Altered Functional Connectivity of Basal Ganglia in Mild Cognitive Impairment and Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Acquisition
2.3. Functional Image Preprocessing
2.4. Voxel-Based FCD Analysis
2.5. Functional Connectivity and Graph Theory Analyses
2.6. Statistical Analyses
3. Results
3.1. Demographic and Clinical Features
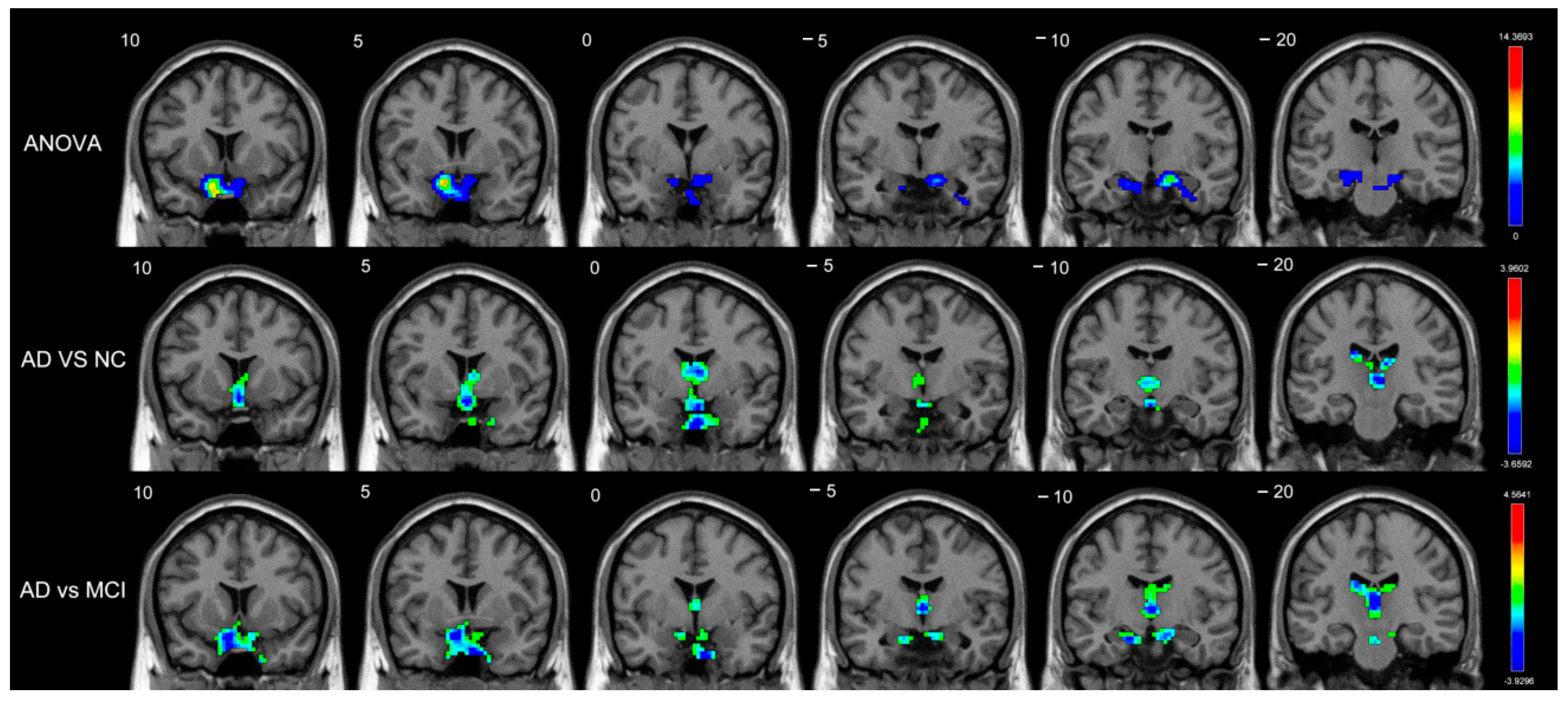
3.2. FCD Differences among the AD, MCI, and Normal Control Groups
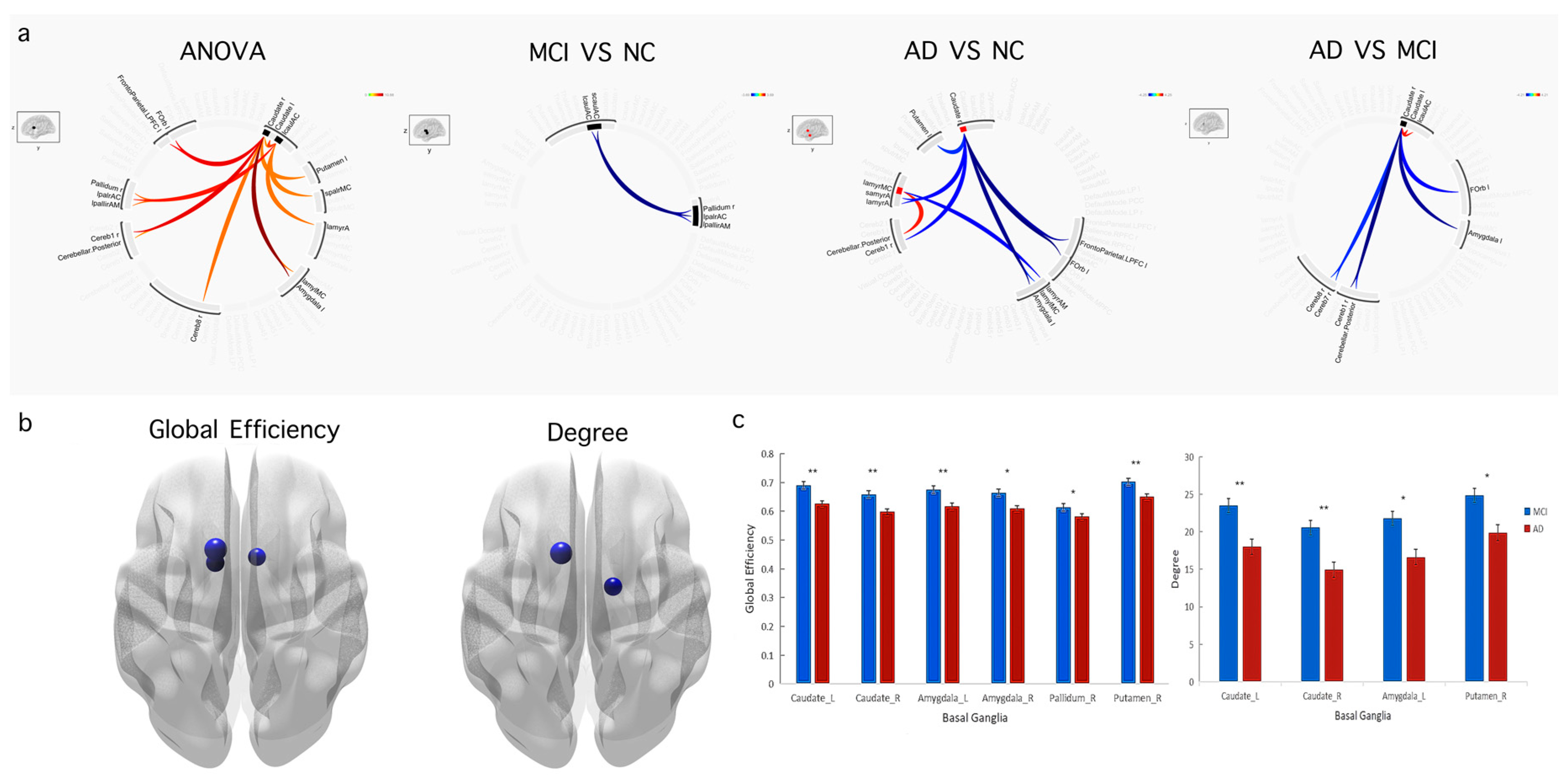
3.3. Functional Connectivity and Graph Theory Differences among the AD, MCI, and Normal Control Groups
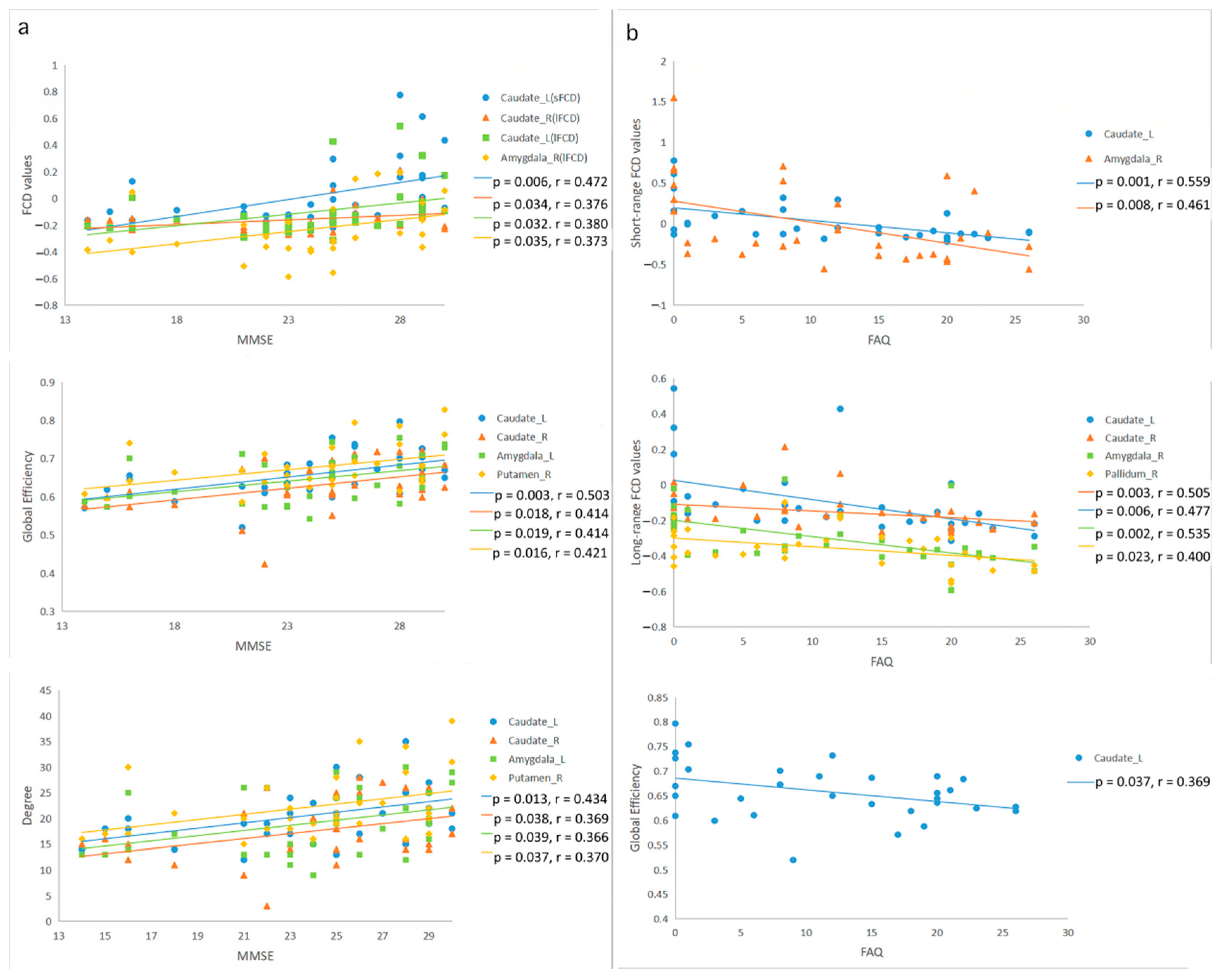
3.4. Correlation among FCD, Graph Theory Indices, and Clinical Cognition Function Assessments
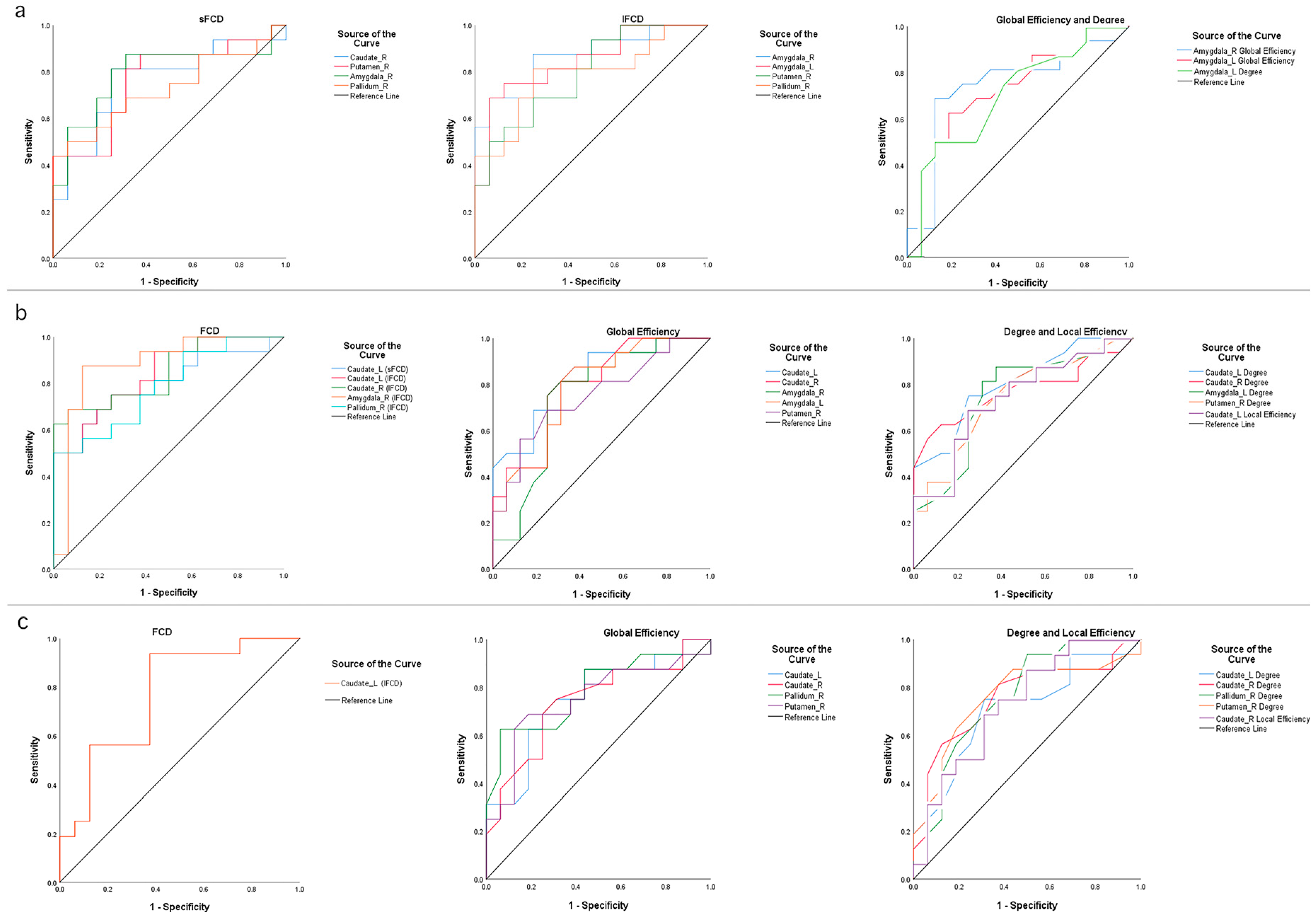
3.5. Classification Performances of FCD and Graph Theory Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gauthier, S.; Rosa-Neto, P.; Morais, J.A.; Webster, C. World Alzheimer Report 2021: Journey through the diagnosis of dementia 2021; Alzheimer’s Disease International: London, UK, 2021. [Google Scholar]
- Spires-Jones, T.L.; Hyman, B.T. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron 2014, 82, 756–771. [Google Scholar] [CrossRef] [PubMed]
- Canuet, L.; Pusil, S.; López, M.E.; Bajo, R.; Pineda-Pardo, J.Á.; Cuesta, P.; Gálvez, G.; Gaztelu, J.M.; Lourido, D.; García-Ribas, G.; et al. Network Disruption and Cerebrospinal Fluid Amyloid-Beta and Phospho-Tau Levels in Mild Cognitive Impairment. J. Neurosci. 2015, 35, 10325–10330. [Google Scholar] [CrossRef] [PubMed]
- Sheline, Y.I.; Raichle, M.E. Resting state functional connectivity in preclinical Alzheimer’s disease. Biol. Psychiatry 2013, 74, 340–347. [Google Scholar] [CrossRef]
- Berron, D.; van Westen, D.; Ossenkoppele, R.; Strandberg, O.; Hansson, O. Medial temporal lobe connectivity and its associations with cognition in early Alzheimer’s disease. Brain 2020, 143, 1233–1248. [Google Scholar] [CrossRef]
