Differential Diagnosis of Major Depressive Disorder and Bipolar Disorder: Genetic and Hormonal Assessment and the Influence of Early-Life Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Hormonal Assessment
2.3. Genetic Assessment
2.4. Data Analysis
3. Results
3.1. Sociodemographic Data
3.2. Hormonal Levels
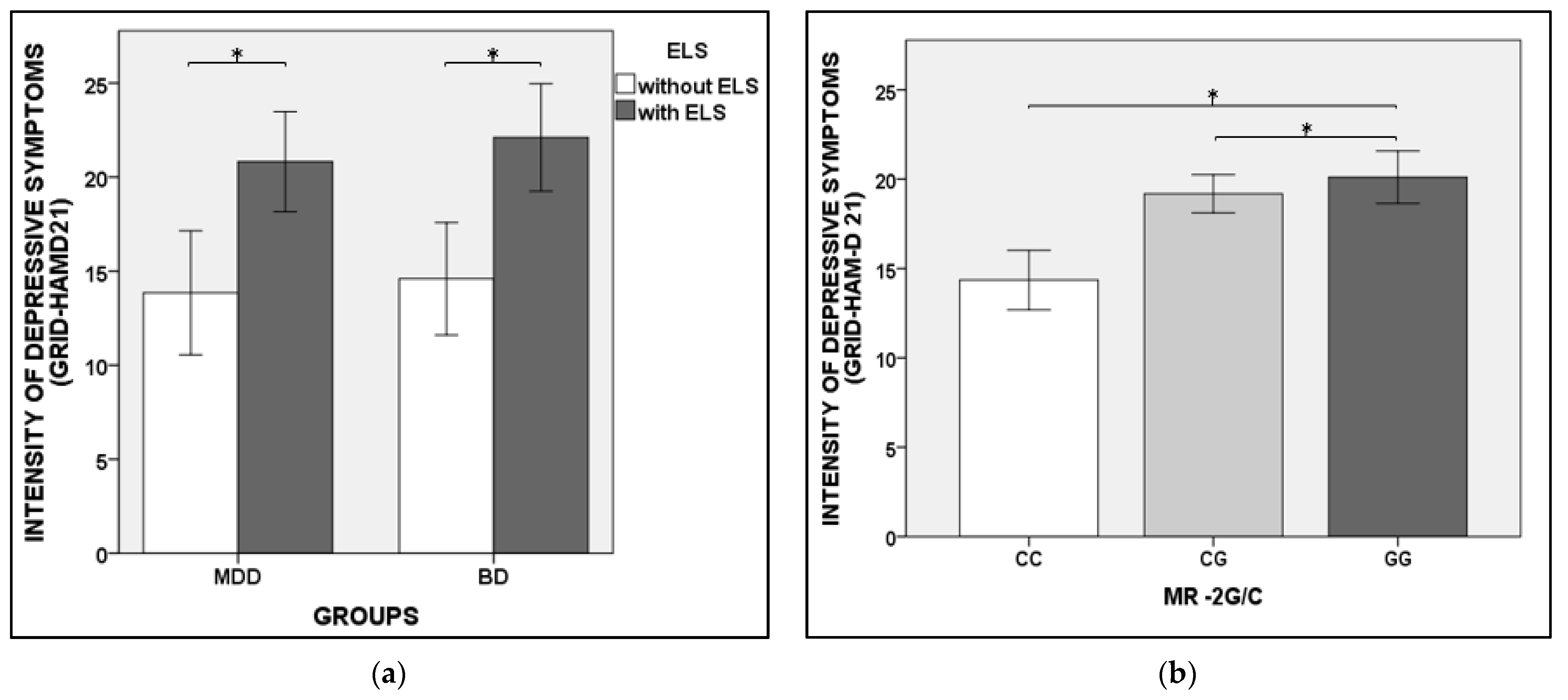
3.3. The Severity of Depressive Symptoms
4. Discussion
Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Genetics Sample | Statistics | Cortisol Sample | Statistics | Aldosterone Sample | Statistics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HC | MDD | BD | HC | MDD | BD | HC | MDD | BD | ||||
| SSRI ADP (%) | -- | 60.26 | 52.44 | X2 = 0.420; p = 0.517 | -- | 59.4 | 53.1 | X2 = 0.535; p = 0.489 | -- | 59.7 | 55.6 | X2 = 0.229; p = 0.763 |
| SRNI ADP (%) | -- | 11.54 | 12.19 | X2 = 0.060; p = 0.807 | -- | 11.6 | 10.9 | X2 = 0.014; p = 1.000 | -- | 11.9 | 12.7 | X2 = 0.017; p = 1.000 |
| MAOI ADP (%) | -- | 0 | 0 | X2 = 0; p = 1.0 | -- | 0 | 0 | 0 | -- | 0 | 0 | 0 |
| Atypical ADP (%) | -- | 15.38 | 8.54 | X2 = 1.491; p = 0.221 | -- | 14.5 | 7.8 | X2 = 0.224; p = 0.278 | -- | 14.9 | 7.3 | X2 = 0.213; p = 0.276 |
| Triciclic ADP (%) | -- | 16.67 | 10.98 | X2 = 0.847; p = 0.357 | -- | 14.5 | 7.8 | X2 = 1.481; p = 0.278 | -- | 16.4 | 9.5 | X2 = 1.358; p = 0.303 |
| Antipsychotic (%) | -- | 30.05 | 39.02 | X2 = 1.355; p = 0.244 | -- | 31.9 | 40.6 | X2 = 1.100; p = 0.367 | -- | 32.8 | 39.7 | X2 = 0.659; p = 0.467 |
| Anticonvulsants (%) | -- | 17.95 | 37.80 | X2 = 9.026; p = 0.003 | -- | 17.4 | 35.9 | X2 = 5.890; p = 0.018 | -- | 17.9 | 38.1 | X2 = 6.607; p = 0.011 |
| Litium (%) | -- | 0 | 19.51 | X2 = 17.829; p < 0.001 c | -- | 0 | 23.1 | X2 = 18.228; p < 0.001 c | -- | 0 | 20.6 | X2 = 15.362; p < 0.001 c |
| Ansiolitic/ hypnotic (%) | -- | 42.31 | 47.56 | X2 = 0.646; p = 0.421 | -- | 40.6 | 46.9 | X2 = 0.535; p = 0.489 | -- | 44.8 | 29.6 | X2 = 0.256; p = 0.725 |
| Oral Hormonal Contraceptive (%) | 40.51 | 19.35 | 18.18 | X2 = 10.534; p = 0.005 a,b | 44.30 | 23.1 | 21.2 | X2 = 9.888; p = 0.007 a,b | 42.5 | 19.2 | 20.0 | X2 = 10.773; p = 0.004 a,b |
| Antidiabetic (%) | 13.3 | 11.5 | 12.8 | X2 = 0.129; p = 0.972 | 5.2 | 4.3 | 3.1 | X2 = 0.382; p = 0.919 | 4.9 | 3.0 | 3.2 | X2 = 0.492; p = 0.761 |
| Antihypertensive (%) | 6.2 | 3.8 | 3.8 | X2 = 0.786; p = 0.722 | 10.3 | 11.6 | 10.9 | X2 = 0.966; p = 0.964 | 9.7 | 9.0 | 9.5 | X2 = 0.028; p = 1.000 |
Appendix B
| Cortisol (Mean ± Standard Error) | Statistics | Aldosterone (Mean ± Standard Error) | Statistics | |||||
|---|---|---|---|---|---|---|---|---|
| HC (n = 97) | MDD (n = 69) | BD (n = 66) | HC (n = 103) | MDD (n = 67) | BD (n = 65) | |||
