A Potential Prognosis Indicator Based on P300 Brain–Computer Interface for Patients with Disorder of Consciousness
Abstract
1. Introduction
- (1)
- We utilized a traditional Bayesian method and CNN model to detect and analyze P300 signals in DOC patients.
- (2)
- We found a positive linear correlation between P300 detection accuracy and CRS-R score which indicated that the patients with the P300 detection accuracy could be used to assess patients’ state of consciousness.
- (3)
- The experiments demonstrated that patients with higher P300 detection accuracies tend to have higher CRS-R score after three months, which indicated better prognosis.
2. Materials and Methods
2.1. Subjects
2.2. Behavioral Assessment
2.3. Paradigm
2.4. Procedure
2.5. Convolutional Neural Network (CNN)
2.6. Bayesian Network (BN)
2.7. Evaluation
2.8. Statistics
2.9. Data Availability
3. Results
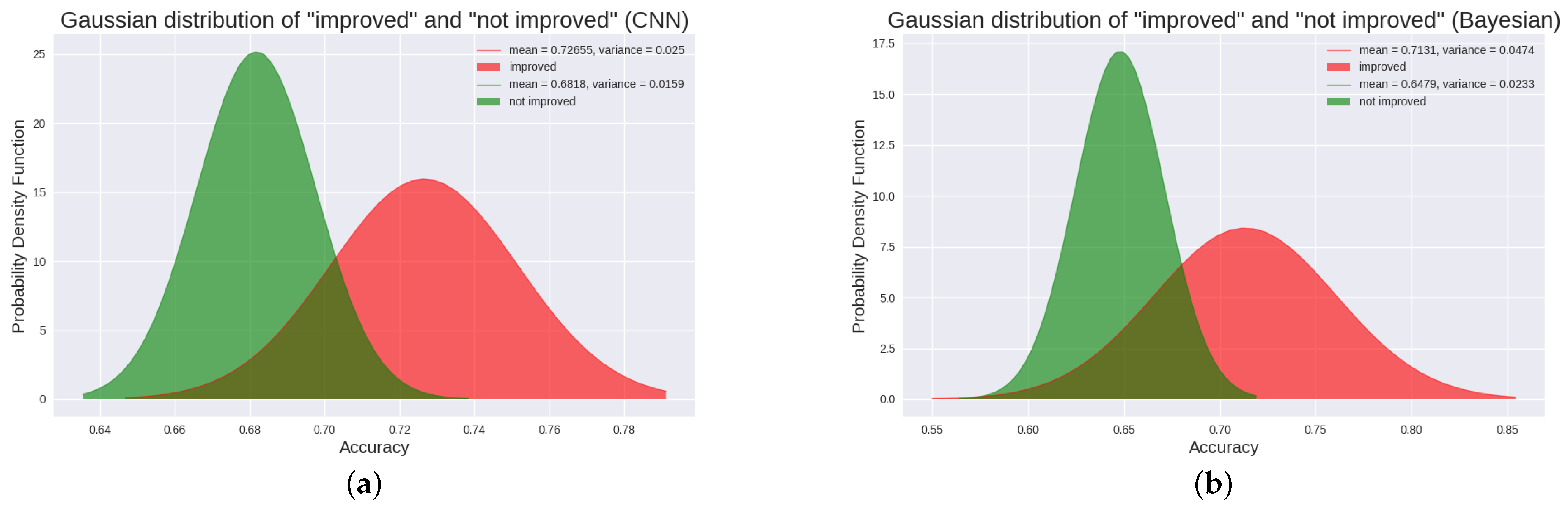
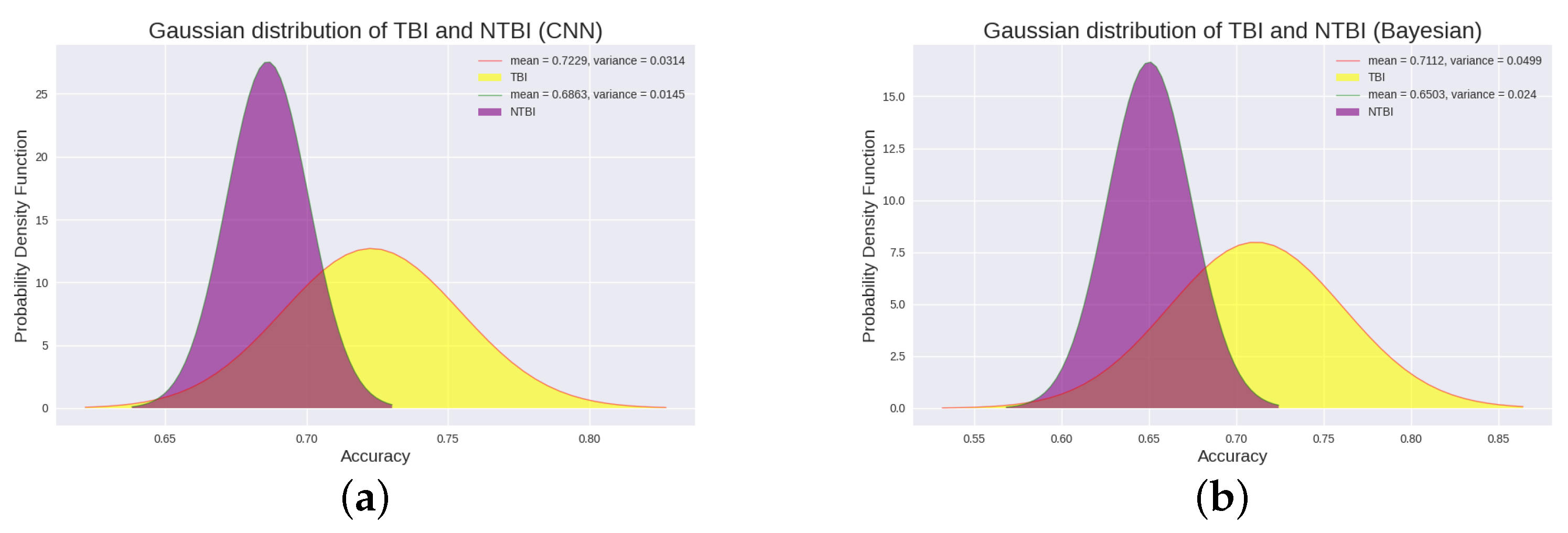
3.1. CNN Results in 18 DOC Patients
3.2. Bayesian Results in 18 DOC Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DOC | Disorders Of Consciousness |
| UWS | Unresponsive Wakefulness Syndrome |
| VS | Vegetative State |
| MCS | Minimally Conscious State |
| MCS+ | Minimally Conscious State Plus |
| MCS- | Minimally Conscious State Minus |
| ERP | Event Related Potential |
| ERPs | Event Related Potentials |
| TBI | Traumatic Brain Injury |
| NTBI | Non-Traumatic Brain Injury |
| LIS | Locked-In Syndrome |
| CRS-R | Coma Recovery Revised-Scale |
| BCI | Brain–Computer Interface |
| BCIs | Brain–Computer Interfaces |
| EEG | Electroencephalogram |
| CNN | Convolutional Neural Network |
| CNNs | Convolutional Neural Networks |
| SSVEPs | Steady state visually evoked potentials |
| SVM | Support Vector Machines |
| LDA | Linear Discriminant Analysis |
References
- Laureys, S.; Celesia, G.G.; Cohadon, F.; Lavrijsen, J.; León-Carrión, J.; Sannita, W.G.; Sazbon, L.; Schmutzhard, E.; von Wild, K.R.; Zeman, A.; et al. Unresponsive wakefulness syndrome: A new name for the vegetative state or apallic syndrome. BMC Med. 2010, 8, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Jennett, B.; Plum, F. Persistent vegetative state after brain damage: A syndrome in search of a name. Lancet 1972, 299, 734–737. [Google Scholar] [CrossRef]
- Giacino, J.T.; Ashwal, S.; Childs, N.; Cranford, R.; Jennett, B.; Katz, D.I.; Kelly, J.P.; Rosenberg, J.H.; Whyte, J.; Zafonte, R.D.; et al. The minimally conscious state: Definition and diagnostic criteria. Neurology 2002, 58, 349–353. [Google Scholar] [CrossRef]
- Bruno, M.A.; Majerus, S.; Boly, M.; Vanhaudenhuyse, A.; Schnakers, C.; Gosseries, O.; Boveroux, P.; Kirsch, M.; Demertzi, A.; Bernard, C.; et al. Functional neuroanatomy underlying the clinical subcategorization of minimally conscious state patients. J. Neurol. 2012, 259, 1087–1098. [Google Scholar] [CrossRef]