- Trzepacz, P.T.; Yu, P.; Bhamidipati, P.K.; Willis, B.; Forrester, T.; Tabas, L.; Schwarz, A.J.; Saykin, A.J. Frontolimbic atrophy is associated with agitation and aggression in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement 2013, 9 (Suppl. S5), S95–S104.e1. [Google Scholar] [CrossRef]
- Jin, D.; Wang, P.; Zalesky, A.; Liu, B.; Song, C.; Wang, D.; Xu, K.; Yang, H.; Zhang, Z.; Yao, H.; et al. Grab-AD: Generalizability and reproducibility of altered brain activity and diagnostic classification in Alzheimer’s Disease. Hum. Brain Mapp. 2020, 41, 3379–3391. [Google Scholar] [CrossRef]
- Haber, S.N.; Kim, K.-S.; Mailly, P.; Calzavara, R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J. Neurosci. 2006, 26, 8368–8376. [Google Scholar] [CrossRef]
- Haber, S.N. Corticostriatal circuitry. Dialogues Clin. Neurosci. 2016, 18, 7–21. [Google Scholar] [CrossRef]
- Barnes, T.D.; Kubota, Y.; Hu, D.; Jin, D.Z.; Graybiel, A.M. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature 2005, 437, 1158–1161. [Google Scholar] [CrossRef]
- Goodman, J.; Ressler, R.L.; Packard, M.G. Enhancing and impairing extinction of habit memory through modulation of NMDA receptors in the dorsolateral striatum. Neuroscience 2017, 352, 216–225. [Google Scholar] [CrossRef]
- Rolinski, M. Aberrant functional connectivity within the basal ganglia of patients with Parkinson’s disease. Neuroimage Clin. 2015, 8, 126–132. [Google Scholar] [CrossRef]
- Luo, C.; Li, Q.; Xia, Y.; Lei, X.; Xue, K.; Yao, Z.; Lai, Y.; Martínez-Montes, E.; Liao, W.; Zhou, D.; et al. Resting state basal ganglia network in idiopathic generalized epilepsy. Hum. Brain Mapp. 2012, 33, 1279–1294. [Google Scholar] [CrossRef] [PubMed]
