Relationships of Motor Changes with Cognitive and Neuropsychiatric Features in FMR1 Male Carriers Affected with Fragile X-Associated Tremor/Ataxia Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Neurological Motor and Cognitive Measures
2.3. Psychiatric Symptom Measures
2.4. FMR1 Molecular Measures
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hagerman, R.J.; Leehey, M.; Heinrichs, W.; Tassone, F.; Wilson, R.; Hills, J.; Grigsby, J.; Gage, B.; Hagerman, P.J. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of the fragile X. Neurology 2001, 57, 127–130. [Google Scholar] [CrossRef]
- Loesch, D.; Hagerman, R. Unstable mutations in the FMR1 gene and the phenotypes. Tandem Repeat Polymorph. 2012, 769, 78–114. [Google Scholar] [CrossRef]
- Jacquemont, S.; Hagerman, R.J.; Leehey, M.A.; Hall, D.A.; Levine, R.A.; Brunberg, J.A.; Zhang, L.; Jardini, T.; Gane, L.W.; Harris, S.W.; et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA 2004, 291, 460–469. [Google Scholar] [CrossRef]
- Brunberg, J.A.; Jacquemont, S.; Hagerman, R.J.; Berry-Kravis, E.M.; Grigsby, J.; Leehey, M.A.; Tassone, F.; Brown, W.T.; Greco, C.M.; Hagerman, P.J. Fragile X premutation carriers: Characteristic MR imaging findings of adult male patients with progressive cerebellar and cognitive dysfunction. Am. J. Neuroradiol. 2002, 23, 1757–1766. [Google Scholar]
- Hagerman, R.J.; Hagerman, P. Fragile X-associated tremor/ataxia syndrome—Features, mechanisms and management. Nat. Rev. Neurol. 2016, 12, 403–412. [Google Scholar] [CrossRef]
- Hermanson, M.; Jhaveri, M.; Stebbins, G.; Dunn, E.; Merkitch, D.; Berry-Kravis, E.; Hall, D. The splenium of the corpus callosum sign in Fragile X Associated Tremor Ataxia Syndrome (FXTAS) (P2.125). Neurology 2015, 84, 125. [Google Scholar]
- Soontarapornchai, K.; Maselli, R.; Fenton-Farrell, G.; Tassone, F.; Hagerman, P.J.; Hessl, D.; Hagerman, R.J. Abnormal nerve conduction features in fragile X premutation carriers. Arch. Neurol. 2008, 65, 495–498. [Google Scholar] [CrossRef]
- Apartis, E.; Blancher, A.; Meissner, W.G.; Guyant-Maréchal, L.; Maltête, D.; de Broucker, T.; Legrand, A.P.; Bouzenada, H.; Thanh, H.T.; Sallansonnet-Froment, M.; et al. FXTAS: New insights and the need for revised diagnostic criteria. Neurology 2012, 79, 1898–1907. [Google Scholar] [CrossRef]
- Adams, J.S.; Adams, P.E.; Nguyen, D.; Brunberg, J.A.; Tassone, F.; Zhang, W.; Koldewyn, K.; Rivera, S.M.; Grigsby, J.; Zhang, L.; et al. Volumetric brain changes in females with fragile X-associated tremor/ataxia syndrome (FXTAS). Neurology 2007, 69, 851–859. [Google Scholar] [CrossRef]
- Loesch, D.Z.; Kotschet, K.; Trost, N.; Greco, C.M.; Kinsella, G.; Slater, H.R.; Venn, A.; Horne, M. White matter changes in basis pontis in small expansion FMR1 allele carriers with parkinsonism. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2011, 156, 502–506. [Google Scholar] [CrossRef]
