Apelin-13 Protects Neurons by Attenuating Early-Stage Postspinal Cord Injury Apoptosis In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Establishment of the Spinal Cord Injury Model
2.3. RNA Extraction, Library Construction, and Sequencing
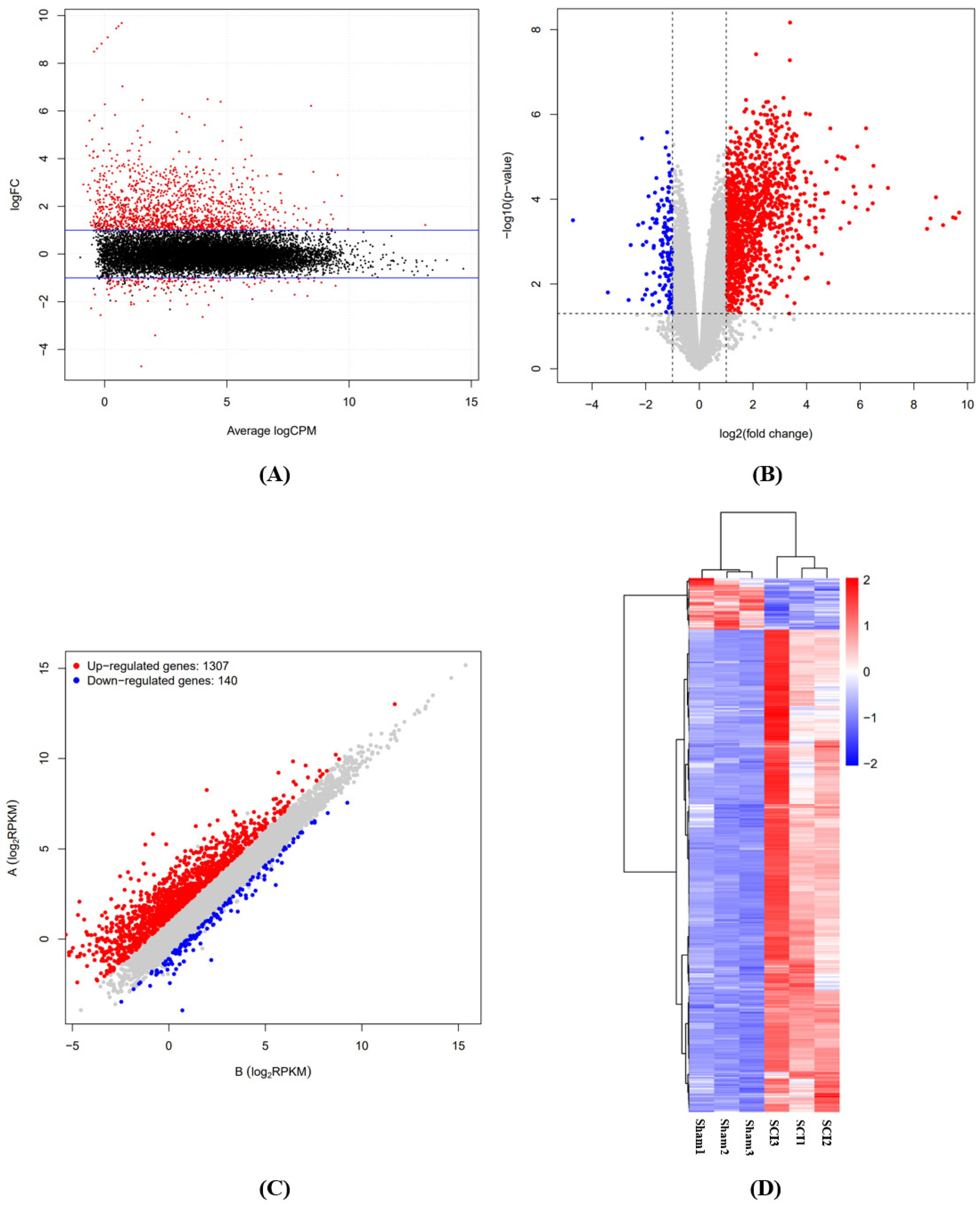
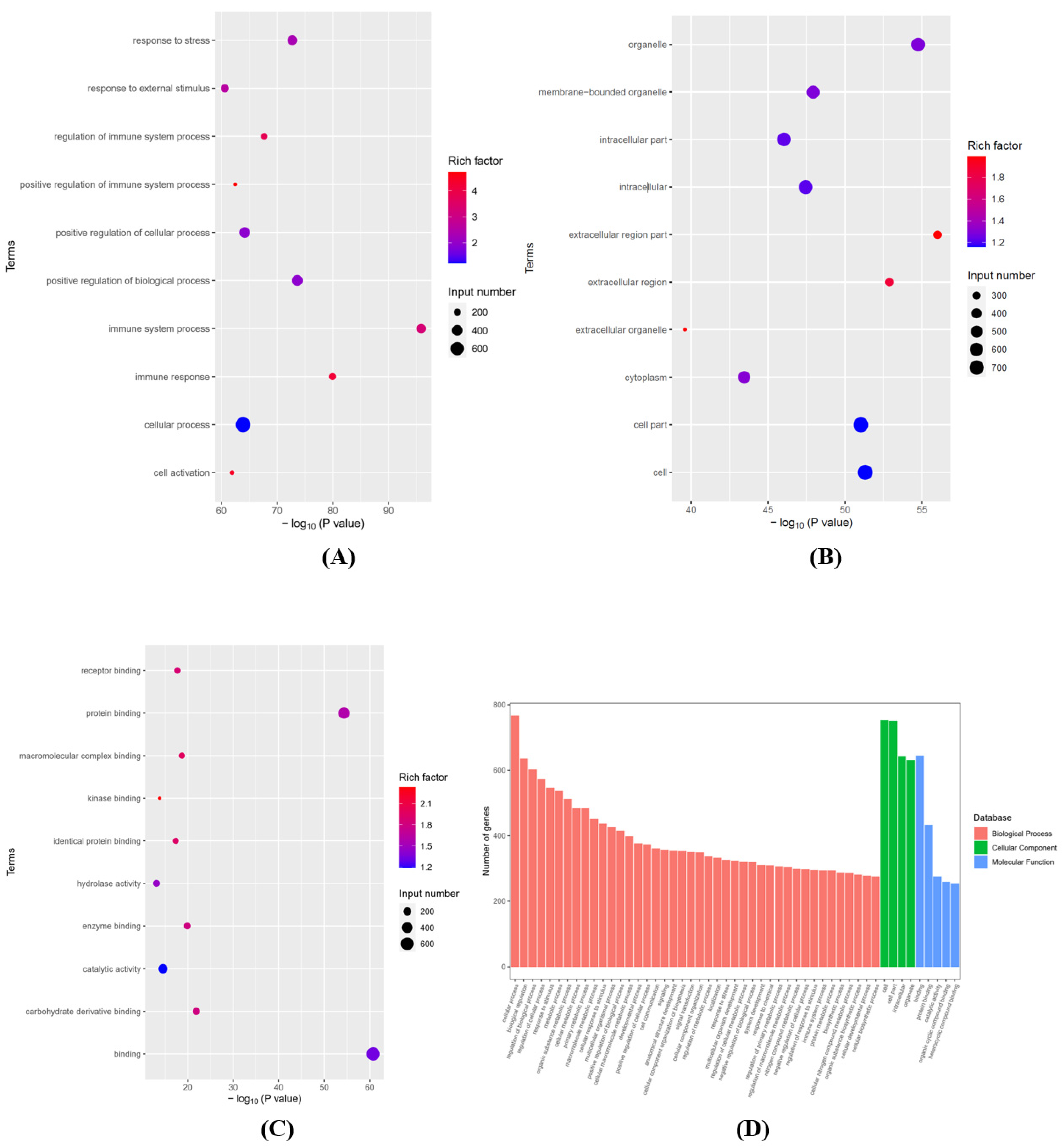
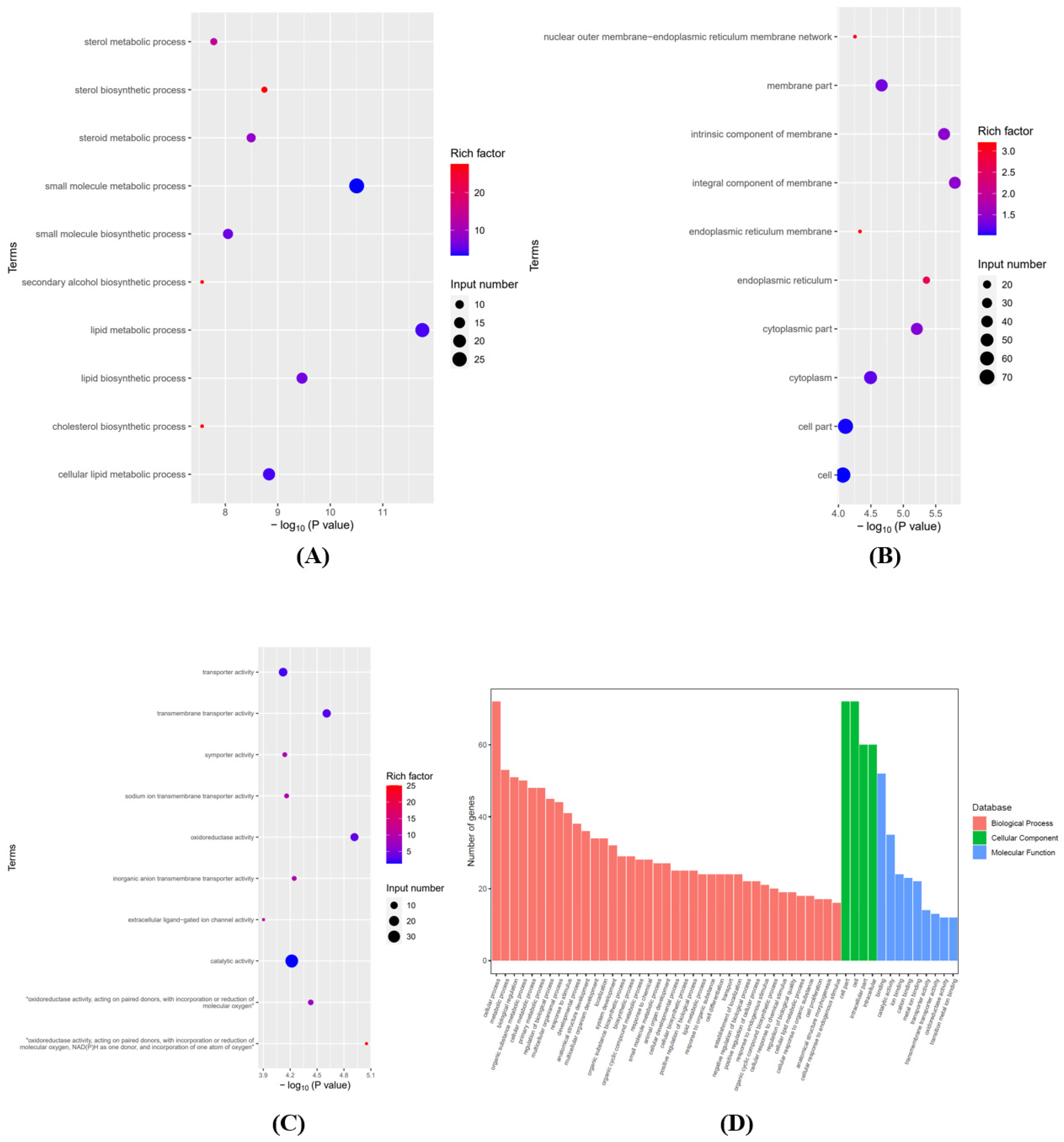
2.4. Differentially Expressed Genes (DEGs) and Function Enrichment Analyses
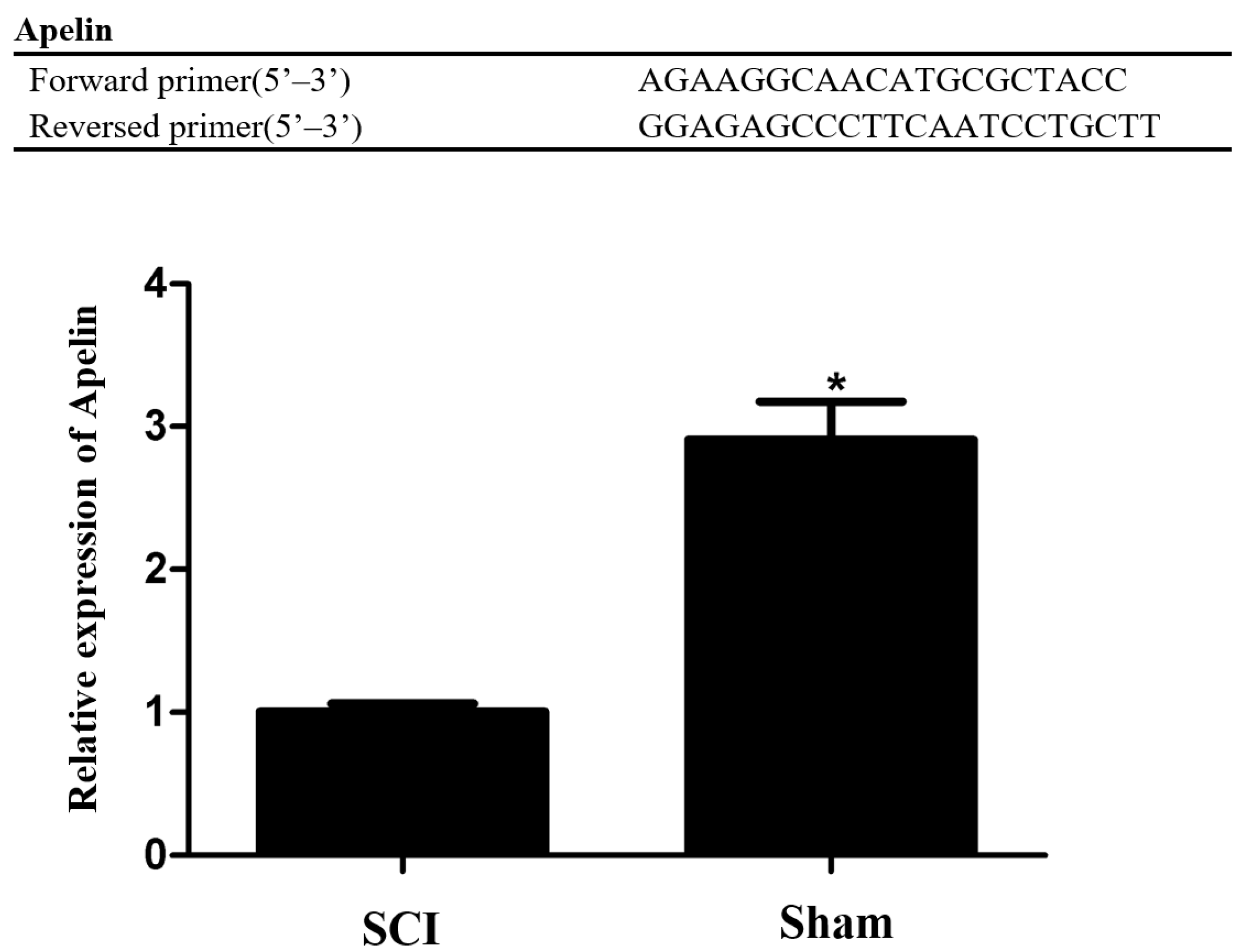
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
2.6. Cell Culture, Differentiation, and Oxygen-Glucose Deprivation (OGD) Model and Treatment
2.7. Cell Viability Assay
2.8. Western Blot Analysis
2.9. TdT-Mediated dTUP Nick End Labeling (TUNEL) Staining
2.10. Flow Cytometry
2.11. Statistical Analysis
3. Results
3.1. Identification of Differentially Expressed mRNAs in the Rat Spinal Cord Following SCI
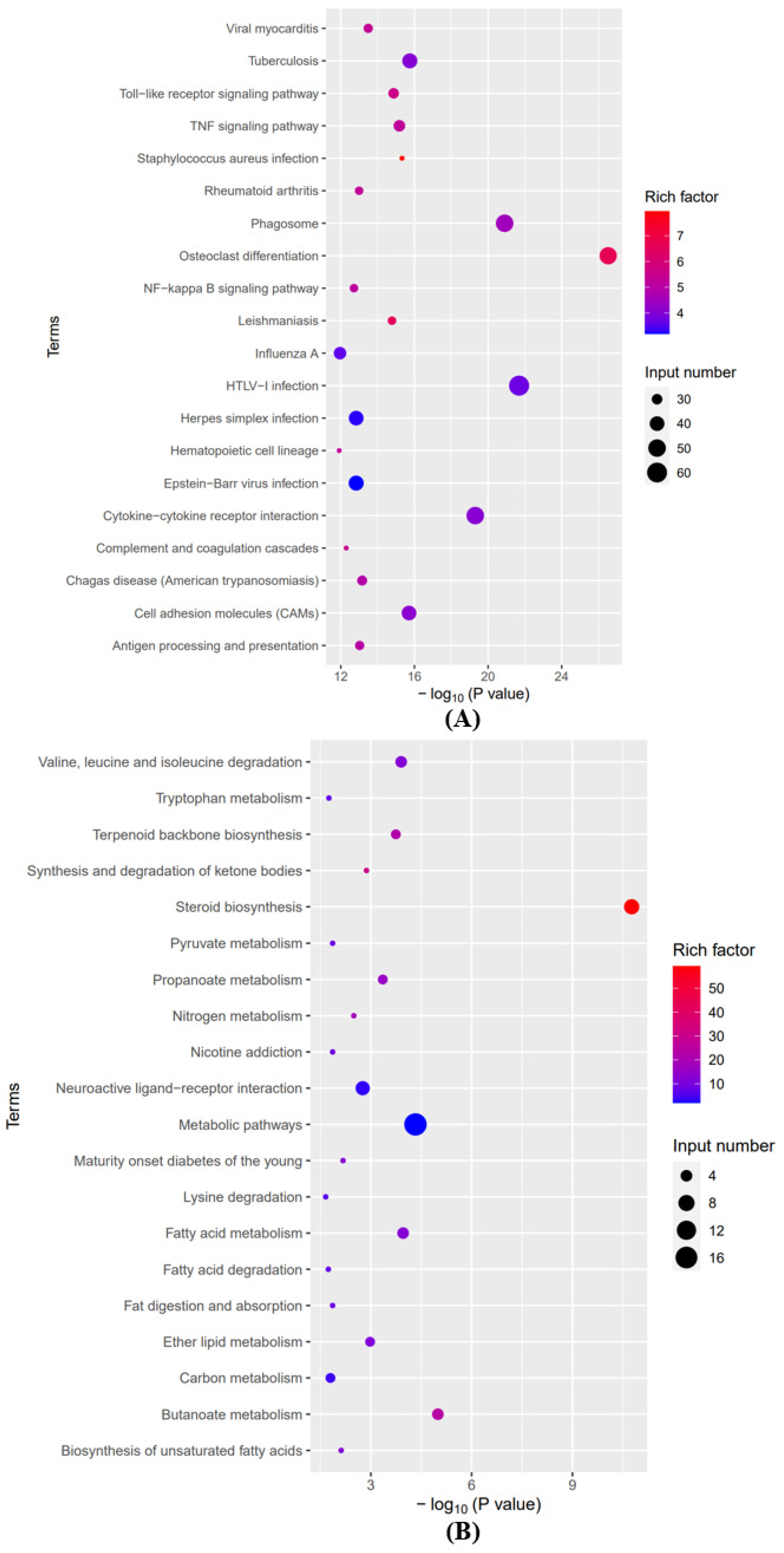
3.2. Analyses of GO and KEGG Pathways in the Differentially Expressed mRNAs
3.3. Selection and Expression Verification of the Differentially Expressed mRNAs
3.4. Effect of Different OGD Injury Times on Apelin Expression in the OGD Model of PC12 Cells In Vitro
3.5. The Cytotoxicity of Apelin-13 at Different Concentrations Was Determined by CCK-8 Assay
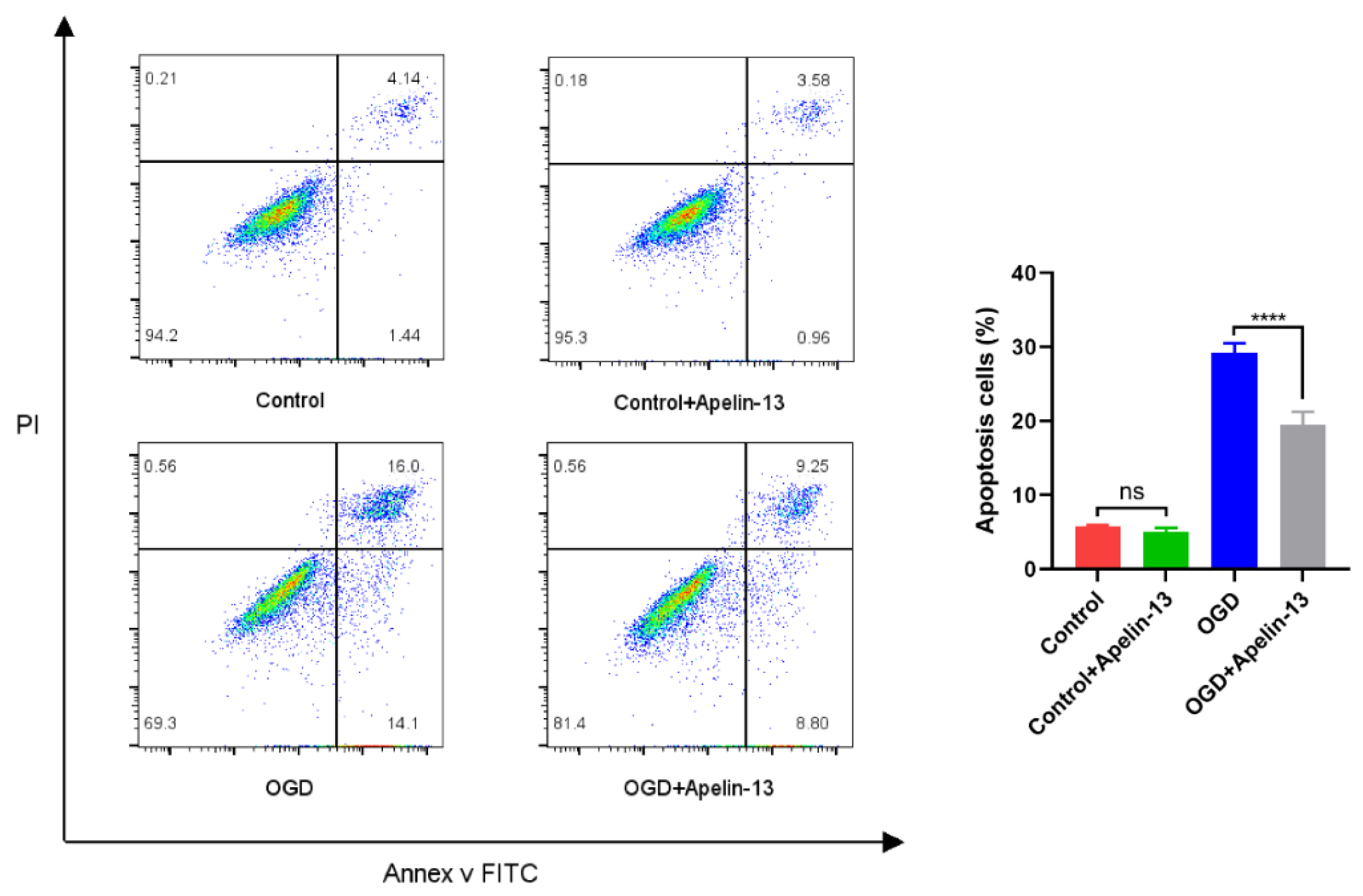
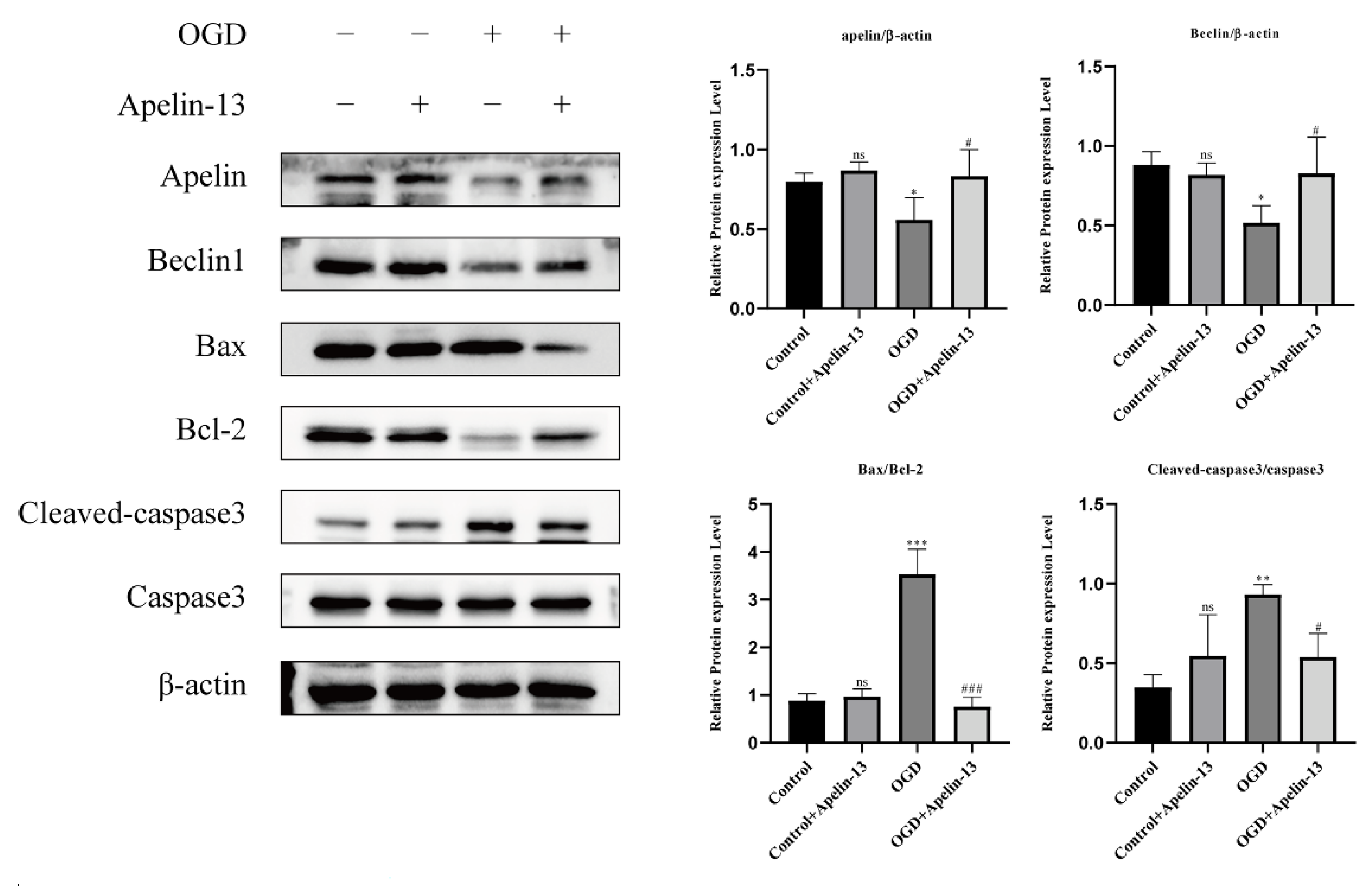