- Marchand, W.R.; Lee, J.N.; Suchy, Y.; Johnson, S.; Thatcher, J.; Gale, P. Aberrant functional connectivity of cortico-basal ganglia circuits in major depression. Neurosci. Lett. 2012, 514, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Watanabe, H.; Bagarinao, E.; Ohdake, R.; Hara, K.; Ogura, A.; Masuda, M.; Kato, T.; Tsuboi, T.; Maesawa, S.; et al. Cerebello-basal ganglia connectivity fingerprints related to motor/cognitive performance in Parkinson’s disease. Parkinsonism. Relat. Disord. 2020, 80, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Szabo, A.B.; Cretin, B.; Gérard, F.; Curot, J.; J Barbeau, E.; Pariente, J.; Dahan, L.; Valton, L. Sleep: The Tip of the Iceberg in the Bidirectional Link Between Alzheimer’s Disease and Epilepsy. Front Neurol. 2022, 13, 836292. [Google Scholar] [CrossRef]
- Roy, J.-C.; Houvenaghel, J.-F.; Duprez, J.; Guillery, M.; Drapier, D.; Robert, G. Dynamics of cognitive action control in late-life depression during action selection. J. Psychiatr. Res. 2021, 143, 276–284. [Google Scholar] [CrossRef]
- Zuo, X.-N.; Di Martino, A.; Kelly, C.; Shehzad, Z.E.; Gee, D.G.; Klein, D.F.; Castellanos, F.X.; Biswal, B.B.; Milham, M.P. The oscillating brain: Complex and reliable. Neuroimage 2010, 49, 1432–1445. [Google Scholar] [CrossRef]
- Spadone, S.; Della Penna, S.; Sestieri, C.; Betti, V.; Tosoni, A.; Perrucci, M.G.; Romani, G.L.; Corbetta, M. Dynamic reorganization of human resting-state networks during visuospatial attention. Proc. Natl. Acad. Sci. USA 2015, 112, 8112–8117. [Google Scholar] [CrossRef]
- He, Y.; Chen, Z.J.; Evans, A.C. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb. Cortex 2007, 17, 2407–2419. [Google Scholar] [CrossRef]
- Buckner, R.L.; Sepulcre, J.; Talukdar, T.; Krienen, F.M.; Liu, H.; Hedden, T.; Andrews-Hanna, J.R.; Sperling, R.A.; Johnson, K.A. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer’s disease. J. Neurosci. 2009, 29, 1860–1873. [Google Scholar] [CrossRef]
- Zhi, D.; Calhoun, V.D.; Lv, L.; Ma, X.; Ke, Q.; Fu, Z.; Du, Y.; Yang, Y.; Yang, X.; Pan, M.; et al. Aberrant Dynamic Functional Network Connectivity and Graph Properties in Major Depressive Disorder. Front Psychiatry 2018, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.J.; Yang, Y.; Wang, Y. Interictal epileptiform discharges changed epilepsy-related brain network architecture in BECTS. Brain Imaging Behav. 