Music Listening, Emotion, and Cognition in Older Adults
Abstract
1. Introduction
1.1. Background Music
1.2. Cognitive Performance after Music Listening
1.3. The Present Study
2. Materials and Methods
2.1. Participants
2.2. Auditory Stimuli
2.3. Measures of Cognition and Emotion
2.4. Procedure
3. Results
3.1. Cognitive Tasks
3.2. Emotion Tasks
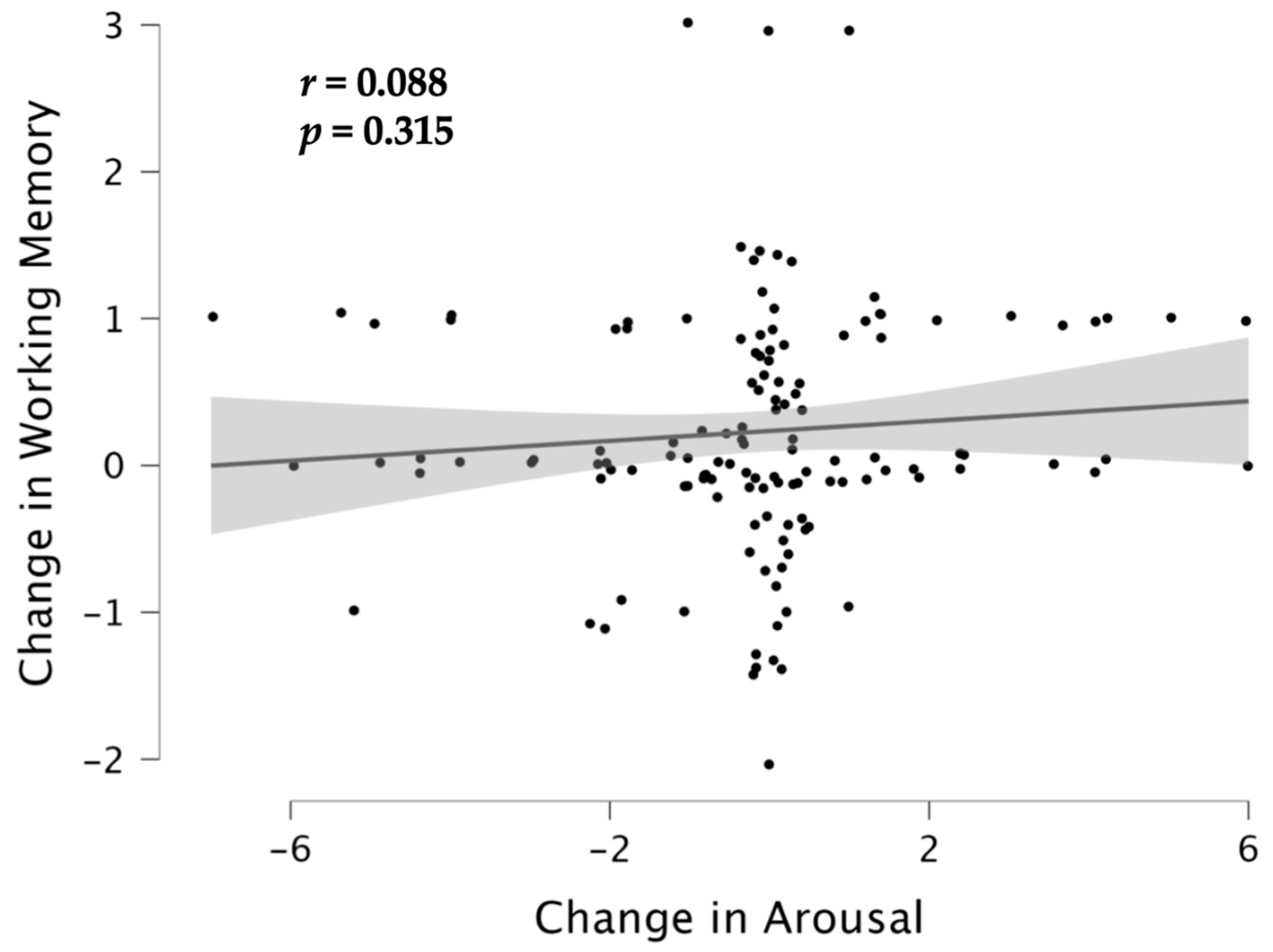
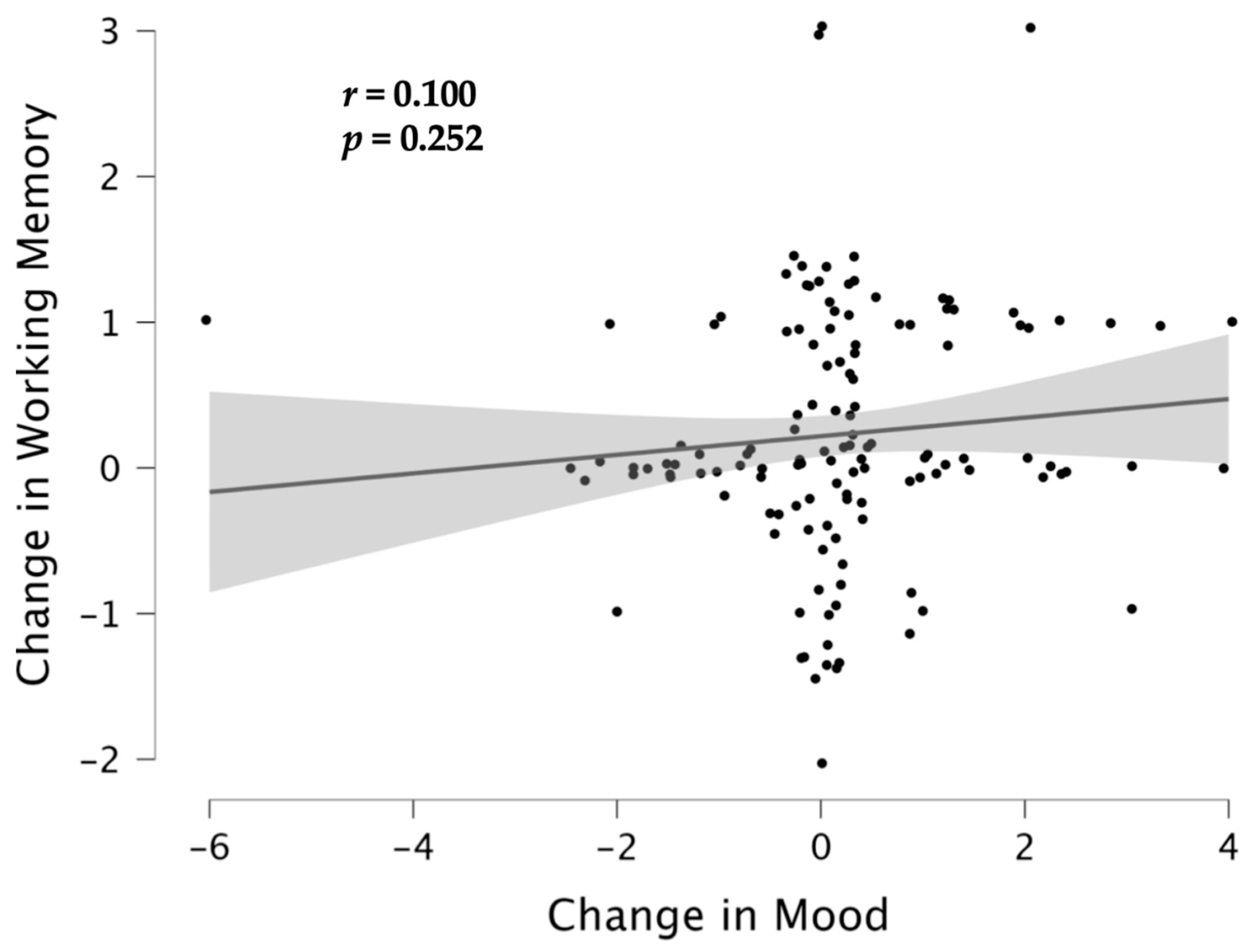
3.3. Associations between Cognition and Emotion
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roman-Caballero, R.; Arnedo, M.; Trivino, M.; Lupianez, J. Musical practice as an enhancer of cognitive function in healthy aging-A systematic review and meta-analysis. PLoS ONE 2018, 13, e0207957. [Google Scholar] [CrossRef] [PubMed]
- Borella, E.; Carbone, E.; Pastore, M.; De Beni, R.; Carretti, B. Working memory training for healthy older adults: The role of individual characteristics in explaining short- and long-term gains. Front. Hum. Neurosci. 2017, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Hallam, S. The effects of listening to music on children’s spatial task performance. Br. Psychol. Soc. Educ. Rev. 2000, 25, 22–26. [Google Scholar]
- Kiss, L.; Linnell, K.J. Making sense of background music listening habits: An arousal and task-complexity account. Psychol. Music 2022, 03057356221089017. [Google Scholar] [CrossRef]
- Kämpfe, J.; Sedlmeier, P.; Renkewitz, F. The impact of background music on adult listeners: A meta-analysis. Psychol. Music 2011, 39, 424–448. [Google Scholar] [CrossRef]
- Braver, T.S.; West, R. Working memory, executive control, and aging. In The Handbook of Aging and Cognition, 3rd ed.; Craik, F.I.M., Salthouse, T.A., Eds.; Psychology Press: London, UK, 2008; pp. 311–372. [Google Scholar] [CrossRef]
- Hasher, L.; Zacks, R. Working memory, comprehension, and aging: A review and a new view. In The Psychology of Learning and Motivation; Bower, G., Ed.; Academic Press: London, UK, 1988; pp. 193–225. [Google Scholar] [CrossRef]
- Borella, E.; Carretti, B.; Cornoldi, C.; De Beni, R. Working memory, control of interference and everyday experience of thought interference: When age makes the difference. Aging Clin. Exp. Res. 2007, 19, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Borella, E.; Delaloye, C.; Lecerf, T.; Renaud, O.; de Ribaupierre, A. Do age differences between young and older adults in inhibitory tasks depend on the degree of activation of information? Eur. J. Cogn. Psychol. 2009, 21, 445–472. [Google Scholar] [CrossRef]