3.6. Apelin-13 Enhanced Autophagy and Decreased Apoptosis In Vitro Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holmes, D. Spinal-cord injury: Spurring regrowth. Nature 2017, 552, S49. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Subedi, M. Pathophysiology, mechanisms and applications of mesenchymal stem cells for the treatment of spinal cord injury. Biomed. Pharmacother. 2017, 91, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xia, X.; Zhu, X.; Cao, J.; Xu, D.; Ni, Y.; Liu, Y.; Yan, S.; Cheng, X.; Liu, Y.; et al. Expression of SGTA correlates with neuronal apoptosis and reactive gliosis after spinal cord injury. Cell Tissue Res. 2014, 358, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-J.; Peng, C.-H.; Zhang, S.; Xu, X.-L.; Shu, G.-F.; Qi, J.; Zhu, Y.-F.; Xu, D.-M.; Kang, X.-Q.; Lu, K.-J.; et al. Polysialic acid-based micelles promote neural regeneration in spinal cord injury therapy. Nano Lett. 2019, 19, 829–838. [Google Scholar] [CrossRef]
- Xu, W.; Li, T.; Gao, L.; Zheng, J.; Yan, J.; Zhang, J.; Shao, A. Apelin-13/APJ system attenuates early brain injury via suppression of endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation and oxidative stress in a AMPK-dependent manner after subarachnoid hemorrhage in rats. J. Neuroinflamm. 2019, 16, 247. [Google Scholar] [CrossRef]