2022, 16, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Vicari, G.; Adenzato, M. Is recursion language-specific? Evidence of recursive mechanisms in the structure of intentional action. Conscious. Cogn. 2014, 26, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Rajmohan, R.; Anderson, R.C.; Fang, D.; Meyer, A.G.; Laengvejkal, P.; Julayanont, P.; Hannabas, G.; Linton, K.; Culberson, J.; Khan, H.M.R.; et al. White Matter Deterioration May Foreshadow Impairment of Emotional Valence Determination in Early-Stage Dementia of the Alzheimer Type. Front Aging Neurosci. 2017, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Habas, C.; Kamdar, N.; Nguyen, D.; Prater, K.; Beckmann, C.F.; Menon, V.; Greicius, M.D. Distinct cerebellar contributions to intrinsic connectivity networks. J. Neurosci. 2009, 29, 8586–8594. [Google Scholar] [CrossRef]
- Kirova, A.M.; Bays, R.B.; Lagalwar, S. Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. Biomed. Res. Int. 2015, 2015, 748212. [Google Scholar] [CrossRef]
- Frank, M.J.; Badre, D. Mechanisms of hierarchical reinforcement learning in corticostriatal circuits 1: Computational analysis. Cereb. Cortex 2012, 22, 509–526. [Google Scholar] [CrossRef]
- Badre, D.; Frank, M.J. Mechanisms of hierarchical reinforcement learning in cortico-striatal circuits 2: Evidence from fMRI. Cereb. Cortex 2012, 22, 527–536. [Google Scholar] [CrossRef]
- D’Esposito, M.; Postle, B.R. The cognitive neuroscience of working memory. Annu. Rev. Psychol. 2015, 66, 115–142. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, X.; Song, H.; Li, K.; Wang, Z. Altered Functional Connectivity of Cognitive-Related Cerebellar Subregions in Alzheimer’s Disease. Front Aging Neurosci. 2017, 9, 143. [Google Scholar] [CrossRef]
- Barrett, D.G.; Denève, S.; Machens, C.K. Optimal compensation for neuron loss. Elife 2016, 5, e12454. [Google Scholar] [CrossRef] [PubMed]