- Wheeler, A.C.; Bailey, D.B., Jr.; Berry-Kravis, E.; Greenberg, J.; Losh, M.; Mailick, M.; Milà, M.; Olichney, J.M.; Rodriguez-Revenga, L.; Sherman, S.; et al. Associated features in females with an FMR1 premutation. J. Neurodev. Disord. 2014, 6, 30. [Google Scholar] [CrossRef]
- Alster, P.; Koziorowski, D.M.; Za Bek, M.; Dzierzȩcki, S.; Ma Dry, J.; Duszyńska-Wa, S.K.; Grygarowicz, H.; Zielonko, J.; Królicki, L.; Friedman, A. Making a difference-positive effect of unilateral VIM Gamma Knife Thalamotomy in the therapy of tremor in Fragile X-Associated Tremor Ataxia Syndrome (FXTAS). Front. Neurol. 2018, 9, 512. [Google Scholar] [CrossRef]
- dos Santos Ghilardi, M.G.; Cury, R.G.; dos Ângelos, J.S.; Barbosa, D.C.; Barbosa, E.R.; Teixeira, M.J.; Fonoff, E.T. Long-term improvement of tremor and ataxia after bilateral DBS of VoP/zona incerta in FXTAS. Neurology 2015, 84, 1904–1906. [Google Scholar] [CrossRef]
- Tassone, F.; Hagerman, R.J.; Taylor, A.K.; Gane, L.W.; Godfrey, T.E.; Hagerman, P.J. Elevated levels of FMR1 mRNA in carrier males: A new mechanism of involvement in the fragile-X syndrome. Am. J. Hum. Genet. 2000, 66, 6–15. [Google Scholar] [CrossRef]
- Greco, C.M.; Hagerman, R.J.; Tassone, F.; Chudley, A.E.; del Bigio, M.R.; Jacquemont, S.; Leehey, M.; Hagerman, P.J. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain 2002, 125, 1760–1771. [Google Scholar] [CrossRef]
- Tassone, F.; Hagerman, R.J.; Garcia-Arocena, D.; Khandjian, E.W.; Greco, C.M.; Hagerman, P.J. Intranuclear inclusions in neural cells with premutation alleles in fragile X associated tremor/ataxia syndrome. J. Med. Genet. 2004, 41, e43. [Google Scholar] [CrossRef]
- Jin, P.; Zarnescu, D.C.; Zhang, F.; Pearson, C.; Lucchesi, J.C.; Moses, K.; Warren, S.T. RNA-mediated neurodegeneration caused by the fragile X premutation rCGG repeats in drosophila. Neuron 2003, 39, 739–747. [Google Scholar] [CrossRef]
- Polussa, J.; Schneider, A.; Hagerman, R. Molecular advances leading to treatment implications for fragile X premutation carriers. Brain Disord. Ther. 2014, 3, 1000119. [Google Scholar]
- Hagerman, P.J.; Hagerman, R.J. Fragile X-associated tremor/ataxia syndrome. Ann. N. Y. Acad. Sci. 2015, 1338, 58–70. [Google Scholar] [CrossRef]
- Tassone, F.; Adams, J.; Berry-Kravis, E.M.; Cohen, S.S.; Brusco, A.; Leehey, M.A.; Li, L.; Hagerman, R.J.; Hagerman, P.J. CGG repeat length correlates with age of onset of motor signs of the fragile X-associated tremor/ataxia syndrome (FXTAS). Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2007, 144B, 566–569. [Google Scholar] [CrossRef]