- Yamaleyeva, L.M.; Shaltout, H.; Varagic, J. Apelin-13 in blood pressure regulation and cardiovascular disease. Curr. Opin. Nephrol. Hypertens. 2016, 25, 396–403. [Google Scholar] [CrossRef]
- Shao, Z.-Q.; Dou, S.-S.; Zhu, J.-G.; Wang, H.-Q.; Wang, C.-M.; Cheng, B.-H.; Bai, B. Apelin-13 inhibits apoptosis and excessive autophagy in cerebral ischemia/reperfusion injury. Neural Regen. Res. 2021, 16, 1044–1051. [Google Scholar]
- Chen, L.; Zhou, T.; White, T.; O’Brien, A.; Chakraborty, S.; Liangpunsakul, S.; Yang, Z.; Kennedy, L.; Saxena, R.; Wu, C.; et al. The Apelin-Apelin Receptor Axis Triggers Cholangiocyte Proliferation and Liver Fibrosis During Mouse Models of Cholestasis. Hepatology 2020, 73, 2411–2428. [Google Scholar] [CrossRef]
- Bircan, B.; Çakır, M.; Kırbağ, S.; Gül, H.F. Effect of apelin hormone on renal ischemia/reperfusion induced oxidative damage in rats. Ren. Fail. 2016, 38, 1122–1128. [Google Scholar] [CrossRef]
- Dong, H.; Dong, B.; Zhang, N.; Liu, S.; Zhao, H. microRNA-182 Negatively Influences the Neuroprotective Effect of Apelin Against Neuronal Injury in Epilepsy. Neuropsychiatr. Dis. Treat. 2020, 16, 327–338. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, X.; Zhou, Y.; Feng, Y.; Yang, Y.; Wang, X. Intrathecally Administered Apelin-13 Alleviated Complete Freund’s Adjuvant-Induced Inflammatory Pain in Mice. Front. Pharmacol. 2020, 11, 1335. [Google Scholar] [CrossRef]