- Ammassari-Teule, M. Neural compensation in presymptomatic hAPP mouse models of Alzheimer’s disease. Learn. Mem. 2020, 27, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Gillan, C.M.; Robbins, T.W. Goal-directed learning and obsessive-compulsive disorder. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130475. [Google Scholar] [CrossRef]
- Goodman, J.; Packard, M.G. Memory Systems and the Addicted Brain. Front. Psychiatry 2016, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Goodman, J.; McClay, M.; Dunsmoor, J.E. Threat-induced modulation of hippocampal and striatal memory systems during navigation of a virtual environment. Neurobiol. Learn Mem. 2020, 168, 107160. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, L.; Tegenthoff, M.; Höffken, O.; Wolf, O.T. Mineralocorticoid receptor blockade prevents stress-induced modulation of multiple memory systems in the human brain. Biol. Psychiatry 2013, 74, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Goode, T.D.; Leong, K.-C.; Goodman, J.; Maren, S.; Packard, M.G. Enhancement of striatum-dependent memory by conditioned fear is mediated by beta-adrenergic receptors in the basolateral amygdala. Neurobiol. Stress 2016, 3, 74–82. [Google Scholar] [CrossRef]
- Vytal, K.E.; Overstreet, C.; Charney, D.R.; Robinson, O.J.; Grillon, C. Sustained anxiety increases amygdala-dorsomedial prefrontal coupling: A mechanism for maintaining an anxious state in healthy adults. J. Psychiatry Neurosci. 2014, 39, 321–329. [Google Scholar] [CrossRef]
- Bostan, A.C.; Strick, P.L. The basal ganglia and the cerebellum: Nodes in an integrated network. Nat. Rev. Neurosci. 2018, 19, 338–350. [Google Scholar] [CrossRef]
- Qi, Z.; An, Y.; Zhang, M.; Li, H.-J.; Lu, J. Altered Cerebro-Cerebellar Limbic Network in AD Spectrum: A Resting-State fMRI Study. Front. Neural. Circuits 2019, 13, 72. [Google Scholar] [CrossRef]