- Bruno, M.A.; Vanhaudenhuyse, A.; Thibaut, A.; Moonen, G.; Laureys, S. From unresponsive wakefulness to minimally conscious PLUS and functional locked-in syndromes: Recent advances in our understanding of disorders of consciousness. J. Neurol. 2011, 258, 1373–1384. [Google Scholar] [CrossRef]
- Thibaut, A.; Bodien, Y.G.; Laureys, S.; Giacino, J.T. Minimally conscious state “plus”: Diagnostic criteria and relation to functional recovery. J. Neurol. 2020, 267, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Sherer, M.; Christoforou, A.; Maurer-Karattup, P.; Hammond, F.M.; Long, D.; Bagiella, E. Behavioral recovery and early decision-making in patients with prolonged disturbance in consciousness after traumatic brain injury. J. Neurotrauma 2020, 37, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Plum, F.; Posner, J. The Diagnosis of Stupor and Coma; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Monti, M.M.; Laureys, S.; Owen, A.M. The vegetative state. BMJ 2010, 341, c3765. [Google Scholar] [CrossRef]
- Seel, R.T.; Sherer, M.; Whyte, J.; Katz, D.I.; Giacino, J.T.; Rosenbaum, A.M.; Hammond, F.M.; Kalmar, K.; Pape, T.L.B.; Zafonte, R.; et al. Assessment scales for disorders of consciousness: Evidence-based recommendations for clinical practice and research. Arch. Phys. Med. Rehabil. 2010, 91, 1795–1813. [Google Scholar] [CrossRef]
- Schnakers, C.; Vanhaudenhuyse, A.; Giacino, J.; Ventura, M.; Boly, M.; Majerus, S.; Moonen, G.; Laureys, S. Diagnostic accuracy of the vegetative and minimally conscious state: Clinical consensus versus standardized neurobehavioral assessment. BMC Neurol. 2009, 9, 1–5. [Google Scholar] [CrossRef]
- Giacino, J.T.; Kalmar, K.; Whyte, J. The JFK Coma Recovery Scale-Revised: Measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehabil. 2004, 85, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Schnakers, C.; Rodriguez-Moreno, D.; Kalmar, K.; Schiff, N.; Hirsch, J. Behavioral assessment in patients with disorders of consciousness: Gold standard or fool’s gold? Prog. Brain Res. 2009, 177, 33–48. [Google Scholar] [PubMed]
- Demertzi, A.; Vanhaudenhuyse, A.; Bruno, M.A.; Schnakers, C.; Boly, M.; Boveroux, P.; Maquet, P.; Moonen, G.; Laureys, S. Is there anybody in there? Detecting awareness in disorders of consciousness. Expert Rev. Neurother. 2008, 8, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, X.; Hu, Z.; Sun, Z.; Laureys, S.; Di, H. The misdiagnosis of prolonged disorders of consciousness by a clinical consensus compared with repeated coma-recovery scale-revised assessment. BMC Neurol. 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Park, S.J. HealthSOS: Real-time health monitoring system for stroke prognostics. IEEE Access 2020, 8, 213574–213586. [Google Scholar] [CrossRef]
- Hussain, I.; Park, S.J. Big-ECG: Cardiographic predictive cyber-physical system for stroke management. IEEE Access 2021, 9, 123146–123164. [Google Scholar] [CrossRef]
