. [Google Scholar] [CrossRef]
- Optale, G.; Urgesi, C.; Busato, V.; Marin, S.; Piron, L.; Priftis, K.; Gamberini, L.; Capodieci, S.; Bordin, A. Controlling Memory Impairment in Elderly Adults Using Virtual Reality Memory Training: A Randomized Controlled Pilot Study. Neurorehabilit. Neural Repair 2009, 24, 348–357. [Google Scholar] [CrossRef]
- Wang, X.; Kou, X.; Meng, X.; Yu, J. Effects of a virtual reality serious game training program on the cognitive function of people diagnosed with schizophrenia: A randomized controlled trial. Front. Psychiatry 2022, 13, 952828. [Google Scholar] [CrossRef]
- Schroeder, A.; Bogie, B.; Rahman, T.; Thérond, A.; Matheson, H.; Guimond, S. Feasibility and Efficacy of Virtual Reality Interventions to Improve Psychosocial Functioning in Psychosis: Systematic Review. JMIR Mental Health 2022, 9, e28502. [Google Scholar] [CrossRef]
- Du Sert, O.P.; Potvin, S.; Lipp, O.; Dellazizzo, L.; Laurelli, M.; Breton, R.; Lalonde, P.; Phraxayavong, K.; O’Connor, K.; Pelletier, J.F.; et al. Virtual reality therapy for refractory auditory verbal hallucinations in schizophrenia: A pilot clinical trial. Schizophr. Res. 2018, 197, 176–181. [Google Scholar] [CrossRef]
- Keefe, R.S.; Goldberg, T.E.; Harvey, P.D.; Gold, J.M.; Poe, M.P.; Coughenour, L. The Brief Assessment of Cognition in Schizophrenia: Reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr. Res. 2004, 68, 283–297. [Google Scholar] [CrossRef]
- Shi, C.; Kang, L.; Yao, S.; Ma, Y.; Li, T.; Liang, Y.; Cheng, Z.; Xu, Y.; Shi, J.; Xu, X.; et al. The MATRICS Consensus Cognitive Battery (MCCB): Co-norming and standardization in China. Schizophr. Res. 2015, 169, 109–115. [Google Scholar] [CrossRef]
- Nuechterlein, K.H.; Green, M.F.; Kern, R.S.; Baade, L.E.; Barch, D.M.; Cohen, J.D.; Essock, S.; Fenton, W.S.; Frese, F.J.; Gold, J.M.; et al. The MATRICS Consensus Cognitive Battery, part 1: Test selection, reliability, and validity. Am. J. Psychiatry 2008, 165, 203–213. [Google Scholar] [CrossRef] [PubMed]
- McCleery, A.; Ventura, J.; Kern, R.; Subotnik, K.; Gretchen-Doorly, D.; Green, M.; Hellemann, G.; Nuechterlein, K. Cognitive functioning in first-episode schizophrenia: MATRICS Consensus Cognitive Battery (MCCB) Profile of Impairment. Schizophr. Res. 2014, 157, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Pearlson, G.D.; Du, Y.; Yu, Q.; Jones, T.R.; Chen, J.; Jiang, T.; Bustillo, J.; Calhoun, V.D. In Search of Multimodal Neuroimaging Biomarkers of Cognitive Deficits in Schizophrenia. Biol. Psychiatry 2015, 78, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Li, S.; Sun, B.; Lyu, H.; Xu, W.; Jiao, J.; Pan, F.; Hu, J.; Chen, J.; Chen, Y.; et al. Verification of using virtual reality to evaluate deficiencies in cognitive function among patients with schizophrenia in the remission stage: A cross-sectional study. BMC Psychiatry 2021, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.; Rosebrock, L.; Waite, F.; Loe, B.S.; Kabir, T.; Petit, A.; Dudley, R.; Chapman, K.; Morrison, A.; O’Regan, E.; et al. Virtual reality (VR) therapy for patients with psychosis: Satisfaction and side effects. Psychol. Med. 2022, 28, 1–12. [Google Scholar] [CrossRef]