- Rönicke, R.; Mikhaylova, M.; Rönicke, S.; Meinhardt, J.; Schröder, U.H.; Fändrich, M.; Reiser, G.; Kreutz, M.R.; Reymann, K.G. Early neuronal dysfunction by amyloid β oligomers depends on activation of NR2B-containing NMDA receptors. Neurobiol. Aging 2011, 32, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- Simón, A.M.; de Maturana, R.L.; Ricobaraza, A.; Escribano, L.; Schiapparelli, L.; Cuadrado-Tejedor, M.; Pérez-Mediavilla, A.; Avila, J.; Del Río, J.; Frechilla, D. Early changes in hippocampal Eph receptors precede the onset of memory decline in mouse models of Alzheimer’s disease. J. Alzheimers Dis. 2009, 17, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H. MRI morphometry in Alzheimer’s disease. Ageing Res. Rev. 2016, 30, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Cui, B.; Han, Y.; Song, H.; Li, K.; He, Y.; Wang, Z. Disrupted Regional Cerebral Blood Flow, Functional Activity and Connectivity in Alzheimer’s Disease: A Combined ASL Perfusion and Resting State fMRI Study. Front Neurosci. 2019, 13, 738. [Google Scholar] [CrossRef]

| Characteristics | AD (n = 16) | MCI (n = 16) | NC (n = 16) | p Value |
|---|---|---|---|---|
| Age (years) | 76.31 ± 3.98 | 75.56 ± 6.02 | 74.69 ± 4.66 | 0.653 |
| Sex (female/male) | 7/9 | 6/10 | 10/6 | 0.338 |
| MMSE | 20.75 ± 3.71 | 27.50 ± 1.79 | 29.19 ± 1.05 | <0.001 |
| GDS | 1.31 ± 0.95 | 2.44 ± 3.44 | 0.56 ± 1.03 | 0.055 |
| CDR | 0.97 ± 0.34 | 0.50 ± 0.26 | 0.03 ± 0.13 | <0.001 |
| FAQ | 17.81 ± 6.6 | 5.06 ± 5.43 | 0.06 ± 0.25 | <0.001 |
| NPI-Q | 3.75 ± 3.21 | 3.31 ± 4.22 | 0.44 ± 0.73 | 0.008 |
| Indices | Region | Brodmann’s Area | MNI Coordinates | T Value | Cluster(Voxels) |
|---|---|---|---|---|---|
| ANOVA | |||||
| Short-range | Amygdala_R | BA34 | (18,0,−17) | 5.9018 | 3 |
| Long-range | Caudate_R | - | (18,−5,20) | 11.3438 | 66 |
| Caudate_L | - | (−16,−12,23) | 6.4713 | 43 | |
| Amygdala_R | BA34 | (19,1,−17) | 13.2933 | 32 | |
| Pallidum_R | BA48 | (19,0,−5) | 7.8705 | 14 | |
| Putamen_R | BA48 | (33,−9,0) | 6.1615 | 7 | |