- De Ribaupierre, A. Working memory and attentional processes across the life span. In Life Span Development of Human Memory; Graf, P., Otha, N., Eds.; MIT Press: Cambridge, MA, USA, 2001; pp. 59–80. [Google Scholar]
- Rey-Mermet, A.; Gade, M. Inhibition in aging: What is preserved? What declines? A meta-analysis. Psychon. Bull. Rev. 2018, 25, 1695–1716. [Google Scholar] [CrossRef]
- Daneman, M.; Carpenter, P.A. Individual differences in working memory and reading. J. Verbal Learning Verbal Behav. 1980, 19, 450–466. [Google Scholar] [CrossRef]
- Robert, C.; Borella, E.; Fagot, D.; Lecerf, T.; De Ribaupierre, A. Working memory and inhibitory control across the life span: Intrusion errors in the Reading Span Test. Mem. Cognit. 2009, 37, 336–345. [Google Scholar] [CrossRef]
- Reaves, S.; Graham, B.; Grahn, J.; Rabannifard, P.; Duarte, A. Turn off the music! Music impairs visual associative memory performance in older adults. Gerontologist 2016, 56, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.F.; Schellenberg, E.G.; Letnic, A.K. Fast and loud background music disrupts reading comprehension. Psychol. Music 2012, 40, 700–708. [Google Scholar] [CrossRef]
- Västfjäll, D. Emotion induction through music: A review of the musical mood induction procedure. Music Sci. 2001, 5 (Suppl. 1), 173–211. [Google Scholar] [CrossRef]
- Schellenberg, E.G. Cognitive performance after listening to music: A review of the Mozart effect. In Music, Health, and Wellbeing; MacDonald, R.A.R., Kreutz, G., Mitchell, L., Eds.; Oxford University Press: Oxford, UK, 2012; pp. 324–338. [Google Scholar] [CrossRef]
- Fernández-Aguilar, L.; Ricarte, J.; Ros, L.; Latorre, J.M. Emotional differences in young and older adults: Films as mood induction procedure. Front. Psychol. 2018, 9, 1110. [Google Scholar] [CrossRef]
- Vieillard, S.; Gilet, A.L. Age-related differences in affective responses to and memory for emotions conveyed by music: A cross-sectional study. Front. Psychol. 2013, 4, 711. [Google Scholar] [CrossRef]
- Groarke, J.M.; Hogan, M.J. Listening to self-chosen music regulates induced negative affect for both younger and older adults. PLoS ONE 2019, 14, e0218017. [Google Scholar] [CrossRef]
- Lima, C.F.; Castro, S.L. Emotion recognition in music changes across the adult life span. Cogn. Emot. 2011, 25, 585–598. [Google Scholar] [CrossRef]
- Vieillard, S.; Roy, M.; Peretz, I. Expressiveness in musical emotions. Psychol. Res. 2012, 76, 641–653. [Google Scholar] [CrossRef]
