Deficit Symptomatology of Schizophrenia Is Associated with Attenuated Taste Identification: Findings from a Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Assessment of Taste Detection
2.3. Questionnaires and Psychometric Scales
2.4. Statistical Analysis
3. Results
- -
- SDS scale—number of deficit symptoms: r = −0.046, p = 0.517 (Number of correctly recognized samples); r = −0.215, p = 0.002 (Mean intensity of taste); r = 0.004, p = 0.958 (Mean pleasure of taste)
- -
- PANSS scale—positive symptoms: r = −0.049, p = 0.491 (Number of correctly recognized samples); r = −0.022, p = 0.760 (Mean intensity of taste); r = −0.036, p = 0.616 (Mean pleasure of taste)
- -
- PANSS scale—negative symptoms: r = −0.106, p = 0.134 (Number of correctly recognized samples); r = −0.071, p = 0.322 (Mean intensity of taste); r = −0.020, p = 0.784 (Mean pleasure of taste)
- -
- PANSS scale—depressive symptoms: r = −0.009, p = 0.896 (Number of correctly recognized samples); r = −0.029, p = 0.683 (Mean in-tensity of taste); r = −0.112, p = 0.114 (Mean pleasure of taste)
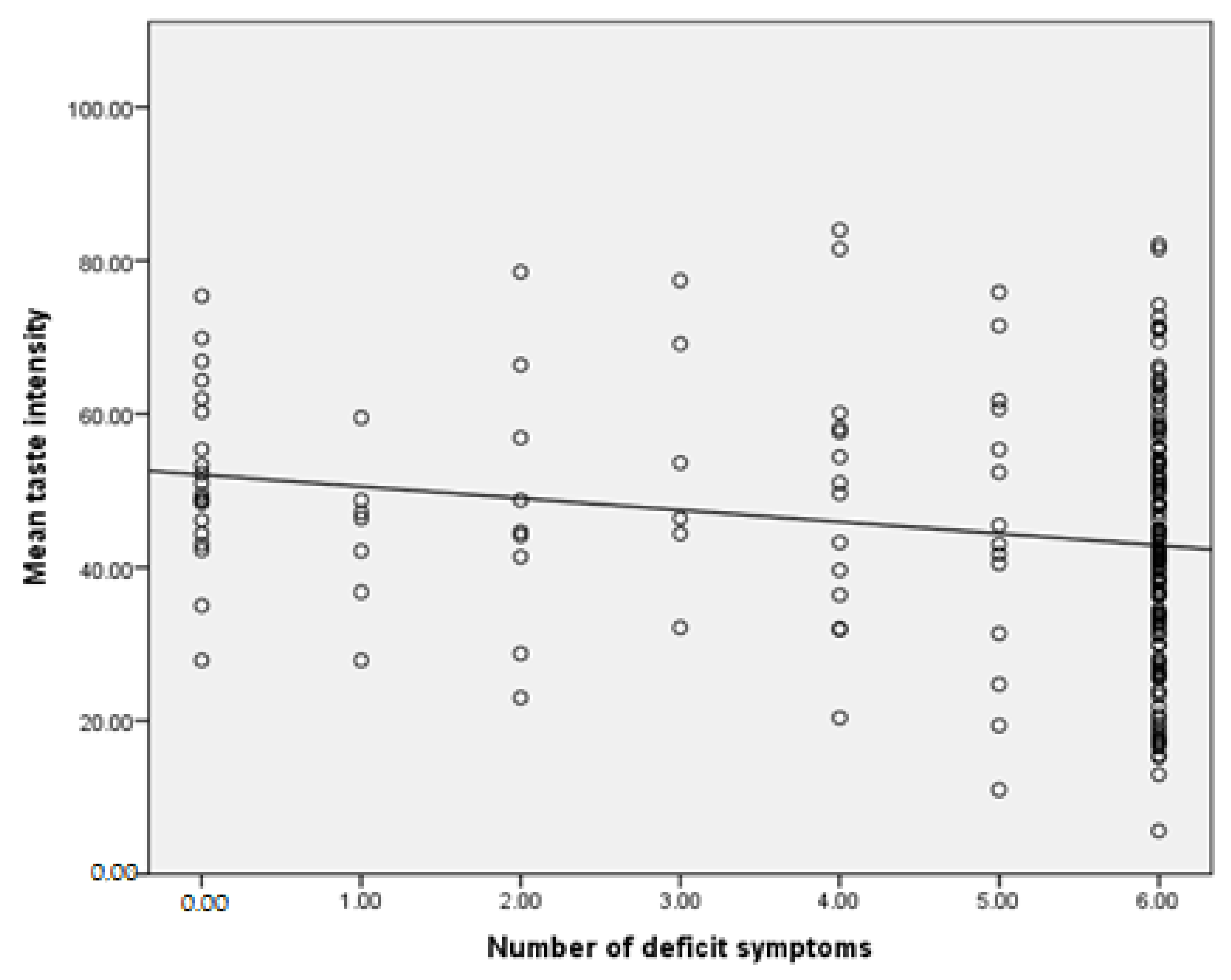
4. Discussion
5. Conclusions
6. Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Häfner, H.; an der Heiden, W. Epidemiology of Schizophrenia. Can. J. Psychiatry. 1997, 42, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Van Os, J.; Kapur, S. Schizophrenia. Lancet 2009, 374, 635–645. [Google Scholar] [CrossRef]
- WHO. Schizophrenia. 2022. Available online: http://www.who.int/mediacentre/factsheets/fs397/en/ (accessed on 10 January 2022).
- Salazar de Pablo, G.; Woods, S.W.; Drymonitou, G.; de Diego, H.; Fusar-Poli, P. Preva lence of Individuals at Clinical High-Risk of Psychosis in the General Population and Clinical Samples: Systematic Review and Meta-Analysis. Brain. Sci. 2021, 11, 1544. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef]
- National Institute of Mental Health. Schizophrenia. Reviewed May 2022. Available online: https://www.nimh.nih.gov/health/topics/schizophrenia (accessed on 10 January 2022).
- McGrath, J.; Saha, S.; Welham, J.; El Saadi, O.; MacCauley, C.; Chant, D. A systematic review of the incidence of schizophrenia: The distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med. 2004, 2, 13. [Google Scholar] [CrossRef]
- Picchioni, M.M.; Murray, R.M. Schizophrenia. BMJ 2007, 335, 91–95. [Google Scholar] [CrossRef]
- Castle, D.; Wessely, S.; Der, G.; Murray, R.M. The incidence of operationally defined schizophrenia in Camberwell, 1965–1984. Br. J. Psychiatry 1991, 159, 790–794. [Google Scholar] [CrossRef]
- Häfner, H.; Maurer, K.; Loffler, W.; Riecher-Rössler, A. The influence of age and sex on the onset and early course of schizophrenia. Br. J. Psychiatry 1993, 162, 80–86. [Google Scholar] [CrossRef]