| MCI vs. NC | |||||
| Long-range | Amygdala_R | BA34 | (21,−2,−17) | 4.4157 | 38 |
| Amygdala_L | - | (−13,2,−15) | 3.3582 | 6 | |
| Putamen_L | BA25 | (−14,7,−9) | 3.2804 | 7 | |
| Pallidum_R | BA25 | (12,3,−5) | 3.1270 | 5 | |
| AD vs. NC | |||||
| Short-range | Caudate_L | - | (−7,1,11) | −2.9712 | 29 |
| Caudate_R | - | (9,5,7) | −2.8622 | 8 | |
| AD vs. MCI | |||||
| Short-range | Caudate_L | - | (−18,−21,23) | −3.2570 | 9 |
| Caudate_R | - | (16,−18,21) | −2.8545 | 4 | |
| Long-range | Caudate_L | - | (−15,14,18) | −3.7649 | 66 |
| Caudate_R | - | (17,6,23) | −3.7601 | 54 | |
| Amygdala_R | BA48 | (19,2,−11) | −3.6551 | 20 | |
| Pallidum_R | BA48 | (19,−3,−2) | −3.4747 | 9 |
| Conditions | Regions | p Value | AUC Value | Sensitivity | Specificity |
|---|---|---|---|---|---|
| NC–MCI | |||||
| Short-range FCD | Caudate_R | 0.012 | 0.762 | 81.3% | 75.0% |
| Amygdala_R | 0.005 | 0.793 | 81.3% | 75.0% | |
| Putamen_R | 0.010 | 0.766 | 87.5% | 62.5% | |
| Pallidum_R | 0.029 | 0.727 | 43.8% | 100.0% | |
| Long-range FCD | Amygdala_R | 0.000 | 0.867 | 87.5% | 75.0% |
| Amygdala_L | 0.001 | 0.844 | 75.0% | 87.5% | |
| Putamen_L | 0.005 | 0.793 | 68.8% | 75.0% | |
| Pallidum_L | 0.006 | 0.785 | 81.3% | 75.0% | |
| Global Efficiency | Amygdala_R | 0.019 | 0.742 | 68.8% | 87.5% |
| Amygdala_L | 0.027 | 0.729 | 62.5% | 81.2% | |
| Degree | Amygdala_L | 0.050 | 0.703 | 50.0% | 87.5% |
| MCI–AD | |||||
| Short-range FCD | Caudate_L | 0.004 | 0.797 | 75.0% | 75.0% |
| Long-range FCD | Caudate_L | 0.001 | 0.840 | 75.0% | 75.0% |
| Caudate_R | 0.001 | 0.848 | 68.8% | 93.7% | |
| Amygdala_R | 0.000 | 0.879 | 87.5% | 87.5% | |
| Pallidum_R | 0.006 | 0.785 | 50.0% | 100.0% | |
| Global Efficiency | Caudate_L | 0.002 | 0.828 | 87.5% | 62.5% |
| Caudate_R | 0.004 | 0.795 | 81.3% | 68.7% | |
| Amygdala_R | 0.018 | 0.746 | 81.3% | 68.7% | |
| Amygdala_L | 0.006 | 0.783 | 87.5% | 62.5% | |
| Putamen_R | 0.016 | 0.750 | 68.8% | 75.0% | |