- Hussain, I.; Park, S.J. Quantitative evaluation of task-induced neurological outcome after stroke. Brain Sci. 2021, 11, 900. [Google Scholar] [CrossRef]
- Arnaldi, D.; Terzaghi, M.; Cremascoli, R.; De Carli, F.; Maggioni, G.; Pistarini, C.; Nobili, F.; Moglia, A.; Manni, R. The prognostic value of sleep patterns in disorders of consciousness in the sub-acute phase. Clin. Neurophysiol. 2016, 127, 1445–1451. [Google Scholar] [CrossRef]
- Azabou, E.; Fischer, C.; Mauguiere, F.; Vaugier, I.; Annane, D.; Sharshar, T.; Lofaso, F. Prospective cohort study evaluating the prognostic value of simple EEG parameters in postanoxic coma. Clin. EEG Neurosci. 2016, 47, 75–82. [Google Scholar] [CrossRef]
- Estraneo, A.; Moretta, P.; Loreto, V.; Lanzillo, B.; Cozzolino, A.; Saltalamacchia, A.; Lullo, F.; Santoro, L.; Trojano, L. Predictors of recovery of responsiveness in prolonged anoxic vegetative state. Neurology 2013, 80, 464–470. [Google Scholar] [CrossRef]
- Kang, X.G.; Li, L.; Wei, D.; Xu, X.X.; Zhao, R.; Jing, Y.Y.; Su, Y.Y.; Xiong, L.Z.; Zhao, G.; Jiang, W. Development of a simple score to predict outcome for unresponsive wakefulness syndrome. Crit. Care 2014, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Xie, Q.; He, Y.; Wang, F.; Di, H.; Laureys, S.; Yu, R.; Li, Y. Detecting awareness in patients with disorders of consciousness using a hybrid brain–computer interface. J. Neural Eng. 2014, 11, 056007. [Google Scholar] [CrossRef] [PubMed]
- Jansen, B.; Hegde, A.; Ruben, J.; Doshi, H.; Boutros, N. Evoked potential generation revealed by nonstationary signal analysis. In Proceedings of the Second Joint 24th Annual Conference and the Annual Fall Meeting of the Biomedical Engineering Society, Engineering in Medicine and Biology, Houston, TX, USA, 23–26 October 2002; Volume 1, pp. 108–109. [Google Scholar]
- Shan, H.; Liu, Y.; Stefanov, T.P. A Simple Convolutional Neural Network for Accurate P300 Detection and Character Spelling in Brain Computer Interface. In Proceedings of the IJCAI, Stockholm, Sweden, 13–19 July 2018; 2018; pp. 1604–1610. [Google Scholar]
- Liu, M.; Wu, W.; Gu, Z.; Yu, Z.; Qi, F.; Li, Y. Deep learning based on batch normalization for P300 signal detection. Neurocomputing 2018, 275, 288–297. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, G.; Mou, X.; Ravi, A.; Li, M.; Wang, Y.; Jiang, N. A convolutional neural network for the detection of asynchronous steady state motion visual evoked potential. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1303–1311. [Google Scholar] [CrossRef]
- He, L.; Liu, B.; Hu, D.; Wen, Y.; Wan, M.; Long, J. Motor imagery EEG signals analysis based on Bayesian network with Gaussian distribution. Neurocomputing 2016, 188, 217–224. [Google Scholar] [CrossRef]
- Esposito, P. BLiTZ—Bayesian Layers in Torch Zoo (a Bayesian Deep Learing Library for Torch). 2020. Available online: https://github.com/piEsposito/blitz-bayesian-deep-learning/ (accessed on 8 August 2022).