- Chan, C.L.; Ngai, E.K.; Leung, P.K.; Wong, S. Effect of the adapted Virtual Reality cognitive training program among Chinese older adults with chronic schizophrenia: A pilot study. Int. J. Geriatr. Psychiatry 2010, 25, 643–649. [Google Scholar] [CrossRef]
- Lopez-Martin, O.; Segura Fragoso, A.; Rodriguez Hernandez, M.; Dimbwadyo Terrer, I.; Polonio-López, B. Effectiveness of a programme based on a virtual reality game for cognitive enhancement in schizophrenia. Gac. Sanit. 2016, 30, 133–136. [Google Scholar]
- Adery, L.H.; Ichinose, M.; Torregrossa, L.J.; Wade, J.; Nichols, H.; Bekele, E.; Bian, D.; Gizdic, A.; Granholm, E.; Sarkar, N.; et al. The acceptability and feasibility of a novel virtual reality based social skills training game for schizophrenia: Preliminary findings. Psychiatry Res. 2018, 270, 496–502. [Google Scholar] [CrossRef]
- Smith, M.J.; Fleming, M.F.; Wright, M.A.; Roberts, A.G.; Humm, L.B.; Olsen, D.; Bell, M.D. Virtual reality job interview training and 6-month employment outcomes for individuals with schizophrenia seeking employment. Schizophr. Res. 2015, 166, 86–91. [Google Scholar] [CrossRef]
- Hasan, A.; Falkai, P.; Wobrock, T.; Lieberman, J.; Glenthoj, B.; Gattaz, W.F.; Thibaut, F.; Möller, H.J.; WFSBP Task force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: Update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J. Biol. Psychiatry 2013, 14, 2–44. [Google Scholar] [CrossRef]
- Sohn, B.K.; Hwang, J.Y.; Park, S.M.; Choi, J.S.; Lee, J.Y.; Lee, J.Y.; Jung, H.Y. Developing a Virtual Reality-Based Vocational Rehabilitation Training Program for Patients with Schizophrenia. Cyberpsychol. Behav. Soc. Netw. 2016, 19, 686–691. [Google Scholar] [CrossRef]
- Puspitosari, W.A.; Wardaningsih, S.; Nanwani, S. Improving the quality of life of people with schizophrenia through community based rehabilitation in Yogyakarta Province, Indonesia: A quasi experimental study. Asian J. Psychiatry 2019, 42, 67–73. [Google Scholar] [CrossRef]
- Garcia-Fernandez, L.; Cabot-Ivorra, N.; Romero-Ferreiro, V.; Pérez-Martín, J.; Rodriguez-Jimenez, R. Differences in theory of mind between early and chronic stages in schizophrenia. J. Psychiatr. Res. 2020, 127, 35–41. [Google Scholar] [CrossRef]


| VRT Group (n = 30) | TAU Group (n = 32) | p | |
|---|---|---|---|
| Age | 46 (37, 50) | 47.5 (37.25, 51.75) | 0.178 b |
| Sex (male/female) | 20/10 | 19/13 | 0.606 c |
| Course of disease (month) | 195.10 ± 107.86 | 249.94 ± 97.55 | 0.040 a |
| Educational years | 10.5 (9, 12) | 9.5 (9, 12) | 0.673 b |
| Age of onset | 22.5 (19, 28.25) | 22 (18.25, 27.75) | 0.389 b |
| MCCB | |||
| SP | 24.23 ± 12.46 | 22.66 ± 13.17 | 0.630 a |
| AV | 33.33 ± 9.18 | 28.72 ± 11.52 | 0.088 a |
| WM | 40.23 ± 17.08 | 42.50 ± 19.78 | 0.632 a |
| VERL | 33.37 ± 7.14 | 34.72 ± 8.02 | 0.487 a |