- Zhou, J.-X.; Shuai, N.-N.; Wang, B.; Jin, X.; Kuang, X.; Tian, S.-W. Neuroprotective gain of Apelin/APJ system. Neuropeptides 2021, 87, 102131. [Google Scholar] [CrossRef]
- Luo, H.; Han, L.; Xu, J. Apelin/APJ system: A novel promising target for neurodegenerative diseases. J. Cell. Physiol. 2020, 235, 638–657. [Google Scholar] [CrossRef]
- Kinjo, T.; Higashi, H.; Uno, K.; Kuramoto, N. Apelin/Apelin Receptor System: Molecular Characteristics, Physiological Roles, and Prospects as a Target for Disease Prevention and Pharmacotherapy. Curr. Mol. Pharmacol. 2020, 14, 210–219. [Google Scholar] [CrossRef]
- Bao, H.-J.; Zhang, L.; Han, W.-C.; Dai, D.-K. Apelin-13 attenuates traumatic brain injuryinduced damage by suppressing autophagy. Neurochem. Res. 2015, 40, 89–97. [Google Scholar] [CrossRef]
- Khaksari, M.; Aboutaleb, N.; Nasirinezhad, F.; Vakili, A.; Madjd, Z. Apelin-13 protects the brain against ischemic reperfusion injury and cerebral edema in a transient model of focal cerebral ischemia. J. Mol. Neurosci. 2012, 48, 201–208. [Google Scholar] [CrossRef]
- Yan, X.-G.; Cheng, B.-H.; Wang, X.; Ding, L.-C.; Liu, H.-Q.; Chen, J.; Bai, B. Lateral intracerebroventricular injection of Apelin-13 inhibits apoptosis after cerebral ischemia/reperfusion injury. Neural Regen. Res. 2015, 10, 766–771. [Google Scholar]