- Leehey, M.A.; Berry-Kravis, E.; Goetz, C.G.; Zhang, L.; Hall, D.A.; Li, L.; Rice, C.D.; Lara, R.; Cogswell, J.; Reynolds, A.; et al. FMR1 CGG repeat length predicts motor dysfunction in premutation carriers. Neurology 2008, 70, 1397–1402. [Google Scholar] [CrossRef]
- Loesch, D.Z.; Bui, M.Q.; Hammersley, E.; Schneider, A.; Storey, E.; Stimpson, P.; Burgess, T.; Francis, D.; Slater, H.; Tassone, F.; et al. Psychological status in female carriers of premutation FMR1 allele showing a complex relationship with the size of CGG expansion. Clin. Genet. 2014, 87, 173–178. [Google Scholar] [CrossRef]
- Hall, D.; Tassone, F.; Klepitskaya, O.; Leehey, M. Fragile X-associated tremor ataxia syndrome in FMR1 gray zone allele carriers. Mov. Disord. 2011, 27, 297–301. [Google Scholar] [CrossRef]
- Loesch, D.Z.; Tassone, F.; Mellick, G.D.; Horne, M.; Rubio, J.P.; Bui, M.Q.; Francis, D.; Storey, E. Evidence for the role of FMR1 gray zone alleles as a risk factor for parkinsonism in females. Mov. Disord. 2018, 33, 1178–1181. [Google Scholar] [CrossRef]
- Cabal-Herrera, A.M.; Tassanakijpanich, N.; Salcedo-Arellano, M.J.; Hagerman, R.J. Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS): Pathophysiology and clinical implications. Int. J. Mol. Sci. 2020, 21, 4391. [Google Scholar] [CrossRef]
- Hall, D.A.; Robertson, E.; Shelton, A.L.; Losh, M.C.; Mila, M.; Moreno, E.G.; Gomez-Anson, B.; Martínez-Cerdeño, V.; Grigsby, J.; Lozano, R.; et al. Update on the clinical, radiographic, and neurobehavioral manifestations in FXTAS and FMR1 premutation carriers. Cerebellum 2016, 15, 578–586. [Google Scholar] [CrossRef]
- Grigsby, J.; Cornish, K.; Hocking, D.; Kraan, C.; Olichney, J.M.; Rivera, S.M.; Schneider, A.; Sherman, S.; Wang, J.Y.; Yang, J.-C. The cognitive neuropsychological phenotype of carriers of the FMR1 premutation. J. Neurodev. Disord. 2014, 6, 1–8. [Google Scholar] [CrossRef]
- Grigsby, J.; Brega, A.G.; Bennett, R.E.; Bourgeois, J.A.; Seritan, A.L.; Goodrich, G.K.; Hagerman, R.J. Clinically significant psychiatric symptoms among male carriers of the fragile X premutation, with and without FXTAS, and the mediating influence of executive functioning. Clin. Neuropsychol. 2016, 30, 944–959. [Google Scholar] [CrossRef]
- Hessl, D.; Tassone, F.; Loesch, D.Z.; Berry-Kravis, E.; Leehey, M.A.; Gane, L.W.; Barbato, I.; Rice, C.; Gould, E.; Hall, D.A.; et al. Abnormal elevation ofFMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2005, 139B, 115–121. [Google Scholar] [CrossRef]
- Hagerman, R.; Hagerman, P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 2013, 12, 786–798. [Google Scholar] [CrossRef]
- Schmahmann, J.D. Disorders of the cerebellum: Ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J. Neuropsychiatry Clin. Neurosci. 2004, 16, 367–378. [Google Scholar] [CrossRef]
- O’Halloran, C.J.; Kinsella, G.; Storey, E. The cerebellum and neuropsychological functioning: A critical review. J. Clin. Exp. Neuropsychol. 2012, 34, 35–56. [Google Scholar] [CrossRef]