| Without ELS | 345.94 (1.10) | 337.29 (1.12) | 211.35 (1.14) | G: F = 1.930; p = 0.149; ELS: F = 0.255; p = 0.614; BD (without ELS < with ELS); * GxELS: F = 3.213; p = 0.043 Without ELS (BD < MDD; BD < HC) * | 319.15 (1.11) | 409.26 (1.33) | 183.65 (1.47) | G: F = 3.618; p = 0.03 BD < MDD *; ELS: F: 0.524; p = 0.471; GxELS: F = 0.214; p = 0.807 |
| With ELS | 283.79 (1.31) | 320.63 (1.12) | 325.09 (1.12) | 296.48 (25.12) | 299.23 (1.22) | 175.39 (1.19) | ||
Appendix C
References
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; (DSM-5); American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- World Health Organization (WHO). Depression and Other Common Mental Disorders: Global Health Estimates; Licence: CC BY-NC-SA3.0 IGO; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Grande, I.; Berk, M.; Birmaher, B.; Vieta, E. Bipolar Disorder. Lancet 2016, 387, 1561–1572. [Google Scholar] [CrossRef]
- Young, A.H.; MacPherson, H. Detection of bipolar disorder. Br. J. Psychiatry 2011, 199, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Mari, J.J.; Pitta, J.C.N. Psiquiatria por Meio de Casos Clínicos; Editora Manole: São Paulo, Brazil, 2010. [Google Scholar]
- Juruena, M.F.; Werne Baes, C.V.; Menezes, I.C.; Graeff, F.G. Early-life stress in depressive patients: Role of glucocorticoid and mineralocorticoid receptors and of hypothalamic-pituitary- adrenal axis activity. Curr. Pharm. Des. 2015, 21, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Murck, H.; Schüssler, P.; Steiger, A. Renin-angiotensin-aldosterone system: The forgotten stress hormone system: Relationship to depression and sleep. Pharmacopsychiatry 2012, 45, 83–95. [Google Scholar] [CrossRef]
- De Kloet, E.R.; Joëls, M. Brain Mineralocorticoid Receptor Function in Control of Salt Balance and Stress-Adaptation. Physiol. Behav. 2017, 178, 13–20. [Google Scholar] [CrossRef]
- Joëls, M.; Karst, H.; Derijk, R.; De Kloet, E.R. The coming out of the brain mineralocorticoid receptor. Trends Neurosci. 2008, 31, 1–7. [Google Scholar] [CrossRef]
- DeRijk, R.H.; Wüst, S.; Meijer, O.C.; Zennaro, M.C.; Federenko, I.S.; Hellhammer, D.H.; Giacchetti, G.; Vreugdenhil, E.; Zitman, F.G.; de Kloet, E.R. A Common Polymorphism in the Mineralocorticoid Receptor Modulates Stress Responsiveness. J. Clin. Endocrinol. Metab. 2006, 91, 5083–5089. [Google Scholar] [CrossRef]
- van Leeuwen, N.; Caprio, M.; Blaya, C.; Fumeron, F.; Sartorato, P.; Ronconi, V.; Giacchetti, G.; Mantero, F.; Fernandes-Rosa, F.L.; Simian, C.; et al. The Functional c.-2G>C Variant of the Mineralocorticoid Receptor Modulates Blood Pressure, Renin, and Aldosterone Levels. Hypertension 2010, 56, 995–1002. [Google Scholar] [CrossRef]
- Carr, C.P.; Martins, C.M.S.; Stingel, A.M.; Lemgruber, V.B.; Juruena, M.F. The Role of Early Life Stress in Adult Psychiatric Disorders: A Systematic Review According to Childhood Trauma Subtypes. J. Nerv. Ment. Dis. 2014, 201, 1007–1020. [Google Scholar] [CrossRef]