- Simpson, G.M.; Yadalam, K.G.; Levinson, D.F.; Stephanos, M.J.; Sing, E.E.; Cooper, T. Single dose pharmoacokinetics of fluphenazine after fluphenazine decanoate administation. J. Clin. Psychopharmacol. 1990, 10, 417–421. [Google Scholar] [CrossRef]
- Ochoa, S.; Usall, J.; Cobo, J.; Labad, X.; Kulkarni, J. Gender differences in schizophrenia and first-episode psychosis: A comprehensive literature review. Schizophr. Res. Treat. 2012, 2012, 916198. [Google Scholar] [CrossRef]
- Flor-Henry, P. Influence of gender in schizophrenia as related to other psychopathological syndromes. Schizophr. Bull. 1990, 16, 211–227. [Google Scholar] [CrossRef]
- Maier, W.; Lichtermann, D.; Minges, J.; Heun, R.; Hallmayer, J.; Benkert, O. Schizoaffective dis order and affective disorders with mood-incongruent psychotic features: Keep separate or combine? Evidence from a family study. Am. J. Psychiatry 1992, 149, 1666–1673. [Google Scholar] [CrossRef]
- Franzek, B.; Beckmann, H. Sex differences and distinct subgroups in schizophrenia. A study of 54 chronic hospitalized schizophrenics. Psychopathology 1992, 25, 90–99. [Google Scholar] [CrossRef]
- Driver, D.I.; Gogtay, N.; Rapoport, J.L. Childhood onset schizophrenia and early onset schizophrenia spectrum disorders. Child. Adolesc. Psychiatr. Clin. N. Am. 2013, 22, 539–555. [Google Scholar] [CrossRef]
- Abidi, S.; Mian, I.; Garcia-Ortega, I.; Lecomte, T.; Raedler, T.; Jackson, K.; Jackson, K.; Pringsheim, T.; Addington, D. Canadian guidelines for the pharmacological treatment of schizophrenia spectrum and other psychotic disorders in children and youth. Can. J. Psychiatry 2017, 62, 635–647. [Google Scholar] [CrossRef]
- Szulc, A.; Samochowiec, J.; Gałecki, P.; Wojnar, M.; Heitzman, J.; Dudek, D. Recommendations for the treatment of schizophrenia with negative symptoms. Standards of pharmacotherapy by the Polish Psychiatric Association (Polskie Towarzystwo Psychiatryczne), part 1. Psychiatr. Pol. 2019, 5, 497–524. [Google Scholar] [CrossRef]
- Szulc, A.; Dudek, D.; Samochowiec, J.; Wojnar, M.; Heitzman, J.; Gałecki, P. Recommendations for the treatment of schizophrenia with negative symptoms. Standards of pharmacotherapy by the Polish Psychiatric Association (Polskie Towarzystwo Psychiatryczne), part 2. Psychiatr. Pol. 2019, 53, 525–540. [Google Scholar] [CrossRef]
- NICE (National Institute for Health and Care Excellence). Available online: https://www.nice.org.uk/guidance/cg178/chapter/1-Recommendations#promoting-recovery-and-possible-future-care-2 (accessed on 12 February 2014).
- Blay, M.; Adam, O.; Bation, R.; Galvao, F.; Brunelin, J.; Mondino, M. Improvement of Insight with Non-Invasive Brain Stimulation in Patients with Schizophrenia: A Systematic Review. J. Clin. Med. 2022, 11, 40. [Google Scholar] [CrossRef]
- Sarkar, S.; Hillner, K.; Velligan, D.I. Conceptualization and treatment of negative symptoms in schizophrenia. World J. Psychiatry 2015, 5, 352–361. [Google Scholar] [CrossRef]