| VIL | 30.57 ± 10.21 | 35.56 ± 15.19 | 0.136 a |
| RPS | 33.5 (31, 38) | 34 (30.25, 42) | 0.344 b |
| SC | 24.00 ± 9.51 | 21.28 ± 8.38 | 0.236 a |
| Composite score | 31.34 ± 5.80 | 31.61 ± 7.89 | 0.882 a |
| PANSS | |||
| PANSS total | 43 (38, 48) | 42 (39, 48.50) | 0.767 b |
| PANSS P | 7 (7, 9) | 7 (7, 9) | 0.944 b |
| PANSS N | 11 (9.25, 13) | 12 (11, 14) | 0.064 b |
| PANSS G | 19 (18, 23) | 19 (17.5, 21) | 0.709 b |
| PANSS P1 | 1.46 ± 0.88 | 1.21 ± 0.58 | 0.401 a |
| PANSS P2 | 1.00 ± 0.00 | 1.35 ± 0.74 | 0.097 a |
| PANSS P3 | 1.15 ± 0.55 | 1.21 ± 0.58 | 0.784 a |
| PANSS N1 | 1.55 ± 0.78 | 1.86 ± 0.67 | 0.264 a |
| PANSS N4 | 1.61 ± 0.77 | 2.00 ± 0.55 | 0.153 a |
| PANSS N6 | 1.31 ± 0.48 | 1.43 ± 0.65 | 0.589 a |
| PANSS G5 | 1.08 ± 0.28 | 1.00 ± 0.00 | 0.309 a |
| PANSS G9 | 1.21 ± 0.43 | 1.08 ± 0.28 | 0.334 a |
| PSP | 70.82 ± 8.34 | 67.08 ± 5.71 | 0.239 a |
| Medicine to Use | Number of Patients in VR Group | Number of Patients in TAU Group | Chi-Square Test |
|---|---|---|---|
| Atypical antipsychotics | 30 | 32 | Value = 0.834 |
| Antidepressants | 8 | 7 | p = 0.934 |
| Mood stabilizers | 8 | 12 | |
| Anxiolytics | 2 | 2 | |
| Sedative-hypnotics | 5 | 7 |
| VRT Group | TAU Group | F | P | |||
|---|---|---|---|---|---|---|
| T0 | T1 | T0 | T1 | |||
| PANSS | ||||||
| PANSS total | 43 (38, 48) | 37 (31, 42) | 42 (39, 48.50) | 39 (35, 41.50) | 1.964 | 0.051 |
| PANSS P | 7 (7, 9) | 7 (7, 8) | 7 (7, 9) | 7 (7, 9) | 3.149 | 0.651 |
| PANSS N | 11 (9.25, 13) | 10 (7, 14) | 12 (11, 14) | 12.5 (11, 14.5) | 4.290 | 0.065 |
| PANSS G | 19 (18, 23) | 17 (16, 21) | 19 (17.5, 21) | 19 (17.25, 20.75) | 4.450 | 0.016 * |
| PSP | 70.82 ± 8.34 | 72.36 ± 8.15 | 67.08 ± 5.71 | 68.25 ± 5.41 | 0.297 | 0.603 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Liu, R.; Sun, B.; Wei, N.; Shen, Z.; Xu, Y.; Huang, M. Effect of Virtual Reality on Cognitive Impairment and Clinical Symptoms among Patients with Schizophrenia in the Remission Stage: A Randomized Controlled Trial. Brain Sci. 2022, 12, 1572. https://doi.org/10.3390/brainsci12111572
Li S, Liu R, Sun B, Wei N, Shen Z, Xu Y, Huang M. Effect of Virtual Reality on Cognitive Impairment and Clinical Symptoms among Patients with Schizophrenia in the Remission Stage: A Randomized Controlled Trial. Brain Sciences. 2022; 12(11):1572. https://doi.org/10.3390/brainsci12111572
Chicago/Turabian StyleLi, Shangda, Renchuan Liu, Bin Sun, Ning Wei, Zhe Shen, Yi Xu, and Manli Huang. 2022. "Effect of Virtual Reality on Cognitive Impairment and Clinical Symptoms among Patients with Schizophrenia in the Remission Stage: A Randomized Controlled Trial" Brain Sciences 12, no. 11: 1572. https://doi.org/10.3390/brainsci12111572
APA StyleLi, S., Liu, R., Sun, B., Wei, N., Shen, Z., Xu, Y., & Huang, M. (2022). Effect of Virtual Reality on Cognitive Impairment and Clinical Symptoms among Patients with Schizophrenia in the Remission Stage: A Randomized Controlled Trial. Brain Sciences, 12(11), 1572. https://doi.org/10.3390/brainsci12111572