- Olson, D.L.; Delen, D. Advanced Data Mining Techniques; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Giacino, J.T.; Fins, J.J.; Laureys, S.; Schiff, N.D. Disorders of consciousness after acquired brain injury: The state of the science. Nat. Rev. Neurol. 2014, 10, 99–114. [Google Scholar] [CrossRef]
- Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; Ling, G.S.; et al. Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Arch. Phys. Med. Rehabil. 2018, 99, 1699–1709. [Google Scholar]
- Diao, Y.; Geng, M.; Fu, Y.; Wang, H.; Liu, C.; Gu, J.; Dong, J.; Mu, J.; Liu, X.; Wang, C. A combination of P300 and eye movement data improves the accuracy of auxiliary diagnoses of depression. J. Affect. Disord. 2022, 297, 386–395. [Google Scholar] [CrossRef]
- Mulligan, E.M.; Simon, J.; Lowe, M.; Santopetro, N.; Flynn, H.; Hajcak, G. The P300 and late positive potential in pregnancy prospectively predict increases in depressive and anxious symptoms in the early postpartum period. J. Affect. Disord. 2022, 317, 193–203. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.; Wang, L.; Li, Y.; Hou, J.; Duan, G.; Guo, T.; Wu, D. Outcome Prediction in Unresponsive Wakefulness Syndrome and Minimally Conscious State by Nonlinear Dynamic Analysis of the EEG. Front. Neurol. 2021, 12, 510424. [Google Scholar] [CrossRef]
- Pan, J.; Wang, L.; Huang, H.; Xiao, J.; Wang, F.; Liang, Q.; Xu, C.; Li, Y.; Xie, Q. A Hybrid Brain-Computer Interface Combining P300 Potentials and Emotion Patterns for Detecting Awareness in Patients with Disorders of Consciousness. IEEE Trans. Cogn. Dev. Syst. 2022; early access. [Google Scholar]
- Bai, Y.; Xia, X.; Li, X.; Wang, Y.; Yang, Y.; Liu, Y.; Liang, Z.; He, J. Spinal cord stimulation modulates frontal delta and gamma in patients of minimally consciousness state. Neuroscience 2017, 346, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Pugin, D.; Hofmeister, J.; Gasche, Y.; Vulliemoz, S.; Lövblad, K.O.; Van De Ville, D.; Haller, S. Resting-state brain activity for early prediction outcome in postanoxic patients in a coma with indeterminate clinical prognosis. Am. J. Neuroradiol. 2020, 41, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Hong, K.S. Most favorable stimulation duration in the sensorimotor cortex for fNIRS-based BCI. Biomed. Opt. Express 2021, 12, 5939–5954. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Hossain, M.A.; Jany, R.; Bari, M.A.; Uddin, M.; Kamal, A.R.M.; Ku, Y.; Kim, J.S. Quantitative Evaluation of EEG-Biomarkers for Prediction of Sleep Stages. Sensors 2022, 22, 3079. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Young, S.; Park, S.J. Driving-induced neurological biomarkers in an advanced driver-assistance system. Sensors 2021, 21, 6985. [Google Scholar] [CrossRef] [PubMed]
- Sutton, S.; Braren, M.; Zubin, J.; John, E. Evoked-potential correlates of stimulus uncertainty. Science 1965, 150, 1187–1188. [Google Scholar] [CrossRef]
- Li, Y.; Pan, J.; Long, J.; Yu, T.; Wang, F.; Yu, Z.; Wu, W. Multimodal BCIs: Target detection, multidimensional control, and awareness evaluation in patients with disorder of consciousness. Proc. IEEE 2015, 104, 332–352. [Google Scholar]