- Rauscher, F.H.; Shaw, G.L.; Ky, C.N. Music and spatial task performance. Nature 1993, 365, 611. [Google Scholar] [CrossRef]
- Steele, K.M.; Bella, S.D.; Peretz, I.; Dunlop, T.; Dawe, L.A.; Humphrey, G.K.; Shannon, R.A.; Kirby, J.L., Jr.; Olmstead, C.G. Prelude or requiem for the ‘Mozart effect’? Nature 1999, 400, 827. [Google Scholar] [CrossRef][Green Version]
- Thompson, W.F.; Schellenberg, E.G.; Husain, G. Arousal, mood, and the Mozart effect. Psychol. Sci. 2001, 12, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Nantais, K.M.; Schellenberg, E.G. The Mozart effect: An artifact of preference. Psychol. Sci. 1999, 10, 370–373. [Google Scholar] [CrossRef]
- Schellenberg, E.G.; Hallam, S. Music listening and cognitive abilities in 10-and 11-year-olds: The blur effect. Ann. N. Y. Acad. Sci. 2005, 1060, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, E.G.; Nakata, T.; Hunter, P.G.; Tamoto, S. Exposure to music and cognitive performance: Tests of children and adults. Psychol. Music 2007, 35, 5–19. [Google Scholar] [CrossRef]
- He, W.J.; Wong, W.C.; Hui, A.N.N. Emotional reactions mediate the effect of music listening on creative thinking: Perspective of the arousal-and-mood hypothesis. Front. Psychol. 2017, 8, 1680. [Google Scholar] [CrossRef] [PubMed]
- Husain, G.; Thompson, W.F.; Schellenberg, E.G. Effects of musical tempo and mode on arousal, mood, and spatial abilities. Music Percept. 2002, 20, 151–171. [Google Scholar] [CrossRef]
- Posner, J.; Russell, J.A.; Peterson, B. The circumplex model of affect: An integrative approach to affective neuroscience, cognitive development, and psychopathology. Dev. Psychopathol. 2005, 17, 715–734. [Google Scholar] [CrossRef]
- Mammarella, N.; Fairfield, B.; Cornoldi, C. Does music enhance cognitive performance in healthy older adults? The Vivaldi effect. Aging Clin. Exp. Res. 2007, 19, 394–399. [Google Scholar] [CrossRef]
- Borella, E.; Carretti, B.; Grassi, M.; Nucci, M.; Sciore, R. Are age-related differences between young and older adults in an affective working memory test sensitive to the music effects? Front. Aging Neurosci. 2014, 6, 298. [Google Scholar] [CrossRef]
- Imbir, K.; Gołąb, M. Affective reactions to music: Norms for 120 excerpts of modern and classical music. Psychol. Music 2017, 45, 432–449. [Google Scholar] [CrossRef]
- Bradley, M.M.; Lang, P.J. Measuring emotion: The self- assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry 1994, 25, 49–59. [Google Scholar] [CrossRef]
- Mehrabian, A.; Russell, J.A. An Approach to Environmental Psychology; The MIT Press: Cambridge, MA, USA, 1974. [Google Scholar]