- Loesch, D.Z.; Annesley, S.J.; Trost, N.; Bui, M.Q.; Lay, S.T.; Storey, E.; De Piazza, S.W.; Sanislav, O.; Francione, L.M.; Hammersley, E.M.; et al. Novel blood biomarkers are associated with white matter lesions in fragile X-associated tremor/ataxia syndrome. Neurodegener. Dis. 2016, 17, 22–30. [Google Scholar] [CrossRef]
- Loesch, D.Z.; Trost, N.; Bui, M.; Hammersley, E.; Lay, S.T.; Annesley, S.J.; Sanislav, O.; Allan, C.Y.; Tassone, F.; Chen, Z.-P.; et al. The spectrum of neurological and white matter changes and premutation status categories of older male carriers of the FMR1 alleles are linked to genetic (CGG and FMR1 mRNA) and cellular stress (AMPK) markers. Front. Genet. 2018, 9, 531. [Google Scholar] [CrossRef]
- Loesch, D.Z.; Kemp, B.E.; Bui, M.Q.; Fisher, P.R.; Allan, C.Y.; Sanislav, O.; Ngoei, K.R.W.; Atkinson, A.; Tassone, F.; Annesley, S.J.; et al. Cellular bioenergetics and AMPK and TORC1 signalling in blood lymphoblasts are biomarkers of clinical status in FMR1 premutation carriers. Front. Psychiatry 2021, 12, 747268. [Google Scholar] [CrossRef]
- Loesch, D.Z.; Tassone, F.; Atkinson, A.; Stimpson, P.; Trost, N.; Pountney, D.L.; Storey, E. Differential progression of motor dysfunction between male and female fragile X premutation carriers reveals novel aspects of sex-specific neural involvement. Front. Mol. Biosci. 2021, 7, 577246. [Google Scholar] [CrossRef]
- Hall, D.A.; Birch, R.C.; Anheim, M.; Jønch, A.E.; Pintado, E.; O’Keefe, J.; Trollor, J.N.; Stebbins, G.T.; Hagerman, R.J.; Fahn, S.; et al. Emerging topics in FXTAS. J. Neurodev. Disord. 2014, 6, 1–11. [Google Scholar] [CrossRef]
- Richards, M.; Marder, K.; Cote, L.; Mayeux, R. Interrater reliability of the unified Parkinson’s disease rating scale motor examination. Mov. Disord. 1994, 9, 89–91. [Google Scholar] [CrossRef]
- Stacy, M.A.; Elble, R.J.; Ondo, W.G.; Wu, S.-C.; Hulihan, J. TRS Study Group Assessment of interrater and intrarater reliability of the Fahn-Tolosa-Marin Tremor Rating Scale in essential tremor. Mov. Disord. 2007, 22, 833–838. [Google Scholar] [CrossRef]
- Storey, E.; Tuck, K.; Hester, R.; Hughes, A.; Churchyard, A. Inter-rater reliability of the International Cooperative Ataxia Rating Scale (ICARS). Mov. Disord. 2004, 19, 190–192. [Google Scholar] [CrossRef]
- Trouillas, P.; Takayanagi, T.; Hallett, M.; Currier, R.; Subramony, S.; Wessel, K.; Bryer, A.; Diener, H.; Massaquoi, S.; Gomez, C.; et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. J. Neurol. Sci. 1997, 145, 205–211. [Google Scholar] [CrossRef]
- Mioshi, E.; Dawson, K.; Mitchell, J.; Arnold, R.; Hodges, J.R. The Addenbrooke’s Cognitive Examination Revised (ACE-R): A brief cognitive test battery for dementia screening. Int. J. Geriatr. Psychiatry 2006, 21, 1078–1085. [Google Scholar] [CrossRef]