- Wu, R.; Mao, S.; Wang, Y.; Zhou, S.; Liu, Y.; Liu, M.; Gu, X.; Yu, B. Differential Circular RNA Expression Profiles Following Spinal Cord Injury in Rats: A Temporal and Experimental Analysis. Front. Neurosci. 2019, 13, 1303. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Jianxia, Z.; Kunlin, W.; Songjun, Z.; da Silva, J.A.T.; Zhao, X.; Tian, C.-E.; Xia, H.; Duan, J. Transcriptome analysis of Cymbidium sinense and its application to the identification of genes associated with floral development. BMC Genom. 2013, 14, 279. [Google Scholar]
- Ran, T.; Riyi, S. Dimercaprol is an acrolein scavenger that mitigates acrolein-mediated PC-12 cells toxicity and reduces acrolein in rat following spinal cord injury. J. Neurochem. 2017, 141, 708–720. [Google Scholar]
- Zhanjun, M.; Yubao, L.; Fengguang, Y.; Li, S.; He, X.; Gao, Y.; Zhang, G.; Ren, E.; Wang, Y.; Kang, X. Rosmarinic acid exerts a neuroprotective effect on spinal cord injury by suppressing oxidative stress and inflammation via modulating the Nrf2/HO-1 and TLR4/NF-κB pathways. Toxicol. Appl. Pharm. 2020, 397, 115014. [Google Scholar]
- Li, H.; Wang, C.; He, T.; Zhao, T.; Chen, Y.-Y.; Shen, Y.-L.; Zhang, X.; Wang, L.-L. Mitochondrial transfer from bone marrow mesenchymal stem cells to motor neurons in spinal cord injury rats via gap junction. Theranostics 2019, 9, 2017–2035. [Google Scholar] [CrossRef]
- Karsy, M.; Hawryluk, G. Modern medical management of spinal cord injury. Curr. Neurol. Neurosci. Rep. 2019, 19, 65. [Google Scholar] [CrossRef]