- Ferguson, H.J.; Brunsdon, V.E.A.; Bradford, E.E.F. The developmental trajectories of executive function from adolescence to old age. Sci. Rep. 2021, 11, 1382. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L. Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron 2004, 44, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Borella, E.; Carretti, B.; Mammarella, I. Do working memory and susceptibility to interference predict individual differences in fluid intelligence? Eur. J. Cogn. Psychol. 2006, 18, 51–69. [Google Scholar] [CrossRef]
- Borella, E.; Carretti, B.; De Beni, R. Working memory and inhibition across the adult life-span. Acta Psychol. 2008, 128, 33–44. [Google Scholar] [CrossRef]
- Arnaud, L.; Lemaire, P.; Allen, P.; Michel, B.F. Strategic aspects of young, healthy older adults’, and Alzheimer patients’ arithmetic performance. Cortex 2008, 44, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Belim, F.; Castro, S.L. The Mozart effect on the episodic memory of healthy adults is null, but low-functioning older adults may be an exception. Front. Psychol. 2020, 11, 538194. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, E. Effects of music listening and relaxation instructions on arousal changes and the working memory task in older adults. J. Music Ther. 2004, 41, 107–127. [Google Scholar] [CrossRef]
- Linnemann, A.; Wenzel, M.; Grammes, J.; Kubiak, T.; Nater, U.M. Music listening and stress in daily life—A matter of timing. Int. J. Behav. Med. 2018, 25, 223–230. [Google Scholar] [CrossRef]
- Craik, F.; Salthouse, T. The Handbook of Aging and Cognition, 3rd ed.; Psychology Press: London, UK, 2008. [Google Scholar] [CrossRef]
- Gallina, P.; Saugo, M.; Antoniazzi, M.; Fortuna, P.; Toffanin, R.; Maggi, S.; Benetollo, P.P. Validazione della scheda per la valutazione multidimensionale dell’anziano (SVAMA) (Validation of a multidimensional assessment profile for older adults). Tend. Nuove 2006, 3, 229–263. [Google Scholar] [CrossRef]
- De Beni, R.; Borella, E.; Carretti, B.; Marigo, C.; Nava, L.A. BAC. Portfolio per la Valutazione del Benessere e Delle Abilità Cognitive Nell’età Adulta e Avanzata [The Assessment of Well-being and Cognitive Abilities in Adulthood and Aging]; Giunti OS: Firenze, Italy, 2008. [Google Scholar]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988, 47, 1063–1070. [Google Scholar] [CrossRef]
- Groarke, J.M.; Hogan, M.J. Development and psychometric evaluation of the adaptive functions of music listening scale. Front. Psychol. 2018, 9, 516. [Google Scholar] [CrossRef] [PubMed]