- Zhang, Y.; Li, R.; Du, J.; Huo, S.; Hao, J.; Song, W. Coherence in P300 as a predictor for the recovery from disorders of consciousness. Neurosci. Lett. 2017, 653, 332–336. [Google Scholar] [CrossRef]
- Spataro, R.; Heilinger, A.; Allison, B.; De Cicco, D.; Marchese, S.; Gregoretti, C.; La Bella, V.; Guger, C. Preserved somatosensory discrimination predicts consciousness recovery in unresponsive wakefulness syndrome. Clin. Neurophysiol. 2018, 129, 1130–1136. [Google Scholar] [CrossRef]
- Giacino, J.T.; Kalmar, K. The vegetative and minimally conscious states: A comparison of clinical features and functional outcome. J. Head Trauma Rehabil. 1997, 12, 36–51. [Google Scholar] [CrossRef]
- Claassen, J.; Doyle, K.; Matory, A.; Couch, C.; Burger, K.M.; Velazquez, A.; Okonkwo, J.U.; King, J.R.; Park, S.; Agarwal, S.; et al. Detection of brain activation in unresponsive patients with acute brain injury. N. Engl. J. Med. 2019, 380, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Faugeras, F.; Rohaut, B.; Valente, M.; Sitt, J.; Demeret, S.; Bolgert, F.; Weiss, N.; Grinea, A.; Marois, C.; Quirins, M.; et al. Survival and consciousness recovery are better in the minimally conscious state than in the vegetative state. Brain Inj. 2018, 32, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Fins, J. Rights Come to Mind: Brain Injury, Ethics, and the Struggle for Consciousness; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Edlow, B.L.; Claassen, J.; Schiff, N.D.; Greer, D.M. Recovery from disorders of consciousness: Mechanisms, prognosis and emerging therapies. Nat. Rev. Neurol. 2021, 17, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, M.; Volpato, C.; Silvoni, S.; Sacchetto, M.; Merico, A.; Piccione, F. Event-related brain potential modulation in patients with severe brain damage. Clin. Neurophysiol. 2011, 122, 719–724. [Google Scholar] [CrossRef]
- Annen, J.; Mertel, I.; Xu, R.; Chatelle, C.; Lesenfants, D.; Ortner, R.; Bonin, E.A.; Guger, C.; Laureys, S.; Müller, F. Auditory and somatosensory p3 are complementary for the assessment of patients with disorders of consciousness. Brain Sci. 2020, 10, 748. [Google Scholar] [CrossRef]
- Risetti, M.; Formisano, R.; Toppi, J.; Quitadamo, L.R.; Bianchi, L.; Astolfi, L.; Cincotti, F.; Mattia, D. On ERPs detection in disorders of consciousness rehabilitation. Front. Hum. Neurosci. 2013, 7, 775. [Google Scholar] [CrossRef]





| CRS-R Scores (Subscores) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Patients | Age (Years Old) | Gender | Etiology | Time Since Injury (Months) | Diagnosis (Before) | Diagnosis (After) | Before the Experiment | After the Experiment |
| P1 | 43 | M | TBI | 5 | UWS | MCS+ | 5 (1-0-1-1-0-2) | 9 (3-1-2-1-0-2) |
| P2 | 51 | M | TBI | 20 | UWS | UWS | 9 (2-1-2-2-0-2) | 9 (2-1-2-2-0-2) |
| P3 | 29 | M | ABI | 8.5 | UWS | UWS | 4 (1-0-1-0-0-2) | 4 (1-0-1-0-0-2) |
| P4 | 37 | M | ABI | 2 | UWS | UWS | 5 (0-0-2-1-0-2) | 5 (0-0-2-1-0-2) |
| P5 | 38 | M | TBI | 1 | UWS | UWS | 7 (1-1-2-1-0-2) | 7 (1-1-2-1-0-2) |
| P6 | 33 | M | TBI | 2 | UWS | UWS | 7 (1-0-2-2-0-2) | 7 (1-0-2-2-0-2) |
| P7 | 40 | M | ABI | 2 | UWS | UWS | 5 (1-0-2-0-0-2) | 7 (1-0-2-2-0-2) |
| P8 | 52 | M | CVD | 4.5 | UWS | MCS- | 5 (1-1-0-1-0-2) | 9 (2-3-0-2-0-2) |
| P9 | 42 | M | CVD | 4 | UWS | MCS+ | 7 (1-1-2-1-0-2) | 11 (3-2-3-1-0-2) |