- Kumar, R.; Lim, J.; Mekary, R.A.; Rattani, A.; Dewan, M.C.; Sharif, S.Y.; Osorio-Fonseca, E.; Park, K.B. Traumatic spinal injury: Global epidemiology and worldwide volume. World Neurosurg. 2018, 113, e345–e363. [Google Scholar] [CrossRef]
- Xia, P.; Gao, X.; Duan, L.; Zhang, W.; Sun, Y.-F. Mulberrin (Mul) reduces spinal cord injury (SCI)-induced apoptosis, inflammation and oxidative stress in rats via miroRNA-337 by targeting Nrf-2. Biomed. Pharmacother. 2018, 107, 1480–1487. [Google Scholar] [CrossRef]
- Bahney, J.; von Bartheld, C.S. The cellular composition and glia-neuron ratio in the spinal cord of a human and a nonhuman primate: Comparison with other species and brain regions. Anat. Rec. 2018, 301, 697–710. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef]
- Khalilzadeh, B.; Shadjou, N.; Kanberoglu, G.S.; Afsharan, H.; de la Guardia, M.; Charoudeh, H.N.; Ostadrahimi, A.; Rashidi, M.-R. Advances in nanomaterial based optical biosensing and bioimaging of apoptosis via caspase-3 activity: A review. Mikrochim. Acta 2018, 185, 434. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Zhu, X.; Qi, X.; Lin, K.; Cheng, L. The effects of icariin on enhancing motor recovery through attenuating pro-inflammatory factors and oxidative stress via mitochondrial apoptotic pathway in the mice model of spinal cord injury. Front. Physiol. 2018, 9, 1617. [Google Scholar] [CrossRef]