- Hovington, C.L.; Bodnar, M.; Joober, R.; Malla, A.K.; Lepage, M. Identifying persistent negative symptoms in first episode psychosis. BMC Psychiatry 2012, 12, 224. [Google Scholar] [CrossRef]
- Abdullah, H.M.; Shahul, H.A.; Hwang, M.Y.; Ferrando, S. Comorbidity in Schizophrenia: Conceptual Issues and Clinical Management. Focus 2020, 18, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, F. Smoking and schizophrenia: Still a burning problem. Schizophr. Bull. 2019, 45 (Suppl. S2), S95. [Google Scholar] [CrossRef]
- Hunt, G.E.; Large, M.M.; Cleary, M.; Lai, H.M.X.; Saunders, J.B. Prevalence of comorbid substance use in schizophrenia spectrum disorders in community and clinical settings, 1990–2017: Systematic review and meta-analysis. Drug Alcohol Depend. 2018, 191, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Samochowiec, J.; Pełka-Wysiecka, J. Deficit schizophrenia—how to diagnose and treat? Przew. Lek. 2012, 1, 110–114. [Google Scholar]
- Kirkpatrick, B.; Buchanan, R.; Ross, D.; Carpenter, W., Jr. A separate disease within the syndrome of schizophrenia. Arch. Gen. Psychiatry 2001, 58, 165–171. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Fenton, W.; Carpenter, W., Jr.; Marder, S. The NIMH-MATRICS consen-sus statement on negative symptoms. Schizophr. Bull 2006, 32, 214–219. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Conley, R.; Kakoyannis, A.; Reep, R.; Roberts, R. Interstitial cells of the white matter in the inferior parietal cortex in schizophrenia: An unbiased cell- counting study. Synapse 1999, 34, 95–102. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Messias, N.; Conley, R.; Roberts, R. Interstitial cells of the white matter in the dorsolateral prefrontal cortex in deficit and nondeficit schizophrenia. J. Nerv. Ment. Dis. 2003, 191, 563–567. [Google Scholar] [CrossRef]
- Podwalski, P.; Tyburski, E.; Szczygieł, K.; Waszczuk, K.; Rek-Owodziń, K.; Mak, M.; Plichta, P.; Bielecki, M.; Rudkowski, K.; Kucharska-Mazur, J.; et al. White Matter Integrity of the Corpus Callosum and Psychopathological Dimensions in Deficit and Non-Deficit Schizophrenia Patients. J. Clin. Med. 2021, 11, 2225. [Google Scholar] [CrossRef]
- Turetsky, B.I.; Moberg, P.J.; Yousem, D.M.; Doty, R.L.; Arnold, S.E.; Gur, R.E. Reduced olfactory bulb volume in patients with schizophrenia. Am. J. Psychiatry 2000, 157, 828–830. [Google Scholar] [CrossRef]
- Turetsky, B.I.; Moberg, P.J.; Arnold, S.E.; Doty, R.L.; Gur, R.E. Low olfactory bulb volume in first-degree relatives of patients with schizophrenia. Am. J. Psychiatry 2003, 160, 703–708. [Google Scholar] [CrossRef]
- Tani, H.; Tada, M.; Maeda, T.; Konishi, M.; Umeda, S.; Terasawa, Y.; Mimura, M.; Takahashi, T.; Uchida, H. Comparison of emotional processing assessed with fear conditioning by interpersonal conflicts in patients with depression and schizophrenia. Psychiatry Clin. Neurosci. 2019, 73, 116–125. [Google Scholar] [CrossRef]