- Wechsler, D. The Wechsler Adult Intelligence Scale. Administration and Scoring Manual, 3rd ed.; The Psychological Corporation: Orlando, FL, USA, 1997. [Google Scholar]
- Smith, A. Symbol Digit Modalities Test; Western Psychological Services: Los Angeles, CA, USA, 1982. [Google Scholar]
- Derogatis, L. SCL-90-R: Symptom Checklist-90-R. Administration, Scoring and Procedures Manual; NCS Pearson: Minneapolis, MN, USA, 1994. [Google Scholar]
- Henry, J.D.; Crawford, J.R. The short-form version of the Depression Anxiety Stress Scales (DASS-21): Construct validity and normative data in a large non-clinical sample. Br. J. Clin. Psychol. 2005, 44, 227–239. [Google Scholar] [CrossRef]
- Tassone, F.; Pan, R.; Amiri, K.; Taylor, A.K.; Hagerman, P.J. A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J. Mol. Diagn. 2008, 10, 43–49. [Google Scholar] [CrossRef]
- Filipovic-Sadic, S.; Sah, S.; Chen, L.; Krosting, J.; Sekinger, E.; Zhang, W.; Hagerman, P.J.; Stenzel, T.T.; Hadd, A.G.; Latham, G.J.; et al. A novel FMR1 PCR method for the routine detection of low abundance expanded alleles and full mutations in fragile X syndrome. Clin. Chem. 2010, 56, 399–408. [Google Scholar] [CrossRef]
- Postuma, R.B.; Lang, A.; Gagnon, J.F.; Pelletier, A.; Montplaisir, J.Y. How does parkinsonism start? Prodromal parkinsonism motor changes in idiopathic REM sleep behaviour disorder. Brain 2012, 135, 1860–1870. [Google Scholar] [CrossRef]
- Fitzpatrick, L.E.; Jackson, M.; Crowe, S.F. Characterization of cerebellar ataxia in chronic alcoholics using the International Cooperative Ataxia Rating Scale (ICARS). Alcohol. Clin. Exp. Res. 2012, 36, 1942–1951. [Google Scholar] [CrossRef]
- So, M.; Foxe, D.; Kumfor, F.; Murray, C.; Hseih, S.; Savage, G.; Ahmed, R.M.; Burrell, J.R.; Hodges, J.R.; Irish, M.; et al. Addenbrooke’s Cognitive Examination III: Psychometric characteristics and relations to functional ability in dementia. J. Int. Neuropsychol. Soc. 2018, 24, 854–863. [Google Scholar] [CrossRef]
- Harrison, A.G.; Armstrong, I.T.; E Harrison, L.; Lange, R.T.; Iverson, G. Comparing Canadian and American Normative Scores on the Wechsler Adult Intelligence Scale-Fourth Edition. Arch. Clin. Neuropsychol. 2014, 29, 737–746. [Google Scholar] [CrossRef]
- Leung, J.L.M.; Lee, G.T.H.; Lam, Y.H.; Chan, R.C.C.; Wu, J.Y.M. The use of the Digit Span Test in screening for cognitive impairment in acute medical impatients. Int. Psychogeriatr. 2011, 23, 1569–1574. [Google Scholar] [CrossRef]
- Kiely, K.M.; Butterworth, P.; Watson, N.; Wooden, M. The Symbol Digit Modalities Test: Normative data from a large nationally representative sample of australians. Arch. Clin. Neuropsychol. 2014, 29, 767–775. [Google Scholar] [CrossRef]