- Moldoveanu, T.; Follis, A.V.; Kriwacki, R.W.; Green, D.R. Many players in BCL-2 family affairs. Trends Biochem. Sci. 2014, 39, 101–111. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Lin, J.; Muharram, A.; Liu, W.-G. Beclin-1-mediated autophagy protects spinal cord neurons against mechanical injury-induced apoptosis. Apoptosis 2014, 19, 933–945. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, L.; Zheng, X.; Chen, G.; Pan, R.; Li, J.; Liu, W. Autophagy protects against PI3K/Akt/mTOR-mediated apoptosis of spinal cord neurons after mechanical injury. Neurosci. Lett. 2017, 656, 158–164. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Wang, Z.; Liu, W. Ezetimibe protects against spinal cord injury by regulating autophagy and apoptosis through inactivation of PI3K/AKT/mTOR signaling. Am. J. Transl. Res. 2020, 12, 2685–2694. [Google Scholar]
- Booth, L.A.; Tavallai, S.; Hamed, H.A.; Cruickshanks, N.; Dent, P. The role of cell signalling in the crosstalk between autophagy and apoptosis. Cell. Signal. 2013, 26, 549–555. [Google Scholar] [CrossRef]




| Concentration of Apelin-13 (μM) | 450 nm OD-Value (Mean ± SD) | Cell Viability Percentage (%) (Mean ± SD) |
|---|---|---|
| 0 | 1.113 ± 0.057 | 100.000 |
| 0.001 | 1.142 ± 0.061 | 102.900 ± 1.481 |
| 0.01 | 1.173 ± 0.069 | 105.905 ± 0.719 |
| 0.1 | 1.188 ± 0.080 | 107.432 ± 0.487 |
| 1 | 1.229 ± 0.098 | 111.414 ± 2.188 |