- Silton, R.L.; Heller, W.; Towers, D.N.; Engels, A.S.; Spielberg, J.M.; Edgar, J.C.; Sass, S.M.; Stewart, J.L.; Sutton, B.P.; Banich, M.T.; et al. The time course of activity in dorsolateral prefrontal cortex and anterior cingulate cortex during top-down attentional control. Neuroimage 2010, 50, 1292–1302. [Google Scholar] [CrossRef]
- Kerns, J.G.; Cohen, J.D.; MacDonald III, A.W.; Cho, R.C.; Stenger, V.A.; Carter, C.S. Anterior cingulate conflict monitoring and adjustments in control. Science 2004, 303, 1023–1026. [Google Scholar] [CrossRef]
- Menon, V.; Adleman, N.E.; White, C.D.; Glover, G.H.; Reiss, A.L. Error-related brain activation during a Go/NoGo response inhibition task. Hum. Brain. Mapp. 2001, 12, 131–143. [Google Scholar] [CrossRef]
- Gehring, W.J.; Knight, R.T. Prefrontal-cingulate interactions in action monitoring. Nat. Neurosci. 2000, 3, 516–520. [Google Scholar] [CrossRef]
- Morris, R.; Griffiths, O.; Le Pelley, M.E.; Weickert, T.W. Attention to irrelevant cues is related to positive symptoms in schizophrenia. Schizophr. Bull. 2013, 39, 575–582. [Google Scholar] [CrossRef]
- Ilankovic, L.M.; Allen, P.P.; Engel, R.; Kambeitz, J.; Riedel, M.; Müller, N.; Hennig-Fast, K. Attentional modulation of external speech attribution in patients with hallucinations and delusions. Neuropsychologia 2011, 49, 805–812. [Google Scholar] [CrossRef]
- Mlakar, J.; Jensterle, J.; Frith, C.D. Central monitoring deficiency and schizophrenic symptoms. Psychol Med. 1994, 24, 557–564. [Google Scholar] [CrossRef]
- Frith, C.D.; Done, D.J. Experiences of alien control in schizophrenia reflect a disorder in the central monitoring of action. Psychol. Med. 1989, 19, 359–363. [Google Scholar] [CrossRef]
- Brebion, G.; David, A.S.; Bressan, R.A.; Ohlsen, R.I.; Pilowsky, L.S. Hallucinations and two types of free-recall intrusion in schizophrenia. Psychol Med. 2009, 39, 917–926. [Google Scholar] [CrossRef]
- Sridharan, D.; Levitin, D.J.; Menon, V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. USA 2008, 105, 12569–12574. [Google Scholar] [CrossRef] [PubMed]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhuo, C.; Liu, F.; Xu, L.; Yua, C. Neural substrates underlying delusions in schizophrenia. Sci. Rep. 2016, 6, 33857. [Google Scholar] [CrossRef] [PubMed]
- Erkwoh, R.; Sabri, O.; Steinmeyer, E.M.; Bull, U.; Sass, H. Psychopathological and SPECT findings in never-treated schizophrenia. Acta. Psychiatr. Scand. 1997, 96, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, E.; Musich, P.R.; Lin, F. Mitochondrial dysfunction in neurodegenerative diseases and the potential countermeasure. CNS Neurosci. Ther. 2019, 25, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.E.; Olsen, E.M.; Tyrka, A.R. Stress and Psychiatric Disorders: The Role of Mitochondria. Annu. Rev. Clin. Psychol. 2020, 16, 165–186. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, A.; Spekker, E.; Polyák, H.; Tóth, F.; Vécsei, L. Mitochondrial Impairment: A Common Motif in Neuropsychiatric Presentation? The Link to the Tryptophan–Kynurenine Metabolic System. Cells 2022, 11, 2607. [Google Scholar] [CrossRef]