- Gossett, A.; Sansone, S.; Schneider, A.; Johnston, C.; Hagerman, R.; Tassone, F.; Rivera, S.M.; Seritan, A.L.; Hessl, D. Psychiatric disorders among women with the fragile X premutation without children affected by fragile X syndrome. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2016, 171, 1139–1147. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Schmahmann, J.D. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. NeuroImage 2009, 44, 489–501. [Google Scholar] [CrossRef]
- Blatt, G.J.; Oblak, A.L.; Schmahmann, J.D. Cerebellar connections with limbic circuits: Anatomy and functional implications. In Handbook of the Cerebellum and Cerebellar Disorders; Manto, M., Schmahmann, J.D., Rossi, F., Gruol, D.L., Koibuchi, N., Eds.; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Battistella, G.; Niederhauser, J.; Fornari, E.; Hippolyte, L.; Perrin, A.G.; Lesca, G.; Forzano, F.; Hagmann, P.; Vingerhoets, F.J.; Draganski, B.; et al. Brain structure in asymptomatic FMR1 premutation carriers at risk for fragile X-associated tremor/ataxia syndrome. Neurobiol. Aging 2013, 34, 1700–1707. [Google Scholar] [CrossRef]
- Wang, J.Y.; Hessl, D.; Schneider, A.; Tassone, F.; Hagerman, R.J.; Rivera, S.M. Fragile X-associated tremor/ataxia syndrome: Influence of the FMR1 gene on motor fiber tracts in males with normal and premutation alleles. JAMA Neurol. 2013, 70, 1022–1029. [Google Scholar] [CrossRef]
- Hocking, D.R.; Loesch, D.Z.; Trost, N.; Bui, M.Q.; Hammersley, E.; Francis, D.; Tassone, F.; Storey, E. Total and regional white matter lesions are correlated with motor and cognitive impairments in carriers of the FMR1 premutation. Front. Neurol. 2019, 10, 832. [Google Scholar] [CrossRef]
- Birch, R.C.; Hocking, D.R.; Cornish, K.M.; Menant, J.C.; Georgiou-Karistianis, N.; Godler, D.E.; Wen, W.; Hackett, A.; Rogers, C.; Trollor, J.N. Preliminary evidence of an effect of cerebellar volume on postural sway in FMR1 premutation males. Genes Brain Behav. 2015, 14, 251–259. [Google Scholar] [CrossRef]
- Birch, R.C.; Hocking, D.R.; Cornish, K.M.; Menant, J.C.; Lord, S.R.; Georgiou-Karistianis, N.; Godler, D.E.; Wen, W.; Rogers, C.; Trollor, J.N. Selective subcortical contributions to gait impairments in males with the FMR1 premutation. J. Neurol. Neurosurg. Psychiatry 2017, 88, 188–190. [Google Scholar] [CrossRef]
- Hocking, D.R.; Birch, R.C.; Bui, Q.M.; Menant, J.C.; Lord, S.R.; Georgiou-Karistianis, N.; Godler, D.E.; Wen, W.; Hackett, A.; Rogers, C.; et al. Cerebellar volume mediates the relationship between FMR1 mRNA levels and voluntary step initiation in males with the premutation. Neurobiol. Aging 2017, 50, 5–12. [Google Scholar] [CrossRef]