- Borella, E.; Carretti, B.; Meneghetti, C.; Carbone, E.; Vincenzi, M.; Madonna, J.C.; Grassi, M.; Fairfield, B.; Mammarella, N. Is working memory training in older adults sensitive to music? Psychol. Res. 2019, 83, 1107–1123. [Google Scholar] [CrossRef] [PubMed]
- Pietschnig, J.; Voracek, M.; Formann, A.K. Mozart effect–Shmozart effect: A meta-analysis. Intelligence 2010, 38, 314–323. [Google Scholar] [CrossRef]
- Hunter, P.G.; Schellenberg, E.G.; Schimmack, U. Feelings and perceptions of happiness and sadness induced by music: Similarities, differences, and mixed emotions. Psychol. Aesthet. Creat. Arts 2010, 4, 47. [Google Scholar] [CrossRef]
- Carretti, B.; Cornoldi, C.; Caldarola, N.; Tencati, C. CO-TT, Comprensione Orale Test e Trattamento [Oral Comprehension, Assessment and Training]; Erickson: Trento, Italy, 2013. [Google Scholar]
- Collerton, J.; Collerton, D.; Arai, Y.; Barrass, K.; Eccles, M.; Jagger, C.; McKeith, I.; Saxby, B.K.; Kirkwood, T.; The Newcastle 85+ Study Core Team. A comparison of computerized and pencil-and-paper tasks in assessing cognitive function in community-dwelling older people in the Newcastle 85+ Pilot Study. J. Am. Geriatr. Soc. 2007, 55, 1630–1635. [Google Scholar] [CrossRef]
- Corsi, P.M. Human Memory and the Medial Temporal Region of the Brain. Ph.D. Thesis, McGill University, Montreal, QC, Canada, 1972, unpublished. [Google Scholar]
- Novelli, G.; Papagno, C.; Capitani, E.; Laiacona, M. Tre test clinici di memoria verbale a lungo termine: Taratura su soggetti normali. Arch. Psicol. Neurol. Psichiatr. 1986, 47, 278–296. [Google Scholar]
- Amodio, P.; Wenin, H.; Del Piccolo, F.; Mapelli, D.; Montagnese, S.; Pellegrini, A.; Musto, C.; Gatta, A.; Umiltà, C. Variability of trail making test, symbol digit test and line trait test in normal people. A normative study taking into account age-dependent decline and sociobiological variables. Aging Clin. Exp. Res. 2002, 14, 117–131. [Google Scholar] [CrossRef]
- Caviola, S.; Gerotto, G.; Lucangeli, D.; Mammarella, I.C. Test AC-FL: Prove di Fluenza per le Abilità di Calcolo [AC-FL Test: Math Fluency Abilities Test]; Erickson: Trento, Italy, 2016. [Google Scholar]
- Lang, P.J.; Öhman, A.; Vaitl, D. The International Affective Picture System; The Center for Research in Psychophysiology, University of Florida: Gainesville, FL, USA, 1988. [Google Scholar]
- JASP Team. JASP, version 0.16.3; Computer Software; JASP Team: Tokyo, Japan, 2022.