- Park, C.; Park, S.K. Molecular links between mitochondrial dysfunctions and schizophrenia. Mol. Cells 2012, 33, 105–110. [Google Scholar] [CrossRef]
- Rajasekaran, A.; Venkatasubramanian, G.; Berk, M.; Debnath, M. Mitochondrial dysfunction in schizophrenia: Pathways, mechanisms and implications. Neurosci. Biobehav. Rev. 2015, 48, 10–21. [Google Scholar] [CrossRef]
- Holper, L.; Ben-Shachar, D.; Mann, J.J. Multivariate meta-analyses of mitochondrial complex I and IV in major depressive disorder, bipolar disorder, schizophrenia, Alzheimer disease, and Parkinson disease. Neuropsychopharmacology 2019, 44, 837–849. [Google Scholar] [CrossRef]
- Chen, T.; Wang, Y.; Zhang, J.; Wang, Z.; Xu, J.; Li, Y.; Yang, Z.; Liu, D. Abnormal Concentration of GABA and Glutamate in The Prefrontal Cortex in Schizophrenia.-An in Vivo 1H-MRS Study. Shanghai Arch. Psychiatry 2017, 29, 277–286. [Google Scholar] [CrossRef]
- Fan, F.M.; Tan, S.P.; Yang, F.D.; Tan, Y.L.; Zhao, Y.L.; Chen, N.; Li, B.B.; Song, C.S.; Wang, Y.H.; Jin, Z.; et al. Ventral medial prefrontal functional connectivity and emotion regulation in chronic schizophrenia: A pilot study. Neurosci. Bull. 2013, 29, 59–74. [Google Scholar] [CrossRef]
- Hiser, J.; Koenigs, M. The multifaceted role of ventromedial prefrontal cortex in emotion, decision-making, social cognition, and psychopathology. Biol. Psychiatry. 2018, 83, 638–647. [Google Scholar] [CrossRef]
- Small, D.M.; Zatorre, R.J.; Dagher, A.; Evans, A.C.; Jones-Gotman, M. Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain 2001, 124, 1720–1733. [Google Scholar] [CrossRef]
- De Araujo, I.E.; Rolls, E.T.; Kringelbach, M.L.; McGlone, F.; Phillips, N. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur. J. Neurosci. 2003, 18, 2059–2068. [Google Scholar] [CrossRef]
- Kringelbach, M.L.; O’Doherty, J.; Rolls, E.T.; Andrews, C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb. Cortex. 2003, 13, 1064–1071. [Google Scholar] [CrossRef]
- McClure, S.M.; Li, J.; Tomlin, D.; Cypert, K.S.; Montague, L.M.; Montague, P.R. Neural correlates of behavioral preference for culturally familiar drinks. Neuron 2004, 44, 379–387. [Google Scholar] [CrossRef]
- Plassmann, H.; O’Doherty, J.; Shiv, B.; Rangel, A. Marketing actions can modulate neural representations of experienced pleasantness. Proc. Natl. Acad. Sci. USA 2008, 105, 1050–1054. [Google Scholar] [CrossRef]
- Rudenga, K.J.; Small, D.M. Ventromedial Prefrontal Cortex Response to Concentrated Sucrose Reflects Liking Rather Than Sweet Quality Coding. Chem. Senses 2013, 38, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Fabius, J.H.; Moravkova, K.; Fracasso, A.; Borgomaneri, S. The Neurobiological Correlates of Gaze Perception in Healthy Individuals and Neurologic Patients. Biomedicines 2022, 10, 627. [Google Scholar] [CrossRef] [PubMed]