| Local Efficiency | Caudate_L | 0.023 | 0.736 | 68.8% | 75.0% |
| Degree | Caudate_L | 0.003 | 0.805 | 75.0% | 75.0% |
| Caudate_R | 0.009 | 0.771 | 62.5% | 87.5% | |
| Amygdala_L | 0.017 | 0.748 | 81.3% | 68.7% | |
| Putamen_R | 0.020 | 0.740 | 81.3% | 56.2% | |
| NC–AD | |||||
| Long-range FCD | Caudate_L | 0.009 | 0.770 | 93.8% | 62.5% |
| Global Efficiency | Caudate_L | 0.014 | 0.754 | 68.8% | 75.0% |
| Caudate_R | 0.020 | 0.740 | 68.8% | 75.0% | |
| Pallidum_R | 0.007 | 0.781 | 62.5% | 93.7% | |
| Putamen_R | 0.017 | 0.748 | 68.8% | 81.2% | |
| Local Efficiency | Caudate_R | 0.026 | 0.730 | 75.0% | 62.5% |
| Degree | Caudate_L | 0.044 | 0.709 | 75.0% | 68.7% |
| Caudate_R | 0.013 | 0.758 | 81.3% | 62.5% | |
| Pallidum_R | 0.012 | 0.762 | 93.8% | 50.0% | |
| Putamen_R | 0.013 | 0.758 | 68.8% | 75.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Y.; Ye, C.; Chen, Y.; Zhong, X.; Chen, H.; Sun, R.; Zhang, J.; Zhong, Z.; Huang, M. Altered Functional Connectivity of Basal Ganglia in Mild Cognitive Impairment and Alzheimer’s Disease. Brain Sci. 2022, 12, 1555. https://doi.org/10.3390/brainsci12111555
Xiong Y, Ye C, Chen Y, Zhong X, Chen H, Sun R, Zhang J, Zhong Z, Huang M. Altered Functional Connectivity of Basal Ganglia in Mild Cognitive Impairment and Alzheimer’s Disease. Brain Sciences. 2022; 12(11):1555. https://doi.org/10.3390/brainsci12111555
Chicago/Turabian StyleXiong, Yu, Chenghui Ye, Ying Chen, Xiaochun Zhong, Hongda Chen, Ruxin Sun, Jiaqi Zhang, Zhanhua Zhong, and Min Huang. 2022. "Altered Functional Connectivity of Basal Ganglia in Mild Cognitive Impairment and Alzheimer’s Disease" Brain Sciences 12, no. 11: 1555. https://doi.org/10.3390/brainsci12111555
APA StyleXiong, Y., Ye, C., Chen, Y., Zhong, X., Chen, H., Sun, R., Zhang, J., Zhong, Z., & Huang, M. (2022). Altered Functional Connectivity of Basal Ganglia in Mild Cognitive Impairment and Alzheimer’s Disease. Brain Sciences, 12(11), 1555. https://doi.org/10.3390/brainsci12111555