- Kass, R.E.; Raftery, A.E. Bayes Factors. J. Am. Stat. Assoc. 1995, 90, 773–795. [Google Scholar] [CrossRef]
- Larcom, M.J.; Isaacowitz, D.M. Rapid emotion regulation after mood induction: Age and individual differences. J. Gerontol. B Psychol. Sci. Soc. Sci. 2009, 64B, 733–741. [Google Scholar] [CrossRef]
- Laukka, P.; Juslin, P.N. Similar patterns of age-related differences in emotion recognition from speech and music. Motiv. Emot. 2007, 31, 182–191. [Google Scholar] [CrossRef]
- Sutcliffe, R.; Rendell, P.G.; Henry, J.D.; Bailey, P.E.; Ruffman, T. Music to my ears: Age-related decline in musical and facial emotion recognition. Psychol. Aging 2017, 32, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.T.; Halpern, A.R. Age-related patterns in emotions evoked by music. Psychol. Aesthet. Creat. Arts 2015, 9, 248–253. [Google Scholar] [CrossRef]
- Carstensen, L.L.; DeLiema, M. The positivity effect: A negativity bias in youth fades with age. Curr. Opin. Behav. Sci. 2018, 19, 7–12. [Google Scholar] [CrossRef]
- Fagot, D.; Mella, N.; Borella, E.; Ghisletta, P.; Lecerf, T.; De Ribaupierre, A. Intra-individual variability from a lifespan perspective: A comparison of latency and accuracy measures. J. Intell. 2018, 6, 16. [Google Scholar] [CrossRef]
- Haynes, B.I.; Bauermeister, S.; Bunce, D. A systematic review of longitudinal associations between reaction time intraindividual variability and age-related cognitive decline or impairment, dementia, and mortality. J. Int. Neuropsychol. Soc. 2017, 23, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Olderbak, S.; Wilhelm, O. Emotion perception and empathy: An individual differences test of relations. Emotion 2017, 17, 1092–1106. [Google Scholar] [CrossRef]
- Phillips, L.H.; Smith, L.; Gilhooly, K.J. The effects of adult aging and induced positive and negative mood on planning. Emotion 2002, 2, 263–272. [Google Scholar] [CrossRef]
- McCabe, D.P.; Roediger, H.L., III; McDaniel, M.A.; Balota, D.A.; Hambrick, D.Z. The relationship between working memory capacity and executive functioning: Evidence for a common executive attention construct. Neuropsychology 2010, 24, 222–243. [Google Scholar] [CrossRef]
- Von Krause, M.; Radev, S.T.; Voss, A. Mental speed is high until age 60 as revealed by analysis of over a million participants. Nat. Hum. Behav. 2022, 6, 700–708. [Google Scholar] [CrossRef]




| Short Version | Long Version | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mozart | Albinoni | Control | Mozart | Albinoni | Control | ||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | ||