- Sellitto, M.; Terenzi, D.; Starita, F.; di Pellegrino, G.; Battaglia, S. The Cost of Imagined Actions in a Reward-Valuation Task. Brain Sci. 2022, 12, 582. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S. Neurobiological advances of learned fear in humans. Adv. Clin. Exp. Med. 2022, 31, 217–221. [Google Scholar] [CrossRef]
- Stahl, S. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Application, 3rd ed.; Cambridge University Press: New York, NY, USA, 2008. [Google Scholar]
- Gargiulo, P.A.; Landa De Gargiulo, A.I. Glutamate and modeling of schizophrenia symptoms: Review of our Findings: 1990−2014. Pharmacol. Rep. 2014, 66, 343–352. [Google Scholar] [CrossRef]
- Milev, P.; Ho, B.C.; Arndt, S.; Nopoulos, P.; Andreasen, N.C. Initial magnetic resonance imaging volumetric brain measurements and outcome in schizophrenia: A prospective longitudinal study with 5-year follow-up. Biol. Psychiatry 2003, 54, 608–615. [Google Scholar] [CrossRef]
- Andreasen, N.C.; Nopoulos, P.; Magnotta, V.; Pierson, R.; Ziebell, S.; Ho, B.C. Progressive brain change in schizophrenia: A prospective longitudinal study of first-episode schizophrenia. Biol. Psychiatry 2011, 70, 672–679. [Google Scholar] [CrossRef]
- Andreasen, N.C.; Liu, D.; Ziebell, S.; Vora, A.; Ho, B.C. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: A prospective longitudinal MRI study. Am. J. Psychiatry 2013, 170, 609–615. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Editorial of Special Issue ‘Dissecting Neurological and Neuropsychiatric Diseases: Neurodegeneration and Neuroprotection’. Int. J. Mol. Sci. 2022, 23, 6991. [Google Scholar] [CrossRef]
- Stockhorst, U.; Pietrowsky, R. Olfactory perception, communication, and the nose-to-brain pathway. Physiol. Behav. 2004, 83, 3–11. [Google Scholar] [CrossRef]
- Kosaka, T.; Kosaka, K. “Interneurons” in the olfactory bulb revisited. Neurosci. Res. 2011, 69, 93–99. [Google Scholar] [CrossRef]
- Pełka-Wysiecka, J.; Wroński, M.; Bieńkowski, P.; Murawiec, S.; Samochowiec, A.; Samochowiec, J. Odors identification differences in deficit and nondeficit schizophrenia. Pharmacol. Rep. 2016, 68, 390–395. [Google Scholar] [CrossRef]
- Urban-Kowalczyk, M.; Śmigielski, J.; Strzelecki, D. Olfactory identification in patients with schizophrenia—The influence of β-endorphin and calcitonin gene-related peptide concentrations. Eur. Psychiatry 2017, 41, 16–20. [Google Scholar] [CrossRef]
- Kamath, V.; Turetsky, B.I.; Moberg, P.J. Identification of pleasant, neutral, and un-pleasant odors in schizophrenia. Psychiatry Res. 2011, 187, 30–35. [Google Scholar] [CrossRef][Green Version]