- Kraan, C.M.; Hocking, D.R.; Georgiou-Karistianis, N.; Metcalfe, S.A.; Archibald, A.D.; Fielding, J.; Trollor, J.; Bradshaw, J.L.; Cohen, J.; Cornish, K.M. Age and CGG-repeat length are associated with neuromotor impairments in at-risk females with the FMR1 premuation. Neurobiol. Aging 2014, 35, 2179.e7–2179.e13. [Google Scholar] [CrossRef]
- Kraan, C.M.; Hocking, D.R.; Georgiou-Karistianis, N.; Metcalfe, S.A.; Archibald, A.D.; Fielding, J.; Trollor, J.; Bradshaw, J.L.; Cohen, J.; Cornish, K.M. Cognitive-motor interference during postural conrol indicates at-risk cerebellar profiles in females with the FMR1 premutation. Behav. Brain Res. 2013, 253, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Lord, S.; Bunce, J.; Burn, D.; Rochester, L. Gait and cognition: Mapping the global and discrete relationships in ageing and neurodegenerative disease. Neurosci. Biobehav. Rev. 2016, 64, 326–345. [Google Scholar] [CrossRef] [PubMed]
- Woollacott, M.; Shumway-Cook, A. Attention and the control of posture and gait: A review of an emerging area of research. Gait Posture 2002, 16, 1–14. [Google Scholar] [CrossRef]
| Normative Values 1 | |||||
|---|---|---|---|---|---|
| Variable | Mean | SD | Range | Mean | SD |
| Characteristic | |||||
| Age | 64.6 | 7.7 | 48–80 | ||
| Years of Education | 12.2 | 3.8 | 6–22 | ||
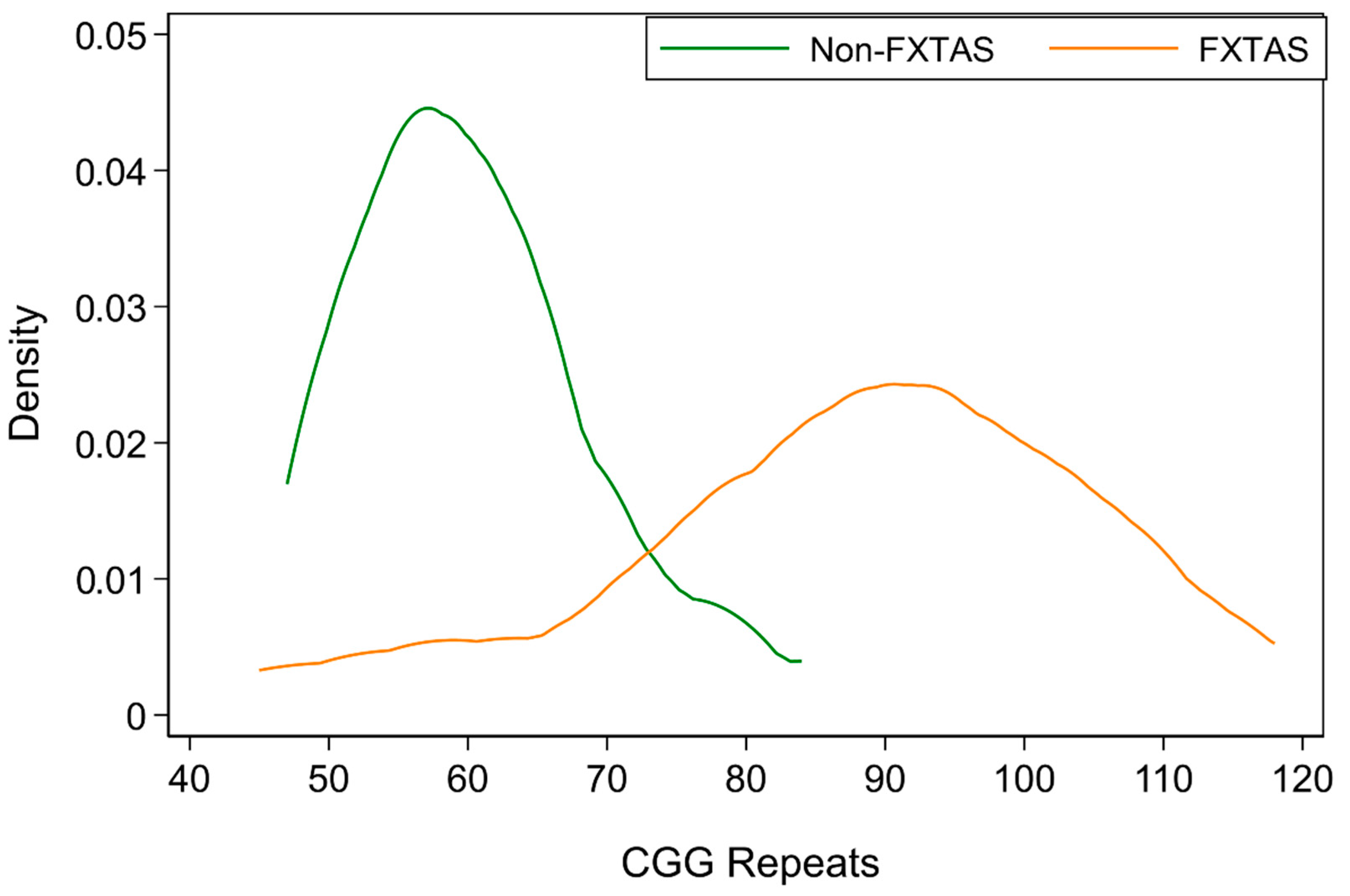
| CGG repeats | 87.7 | 17.5 | 45–118 | ||
| Motor Scores | |||||
| UPDRS | 17.7 | 13.4 | 2–39 | 1.9 | 2.0 2 |
| ICARS GAIT | 9.3 | 4.7 | 2–22 | 2.0 | 2.0 3 |
| ICARS KINETIC | 15.3 | 7.7 | 6–29 | 1.8 | 1.9 3 |
| ICARS Total | 27.9 | 13.8 | 10–57 | 4.1 | 2.2 3 |
| Cognitive scores | |||||
| ACE-III Total | 73.6 | 14.7 | 48–94 | 95.7 | 3.3 4 |
| MR SS | 10.0 | 4.0 | 3–18 | 10.4 | 2.9 5 |
| Pro-rated IQ | 97.1 | 16.4 | 69–131 | - | - |
| DS Backwards | 5.5 | 1.7 | 3–10 | 3.1 | 1.2 6 |
| SDMT | 29.3 | 14.7 | 16–59 | 42.3 | 8.1 7 |
| Neuropsychiatric scores | |||||
| SCL-90-R Depression | 59.1 | 13.9 | 38–81 | 49.0 | 11.2 8 |
| SCL-90-R Anxiety | 53.1 | 13.9 | 40–62 | 46.0 | 11.2 8 |
| SCL-90-R GSI | 56.8 | 11.6 | 34–81 | 50.5 | 8.6 8 |
| DASS Anxiety | 7.2 | 6.7 | 0–24 | 6.3 | 7.0 9 |
| DASS Depression | 11.5 | 11.8 | 0–36 | 4.7 | 4.9 9 |
| FXTAS | |||||
|---|---|---|---|---|---|
| N | Coef. | Se | p-Value | ||
| Cognitive scores | |||||
| ACE-III Total | 16 | −0.325 | 0.130 | 0.012 | |
| MR SS | 19 | −0.085 | 0.027 | 0.001 | |
| * DS Backwards | 22 | −0.022 | 0.021 | 0.301 | |
| PRO-rated IQ | 18 | −0.250 | 0.110 | 0.023 | |
| SDMT Score | 17 | −0.287 | 0.082 | <0.001 | |
| Motor scores | |||||
| ICARS TOTAL | 17 | 0.308 | 0.082 | <0.001 | |
| ICARS GAIT | 17 | 0.104 | 0.027 | <0.001 | |
| ICARS KINETIC | 17 | 0.138 | 0.060 | 0.022 | |
| UPDRS | 16 | 0.288 | 0.082 | <0.001 | |
| Neuropsychiatric scores | |||||
| DASS Anxiety | 12 | 0.115 | 0.032 | <0.001 | |
| DASS Depression | 12 | 0.240 | 0.043 | <0.001 | |
| SCL-90-R Depression | 11 | 0.376 | 0.126 | 0.003 | |