| Cognition | |||||||||||||
| Working Memory | Pre | 2.73 | 1.24 | 2.59 | 1.10 | 2.50 | 0.96 | 2.32 | 0.57 | 2.82 | 1.01 | 2.64 | 1.00 |
| Post | 3.32 | 1.52 | 2.68 | 1.49 | 2.50 | 0.67 | 2.91 | 0.91 | 2.86 | 0.89 | 2.68 | 0.78 | |
| Flexibility/Speed | Pre | 118.50 | 20.11 | 117.96 | 30.74 | 119.82 | 23.61 | 122.05 | 31.60 | 123.73 | 32.03 | 124.09 | 35.98 |
| Post | 89.59 | 27.50 | 95.23 | 34.95 | 106.27 | 19.88 | 102.18 | 29.96 | 104.00 | 33.23 | 102.64 | 31.16 | |
| Verbal Fluency | Pre | 11.91 | 4.02 | 13.05 | 3.48 | 12.46 | 3.85 | 12.55 | 3.84 | 12.68 | 4.01 | 12.68 | 3.84 |
| Post | 12.86 | 3.63 | 13.00 | 2.33 | 14.05 | 4.12 | 13.05 | 5.12 | 13.59 | 3.51 | 13.86 | 3.12 | |
| Arithmetic | Pre | 38.91 | 10.94 | 38.59 | 7.06 | 39.18 | 6.32 | 36.27 | 11.27 | 37.86 | 9.53 | 36.82 | 10.51 |
| Post | 41.36 | 9.95 | 40.68 | 5.31 | 41.64 | 5.31 | 39.05 | 10.25 | 40.36 | 7.31 | 38.64 | 9.16 | |
| Emotion | |||||||||||||
| Arousal | Pre | 4.59 | 1.41 | 5.05 | 2.28 | 4.73 | 2.05 | 4.27 | 2.51 | 4.18 | 2.34 | 4.09 | 1.72 |
| Post | 5.14 | 1.91 | 3.09 | 1.41 | 4.59 | 2.04 | 5.36 | 2.48 | 3.32 | 2.17 | 4.09 | 1.44 | |
| Mood | Pre | 6.55 | 0.96 | 6.46 | 1.37 | 6.14 | 0.83 | 5.18 | 1.22 | 6.36 | 1.22 | 5.96 | 1.25 |
| Post | 6.68 | 0.89 | 6.22 | 1.34 | 5.86 | 0.99 | 7.82 | 0.96 | 6.59 | 1.30 | 5.73 | 1.12 | |
| Dominance | Pre | 6.64 | 1.40 | 7.18 | 1.33 | 6.91 | 2.09 | 7.05 | 1.68 | 6.59 | 1.62 | 6.91 | 1.27 |
| Post | 6.59 | 1.37 | 6.55 | 1.47 | 7.05 | 1.21 | 7.14 | 1.75 | 6.50 | 1.57 | 6.95 | 1.53 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vincenzi, M.; Borella, E.; Sella, E.; Lima, C.F.; De Beni, R.; Schellenberg, E.G. Music Listening, Emotion, and Cognition in Older Adults. Brain Sci. 2022, 12, 1567. https://doi.org/10.3390/brainsci12111567
Vincenzi M, Borella E, Sella E, Lima CF, De Beni R, Schellenberg EG. Music Listening, Emotion, and Cognition in Older Adults. Brain Sciences. 2022; 12(11):1567. https://doi.org/10.3390/brainsci12111567
Chicago/Turabian StyleVincenzi, Margherita, Erika Borella, Enrico Sella, César F. Lima, Rossana De Beni, and E. Glenn Schellenberg. 2022. "Music Listening, Emotion, and Cognition in Older Adults" Brain Sciences 12, no. 11: 1567. https://doi.org/10.3390/brainsci12111567
APA StyleVincenzi, M., Borella, E., Sella, E., Lima, C. F., De Beni, R., & Schellenberg, E. G. (2022). Music Listening, Emotion, and Cognition in Older Adults. Brain Sciences, 12(11), 1567. https://doi.org/10.3390/brainsci12111567