| P10 | 26 | M | CVD | 1 | UWS | MCS+ | 7 (1-1-2-1-0-2) | 15 (4-5-2-1-1-2) |
| P11 | 48 | M | TBI | 3.5 | MCS- | MCS+ | 12 (1-2-5-1-0-2) | 16 (3-3-5-2-1-2) |
| P12 | 34 | M | TBI | 1.5 | MCS- | MCS+ | 9 (1-1-5-1-0-1) | 15 (4-4-5-1-0-1) |
| P13 | 37 | M | TBI | 4 | MCS- | MCS+ | 9 (1-3-2-1-0-2) | 19 (3-5-6-2-1-2) |
| P14 | 20 | M | TBI | 4 | MCS- | MCS- | 7 (1-0-3-1-0-2) | 7 (1-0-3-1-0-2) |
| P15 | 19 | M | ABI | 1.5 | MCS- | MCS- | 8 (1-1-3-1-0-2) | 8 (1-1-3-1-0-2) |
| P16 | 17 | M | TBI | 2 | MCS- | MCS+ | 8 (1-1-3-1-0-2) | 18 (4-5-3-1-2-3) |
| P17 | 46 | F | TBI | 1.5 | MCS- | MCS+ | 7 (1-0-3-1-0-2) | 19 (3-5-6-2-1-2) |
| P18 | 46 | M | CVD | 2 | MCS- | MCS+ | 9 (1-1-4-1-0-2) | 20 (4-5-6-2-1-2) |
| Patients | Total Number of Samples | Number of P300 | Number of Non-P300 |
|---|---|---|---|
| P1 | 1100 | 550 | 550 |
| P2 | 900 | 450 | 450 |
| P3 | 2880 | 1395 | 1485 |
| P4 | 2880 | 1398 | 1482 |
| P5 | 2880 | 1376 | 1504 |
| P6 | 2880 | 1387 | 1493 |
| P7 | 2880 | 1378 | 1502 |
| P8 | 3400 | 1647 | 1753 |
| P9 | 2880 | 1388 | 1492 |
| P10 | 2880 | 1389 | 1491 |
| P11 | 1080 | 540 | 540 |
| P12 | 1100 | 550 | 550 |
| P13 | 760 | 380 | 380 |
| P14 | 1100 | 550 | 550 |
| P15 | 2880 | 1379 | 1501 |
| P16 | 3000 | 1454 | 1546 |
| P17 | 2880 | 1386 | 1494 |
| P18 | 2880 | 1389 | 1491 |
| Patients | Age (Years Old) | Etiology | Time Since Injury (Months) | Diagnosis (Before) | Diagnosis (After) | Improved/Not Improved | Accuracy Rate | F1_SCORE |
|---|---|---|---|---|---|---|---|---|
| P1 | 43 | TBI | 5 | UWS | MCS+ | improved | 74.00% | 0.7436 |
| P2 | 51 | TBI | 20 | UWS | UWS | not improved | 67.22% | 0.6308 |
| P3 | 29 | ABI | 8.5 | UWS | UWS | not improved | 68.30% | 0.5803 |
| P4 | 37 | ABI | 2 | UWS | UWS | not improved | 67.95% | 0.5882 |
| P5 | 38 | TBI | 1 | UWS | UWS | not improved | 71.42% | 0.6224 |
| P6 | 33 | TBI | 2 | UWS | UWS | not improved | 68.99% | 0.5800 |
| P7 | 40 | ABI | 2 | UWS | UWS | not improved | 65.91% | 0.5452 |
| P8 | 52 | CVD | 4.5 | UWS | MCS- | improved | 69.55% | 0.5843 |
| P9 | 42 | CVD | 4 | UWS | MCS+ | improved | 70.14% | 0.6160 |
| P10 | 26 | CVD | 1 | UWS | MCS+ | improved | 70.00% | 0.6130 |
| P11 | 48 | TBI | 3.5 | MCS- | MCS+ | improved | 73.70% | 0.7373 |
| P12 | 34 | TBI | 1.5 | MCS- | MCS+ | improved | 74.73% | 0.7262 |
| P13 | 37 | TBI | 4 | MCS- | MCS+ | improved | 74.74% | 0.7485 |
| P14 | 20 | TBI | 4 | MCS- | MCS- | not improved | 67.95% | 0.5863 |
| P15 | 19 | ABI | 1.5 | MCS- | MCS- | not improved | 67.67% | 0.5512 |
| P16 | 17 | TBI | 2 | MCS- | MCS+ | improved | 75.27% | 0.7162 |
| P17 | 46 | TBI | 1.5 | MCS- | MCS+ | improved | 74.91% | 0.7230 |
| P18 | 46 | CVD | 2 | MCS- | MCS+ | improved | 69.51% | 0.6033 |
| Patients | Precision | Sensitivity | Specificity | NPV | AUPRC | F | F |
|---|---|---|---|---|---|---|---|
| P1 | 0.7854 | 0.7836 | 0.6964 | 0.8141 | 0.8386 | 0.7564 | 0.7590 |
| P2 | 0.7336 | 0.6022 | 0.7422 | 0.6918 | 0.7673 | 0.6806 | 0.6066 |
| P3 | 0.8041 | 0.4558 | 0.8964 | 0.6376 | 0.7617 | 0.6958 | 0.4984 |
| P4 | 0.7833 | 0.4719 | 0.8754 | 0.6374 | 0.7558 | 0.6911 | 0.5124 |
| P5 | 0.8462 | 0.4926 | 0.9170 | 0.6638 | 0.7906 | 0.7396 | 0.5374 |
| P6 | 0.8198 | 0.4511 | 0.9117 | 0.6429 | 0.7676 | 0.7023 | 0.4949 |