| SCL-90-R Anxiety | 13 | 0.212 | 0.127 | 0.096 | |
| SCL-90-R GSIT | 11 | 0.171 | 0.025 | <0.001 | |
| ICARS Total | ICARS Gait | ICARS Kinetic | UPDRS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Coef. | SE | p | Coef. | SE | p | Coef. | SE | p | Coef. | se | p | |
| Cognitive scores | |||||||||||||
| ACE-III Total | 18 | −0.42 | 0.26 | 0.105 | −2.09 | 0.32 | <0.001 | −0.36 | 0.50 | 0.468 | −0.39 | 0.33 | 0.243 |
| MR.Sc 1 | 18 | −0.02 | 0.07 | 0.743 | −0.20 | 0.22 | 0.348 | 0.01 | 0.09 | 0.953 | −0.05 | 0.08 | 0.525 |
| DS Backwards | 18 | −0.02 | 0.03 | 0.392 | −0.14 | 0.07 | 0.050 | −0.01 | 0.05 | 0.813 | −0.01 | 0.03 | 0.859 |
| PRO-rated IQ 1 | 17 | −0.02 | 0.21 | 0.908 | −0.56 | 0.83 | 0.499 | 0.16 | 0.35 | 0.643 | −0.06 | 0.39 | 0.883 |
| SDMT 2 | 16 | −0.05 | 0.24 | 0.843 | −1.52 | 0.43 | <0.001 | 0.29 | 0.31 | 0.355 | −0.24 | 0.29 | 0.399 |
| Neuropsychiatric scores | |||||||||||||
| DASS Anxiety | 12 | 0.21 | 0.04 | <0.001 | 0.50 | 0.13 | <0.001 | 0.44 | 0.14 | 0.001 | 0.28 | 0.06 | <0.001 |
| DASS Depression | 12 | 0.62 | 0.18 | 0.001 | 1.67 | 0.22 | <0.001 | 1.11 | 0.46 | 0.017 | 0.66 | 0.18 | <0.001 |
| SCL90 Depression | 11 | 0.61 | 0.20 | 0.002 | 1.61 | 0.49 | 0.001 | 1.27 | 0.44 | 0.004 | 0.68 | 0.22 | 0.002 |
| SCL90 Anxiety | 11 | 0.49 | 0.21 | 0.017 | 0.76 | 0.53 | 0.149 | 1.11 | 0.33 | 0.001 | 0.62 | 0.17 | <0.001 |
| SCL90 GSIT 1 | 10 | 0.35 | 0.18 | 0.047 | 0.70 | 0.39 | 0.071 | 0.87 | 0.55 | 0.116 | 0.38 | 0.13 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hocking, D.R.; Loesch, D.Z.; Stimpson, P.; Tassone, F.; Atkinson, A.; Storey, E. Relationships of Motor Changes with Cognitive and Neuropsychiatric Features in FMR1 Male Carriers Affected with Fragile X-Associated Tremor/Ataxia Syndrome. Brain Sci. 2022, 12, 1549. https://doi.org/10.3390/brainsci12111549
Hocking DR, Loesch DZ, Stimpson P, Tassone F, Atkinson A, Storey E. Relationships of Motor Changes with Cognitive and Neuropsychiatric Features in FMR1 Male Carriers Affected with Fragile X-Associated Tremor/Ataxia Syndrome. Brain Sciences. 2022; 12(11):1549. https://doi.org/10.3390/brainsci12111549
Chicago/Turabian StyleHocking, Darren R., Danuta Z. Loesch, Paige Stimpson, Flora Tassone, Anna Atkinson, and Elsdon Storey. 2022. "Relationships of Motor Changes with Cognitive and Neuropsychiatric Features in FMR1 Male Carriers Affected with Fragile X-Associated Tremor/Ataxia Syndrome" Brain Sciences 12, no. 11: 1549. https://doi.org/10.3390/brainsci12111549
APA StyleHocking, D. R., Loesch, D. Z., Stimpson, P., Tassone, F., Atkinson, A., & Storey, E. (2022). Relationships of Motor Changes with Cognitive and Neuropsychiatric Features in FMR1 Male Carriers Affected with Fragile X-Associated Tremor/Ataxia Syndrome. Brain Sciences, 12(11), 1549. https://doi.org/10.3390/brainsci12111549