- Moberg, P.J.; Doty, R.L.; Turetsky, B.I.; Wylonis, L.; Cannon, T.D.; Acosta, T.A.; Gur, R.E. Olfactory functioning in siblings discordent for schizophrenia. Biol. Psychiatry 1996, 39, 571–572. [Google Scholar] [CrossRef]
- Faurion, A. Are umami taste receptor sites structurally related to glutamate CNS receptor sites? Physiol. Behav. 1991, 49, 905–912. [Google Scholar] [CrossRef]
- Chandrashekar, J.; Hoon, M.A.; Ryba, N.J.; Zuker, C.S. The receptors and cells for mammalian taste. Nature 2006, 444, 288–294. [Google Scholar] [CrossRef]
- WHO. International Statistical Classification of Diseases and Health Problems. Tenth Revision. Classification of Mental and Behavioral Disorders in the ICD-10. Research Diagnostic Criteria; Institute of Psychiatry and Neurology, University Medical Publishing House “Vesalius”: Krakow, Warsaw, 1998; pp. 69–71. [Google Scholar]
- Kirkpatrick, B.; Buchanan, R.W.; McKenney, P.D.; Alphs, L.D.; Carpenter, W.T., Jr. The Schedule for the deficit syndrome: An instrument for research in schizophrenia. Psychiatry Res. 1989, 30, 119–123. [Google Scholar] [CrossRef]
- Galderisi, S.; Mucci, A.; Buchanan, R.W.; Arango, C. Negative symptoms of schizophrenia: New developments and unanswered research questions. Lancet Psychiatry 2018, 5, 664–677. [Google Scholar] [CrossRef]
- Kirschner, M.; Aleman, A.; Kaiser, S. Secondary negative symptoms—A review of mechanisms, assessment and treatment. Schizophr. Res. 2017, 186, 29–38. [Google Scholar] [CrossRef]
- Kirkpatrick, B. Progress in the study of negative symptoms. Schizophr. Bull. 2014, 40 (Suppl S2), S101–S106. [Google Scholar] [CrossRef]
- Moller, H.J. Clinical evaluation of negative symptoms in schizophrenia. Eur. Psychiatry 2007, 22, 380–386. [Google Scholar] [CrossRef]
- Wójciak, P. Objawy negatywne schizofrenii pierwotne i wtórne, zespół deficytowy, uporczywe objawy negatywne. Neuropsychiatr. I Neuropsychol. 2017, 3, 108–117. [Google Scholar] [CrossRef]
- Kalisz, A.; Mętel, D.; Daren, A.; Błądziński, P.; Kruk, D.; Cechnicki, A. Objawy negatywne, przetrwałe objawy negatywne i zespół deficytowy a nasilenie objawów schizofrenii i poziom funkcjonowania przez 20 lat. Adv. Psychiatry Neurol. 2020, 29, 25–38. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.O.; Lewis, A. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr. Bull 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Leucht, S.; Kissling, W.; Davis, J.M. The PANSS should be rescaled. Schizophr. Bull 2010, 36, 461–462. [Google Scholar] [CrossRef]
- Jarema, M. Standards of Pharmacological Treatment of Some Mental Disorders; Via Medica: Gdańsk, Poland, 2015. [Google Scholar]
- Szafrański, T. W Labiryncie Ekwiwalencji. Krótki, Subiektywny Przewodnik. Psychiatra. Pismo dla Praktyków. Grudzień 2014-Luty 2015 / NR 7. pp. 44–45. Available online: https://www.psychiatraonline.pl/wp-content/uploads/2017/06/PSYCHIATRA_7_eBOOK_COVERED.pdf (accessed on 10 January 2022).