| P7 | 0.7654 | 0.4242 | 0.8798 | 0.6199 | 0.7342 | 0.6586 | 0.4655 |
| P8 | 0.8067 | 0.4613 | 0.9102 | 0.6511 | 0.7628 | 0.6984 | 0.5034 |
| P9 | 0.8392 | 0.4935 | 0.8950 | 0.6525 | 0.7884 | 0.7308 | 0.5353 |
| P10 | 0.8258 | 0.4888 | 0.8969 | 0.6518 | 0.7805 | 0.7246 | 0.5317 |
| P11 | 0.7892 | 0.7044 | 0.7037 | 0.8084 | 0.8372 | 0.7551 | 0.7485 |
| P12 | 0.8172 | 0.7200 | 0.7745 | 0.7995 | 0.8386 | 0.7675 | 0.7141 |
| P13 | 0.7958 | 0.7868 | 0.7079 | 0.8275 | 0.8446 | 0.7637 | 0.7626 |
| P14 | 0.7918 | 0.4697 | 0.8743 | 0.6379 | 0.7584 | 0.6930 | 0.5098 |
| P15 | 0.7909 | 0.4334 | 0.9023 | 0.6362 | 0.7485 | 0.6665 | 0.4734 |
| P16 | 0.8465 | 0.6727 | 0.8327 | 0.7693 | 0.8414 | 0.7801 | 0.6822 |
| P17 | 0.8108 | 0.7164 | 0.7818 | 0.8006 | 0.8345 | 0.7632 | 0.7108 |
| P18 | 0.8176 | 0.4801 | 0.8955 | 0.6486 | 0.7742 | 0.7151 | 0.5226 |
| Patients | Age (Years Old) | Etiology | Time Since Injury (Months) | Diagnosis (Before) | Diagnosis (After) | Improved/Not Improved | Accuracy Rate | F1_SCORE |
|---|---|---|---|---|---|---|---|---|
| P1 | 43 | TBI | 5 | UWS | MCS+ | improved | 75.55% | 0.7803 |
| P2 | 51 | TBI | 20 | UWS | UWS | not improved | 67.56% | 0.6035 |
| P3 | 29 | ABI | 8.5 | UWS | UWS | not improved | 62.88% | 0.5327 |
| P4 | 37 | ABI | 2 | UWS | UWS | not improved | 62.08% | 0.5966 |
| P5 | 38 | TBI | 1 | UWS | UWS | not improved | 68.06% | 0.6259 |
| P6 | 33 | TBI | 2 | UWS | UWS | not improved | 62.81% | 0.6099 |
| P7 | 40 | ABI | 2 | UWS | UWS | not improved | 63.47% | 0.6046 |
| P8 | 52 | CVD | 4.5 | UWS | MCS- | improved | 64.00% | 0.5968 |
| P9 | 42 | CVD | 4 | UWS | MCS+ | improved | 68.96% | 0.6663 |
| P10 | 26 | CVD | 1 | UWS | MCS+ | improved | 67.43% | 0.6234 |
| P11 | 48 | TBI | 3.5 | MCS- | MCS+ | improved | 75.28% | 0.7252 |
| P12 | 34 | TBI | 1.5 | MCS- | MCS+ | improved | 77.00% | 0.7702 |
| P13 | 37 | TBI | 4 | MCS- | MCS+ | improved | 74.34% | 0.7403 |
| P14 | 20 | TBI | 4 | MCS- | MCS- | not improved | 66.53% | 0.6269 |
| P15 | 19 | ABI | 1.5 | MCS- | MCS- | not improved | 64.90% | 0.5692 |
| P16 | 17 | TBI | 2 | MCS- | MCS+ | improved | 68.23% | 0.6966 |
| P17 | 46 | TBI | 1.5 | MCS- | MCS+ | improved | 75.82% | 0.7443 |
| P18 | 46 | CVD | 2 | MCS- | MCS+ | improved | 66.49% | 0.6842 |
| Patients | Precision | Sensitivity | Specificity | NPV | AUPRC | F | F |
|---|---|---|---|---|---|---|---|
| P1 | 0.7310 | 0.9000 | 0.6109 | 0.9077 | 0.8405 | 0.7425 | 0.8426 |
| P2 | 0.7517 | 0.5444 | 0.8067 | 0.6809 | 0.7620 | 0.6768 | 0.5606 |
| P3 | 0.6846 | 0.4735 | 0.7741 | 0.6223 | 0.7065 | 0.6047 | 0.4908 |
| P4 | 0.6144 | 0.6372 | 0.6053 | 0.6884 | 0.7138 | 0.5997 | 0.6138 |
| P5 | 0.7520 | 0.5803 | 0.7731 | 0.6763 | 0.7665 | 0.6870 | 0.5919 |
| P6 | 0.6494 | 0.6385 | 0.6195 | 0.6687 | 0.7311 | 0.6240 | 0.6193 |
| P7 | 0.6450 | 0.6211 | 0.6473 | 0.6856 | 0.7237 | 0.6211 | 0.6078 |
| P8 | 0.6668 | 0.5864 | 0.6904 | 0.6496 | 0.7267 | 0.6262 | 0.5863 |
| P9 | 0.6969 | 0.6905 | 0.6882 | 0.7472 | 0.7682 | 0.6768 | 0.6748 |
| P10 | 0.7192 | 0.6017 | 0.7418 | 0.7037 | 0.7564 | 0.6689 | 0.6033 |
| P11 | 0.8221 | 0.7093 | 0.7963 | 0.7876 | 0.8384 | 0.7696 | 0.7084 |
| P12 | 0.8536 | 0.7600 | 0.7800 | 0.7915 | 0.8668 | 0.8098 | 0.7561 |