| 10 | 1.169 ± 0.042 | 105.538 ± 2.131 |
| Group | 450 nm OD-Value (Mean ± SD) | Cell Viability Percentage (%) (Mean ± SD) |
|---|---|---|
| Control | 1.137 ± 0.016 | 100.000 |
| Control+Apelin-13 | 1.251 ± 0.010 | 111.111 ± 4.332 |
| OGD | 0.753 ± 0.022 | 57.680 ± 3.912 |
| OGD+Apelin-13 | 0.892 ± 0.021 | 73.451 ± 3.941 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.; Zhao, Y.; Guo, S.; Wu, Z.; Li, W.; Wu, R.; Wang, Z.; Liu, W. Apelin-13 Protects Neurons by Attenuating Early-Stage Postspinal Cord Injury Apoptosis In Vitro. Brain Sci. 2022, 12, 1515. https://doi.org/10.3390/brainsci12111515
Lin T, Zhao Y, Guo S, Wu Z, Li W, Wu R, Wang Z, Liu W. Apelin-13 Protects Neurons by Attenuating Early-Stage Postspinal Cord Injury Apoptosis In Vitro. Brain Sciences. 2022; 12(11):1515. https://doi.org/10.3390/brainsci12111515
Chicago/Turabian StyleLin, Taotao, Yujie Zhao, Shengyu Guo, Zhengru Wu, Wenwen Li, Rongcan Wu, Zhenyu Wang, and Wenge Liu. 2022. "Apelin-13 Protects Neurons by Attenuating Early-Stage Postspinal Cord Injury Apoptosis In Vitro" Brain Sciences 12, no. 11: 1515. https://doi.org/10.3390/brainsci12111515
APA StyleLin, T., Zhao, Y., Guo, S., Wu, Z., Li, W., Wu, R., Wang, Z., & Liu, W. (2022). Apelin-13 Protects Neurons by Attenuating Early-Stage Postspinal Cord Injury Apoptosis In Vitro. Brain Sciences, 12(11), 1515. https://doi.org/10.3390/brainsci12111515