- Bałczewska, E.; Nowak, A. Taste Disorders—Dysgeusia; Nowa Stomatologia: Kraków, Poland, 2000; pp. 3–8. [Google Scholar]
- Iordachescu, G.; Vlasceanu, G.; Bleoanca, I.; Neagu, C.; Iordachescu, A. Umami taste and the consumer perception. Ann. Univ. Dunarea De Jos Galati. Fascicle VI-Food Technol. 2008, 32, 58–61. [Google Scholar]
- Singh, P.B.; Schuster, B.; Seo, H.S. Variation in umami taste perception in the German and Norwegian population. Eur. J. Clin. Nutr. 2010, 64, 1248–1250. [Google Scholar] [CrossRef]
- Setsu, R.; Hirano, Y.; Tokunaga, M.; Takahashi, T.; Numata, N.; Matsumoto, K.; Ma-suda, Y.; Matsuzawa, D.; Iyo, M.; Shimizu, E.; et al. Increased Subjective Distaste and Altered Insula Activity to Umami Tastant in Patients with Bulimia Nervosa. Front. Psychiatry 2017, 8, 172. [Google Scholar] [CrossRef]
- Wrobel, E.; Skrok-Wolska, D.; Ziolkowski, M.; Korkosz, A.; Habrat, B.; Woronowicz, B.; Kukwa, A.; Kostowski, W.; Bienkowski, P.; Scinska, A. Taste responses to monosodium glutamate after alcohol exposure. Alcohol Alcohol. 2005, 40, 106–111. [Google Scholar] [CrossRef][Green Version]
| Triplet No. | Sample 1 | Sample 2 | Sample 3 |
|---|---|---|---|
| 1 | MSG (0.001%) | W | W |
| 2 | W | W | MSG (0.003%) |
| 3 | W | MSG (0.01%) | W |
| 4 | W | MSG (0.03%) | W |
| 5 | W | W | MSG (0, 1%) |
| 6 | MSG (0.3%) | W | W |
| 7 | W | MSG (1%) | W |
| Mean ± SD or n (%) | |
|---|---|
| Age, years | 39.9 ± 11.2 |
| Sex, males | 98 (48.0) |
| Education: | |
| Higher Secondary Vocational Primary | 36 (18.0) 94 (47.0) 44 (22.0) 26 (13.0) |
| BMI, kg/m2 | 27.6 ± 4.8 |
| Place of residence: | |
| Urban Rural | 185 (92.5) 15 (7.5) |
| Cigarette smoking, yes | 95 (47.5) |
| Illness duration, years | 13.6 ± 9.2 |
| CPZE, mg/day | 820.5 ± 1179.8 |
| PANSS—positive symptoms | 7.7 ± 7.0 |
| PANSS—negative symptoms | 12.6 ± 8.8 |
| PANSS—depressive symptoms | 4.3 ± 4.0 |
| SDS—number of deficit symptoms | 4.7 ± 2.1 |
| Number of Correctly Recognized Samples | Mean Intensity of Taste | Mean Pleasure of Taste | |
|---|---|---|---|
| SDS—number of deficit symptoms | r = −0.046, p = 0.517 | r = −0.215, p = 0.002 | r = 0.004, p = 0.958 |
| PANSS—positive symptoms | r = −0.049, p = 0.491 | r = −0.022, p = 0.760 | r = −0.036, p = 0.616 |
| PANSS—negative symptoms | r = −0.106, p = 0.134 | r = −0.071, p = 0.322 | r = −0.020, p = 0.784 |
| PANSS—depressive symptoms | r = −0.009, p = 0.896 | r = −0.029, p = 0.683 | r = −0.112, p = 0.114 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wroński, M.; Samochowiec, J.; Pełka-Wysiecka, J.; Liśkiewicz, P.; Bieńkowski, P.; Misiak, B. Deficit Symptomatology of Schizophrenia Is Associated with Attenuated Taste Identification: Findings from a Cross-Sectional Study. Brain Sci. 2022, 12, 1520. https://doi.org/10.3390/brainsci12111520
Wroński M, Samochowiec J, Pełka-Wysiecka J, Liśkiewicz P, Bieńkowski P, Misiak B. Deficit Symptomatology of Schizophrenia Is Associated with Attenuated Taste Identification: Findings from a Cross-Sectional Study. Brain Sciences. 2022; 12(11):1520. https://doi.org/10.3390/brainsci12111520
Chicago/Turabian StyleWroński, Michał, Jerzy Samochowiec, Justyna Pełka-Wysiecka, Paweł Liśkiewicz, Przemysław Bieńkowski, and Błażej Misiak. 2022. "Deficit Symptomatology of Schizophrenia Is Associated with Attenuated Taste Identification: Findings from a Cross-Sectional Study" Brain Sciences 12, no. 11: 1520. https://doi.org/10.3390/brainsci12111520
APA StyleWroński, M., Samochowiec, J., Pełka-Wysiecka, J., Liśkiewicz, P., Bieńkowski, P., & Misiak, B. (2022). Deficit Symptomatology of Schizophrenia Is Associated with Attenuated Taste Identification: Findings from a Cross-Sectional Study. Brain Sciences, 12(11), 1520. https://doi.org/10.3390/brainsci12111520