| P13 | 0.7388 | 0.8158 | 0.6711 | 0.8576 | 0.8234 | 0.7281 | 0.7777 |
| P14 | 0.7114 | 0.6118 | 0.7150 | 0.6938 | 0.7550 | 0.6668 | 0.6107 |
| P15 | 0.7171 | 0.5146 | 0.7758 | 0.6512 | 0.7335 | 0.6381 | 0.5298 |
| P16 | 0.6773 | 0.7861 | 0.5872 | 0.7745 | 0.7829 | 0.6763 | 0.7412 |
| P17 | 0.7989 | 0.7582 | 0.7582 | 0.8147 | 0.8390 | 0.7669 | 0.7455 |
| P18 | 0.6405 | 0.7796 | 0.5581 | 0.7620 | 0.7632 | 0.6521 | 0.7343 |
| Study | Time Since Injury | Etiology | Diagnosis | Results | Conclusions |
|---|---|---|---|---|---|
| Zhang et al. [44] | 1.6–21 months | 8 TBI, 10 NTBI | 2 coma, 9 VS/UWS, 7 MCS | All of MCS and four out of nine UWS/VS showed an intact P300 in 8 paradigm. All of the five patients with P300 in both paradigms were finally awake after 12 months, while none of the eight patients without P300 regained consciousness. | A highly significant relationship between P300 and subsequent recovery was found. |
| Rosseal et al. [45] | 35–360 days | 3 TBI, 10 NTBI | 13 UWS | 4 UWS patients demonstrated clear EEG-based indices of task following in one or both paradigms, which did not correlate with clinical factors. The efficacy of somatosensory discrimination strongly correlated with the clinical outcome at 6 months. The BCI system also yielded the expected results with healthy controls. | Neurophysiological correlates of somatosensory discrimination can be detected in clinically unresponsive patients and are associated with recovery of behavioral responsiveness at six months. |
| Ours | 1–20 months | 10 TBI, 8 NTBI | 10 VS/UWS, 8 MCS | The results showed that the P300 detection accuracy of “improved” patients and “not improved” patients, TBI patients and NTBI patients was significantly different (p < 0.05). Moreover, there was a positive correlation between CRS-R score and P300 detection accuracy. | The current clinical significance of our findings is that P300 can be used to effectively classify patients with better or worse recovery of consciousness at a later stage, and predict patients who are more likely to recover, revealing the potential prognostic value of P300. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Huang, B.; Wang, F.; Xie, Q.; Xu, C.; Huang, H.; Pan, J. A Potential Prognosis Indicator Based on P300 Brain–Computer Interface for Patients with Disorder of Consciousness. Brain Sci. 2022, 12, 1556. https://doi.org/10.3390/brainsci12111556
Li J, Huang B, Wang F, Xie Q, Xu C, Huang H, Pan J. A Potential Prognosis Indicator Based on P300 Brain–Computer Interface for Patients with Disorder of Consciousness. Brain Sciences. 2022; 12(11):1556. https://doi.org/10.3390/brainsci12111556
Chicago/Turabian StyleLi, Jingcong, Biao Huang, Fei Wang, Qiuyou Xie, Chengwei Xu, Haiyun Huang, and Jiahui Pan. 2022. "A Potential Prognosis Indicator Based on P300 Brain–Computer Interface for Patients with Disorder of Consciousness" Brain Sciences 12, no. 11: 1556. https://doi.org/10.3390/brainsci12111556
APA StyleLi, J., Huang, B., Wang, F., Xie, Q., Xu, C., Huang, H., & Pan, J. (2022). A Potential Prognosis Indicator Based on P300 Brain–Computer Interface for Patients with Disorder of Consciousness. Brain Sciences, 12(11), 1556. https://doi.org/10.3390/brainsci12111556