- Heim, C.; Newport, D.J.; Mietzko, T.; Miller, A.H.; Nemeroff, C.B. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinoly 2008, 33, 693–710. [Google Scholar] [CrossRef]
- Andrade, L.H.; Wang, Y.P.; Andreoni, S.; Silveira, C.M.; Alexandrino-Silva, C.; Siu, E.R. Mental Disorders in Megacities: Findings from the São Paulo Megacity Mental Health Survey, Brazil. PLoS ONE 2012, 7, e31879. [Google Scholar] [CrossRef] [PubMed]
- Amorim, P. Mini International Neuropsychiatric Interview (MINI): Validação de entrevista breve para diagnóstico de transtornos mentais. Braz. J. Psychiatry 2000, 22, 106–115. [Google Scholar] [CrossRef]
- Henrique-Araújo, R.; Osório, F.L.; Gonçalves, R.M.; Monteiro, I.S.; Williams, J.B.; Kalali, A.; Crippa, A.J.; Oliveira, I.R. Transcultural Adaptation of GRID Hamilton Rating Scale for Depression (GRID-HAMD) to Brazilian Portuguese and Evaluation of the Impact of Training upon Inter-Rater Reliability. Innov. Clin. Neurosci. 2014, 11, 10–18. [Google Scholar] [PubMed]
- Grassi-Oliveira, R.; Stein, L.M.; Pezzi, J.C. Tradução e validação de conteúdo da versão em português do Childhood Trauma Questionnaire. Rev. Saúde. Pública 2006, 40, 249–255. [Google Scholar] [CrossRef]
- Bernstein, D.P.; Stein, J.A.; Newcom, M.D.; Walker, E.; Pogge, D.; Ahluvalia, T.; Stokes, J.; Handelsman, L.; Medrano, M.; Desmond, Z.D. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse. Negl. 2003, 27, 169–190. [Google Scholar] [CrossRef]
- World Medical Association (WMA). World Medical Association Declaration of Helsinki ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Castro, M.; Elias, P.C.; Quidute, A.R.; Halah, F.P.; Moreira, A.C. Out-patient screening for Cushing’s syndrome: The sensitivity of the combination of circadian rhythm and overnight dexamethasone suppression salivary cortisol tests. J. Clin. Endocrinol. Metab. 1999, 84, 878–882. [Google Scholar]
- LeMoult, J.; Humphreys, K.L.; Tracy, A.; Hoffmeister, J.A.; Ip, E.; Gotlib, I.H. Meta-Analysis: Exposure to Early Life Stress and Risk for Depression in Childhood and Adolescence. J. Am. Acad. Child Adolesc. Psychiatry 2020, 59, 842–855. [Google Scholar] [CrossRef]
- Menezes, I.C.; von Werne, B.C.; Lacchini, R.; Juruena, M.F. Genetic biomarkers for differential diagnosis of major depressive disorder and bipolar disorder: A systematic and critical review. Behav. Brain Res. 2019, 357–358, 29–38. [Google Scholar] [CrossRef]
- Murck, H.; Büttner, M.; Kircher, T.; Konrad, C. Genetic, molecular and clinical determinants for the involvement of aldosterone and its receptors in major depression. Nephron. Physiol. 2014, 128, 17–25. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Gama, C.S.; Ceresér, K.M.; Yatham, L.N.; Fries, G.R.; Colpo, G.; Lucena, D.; Kunz, M.; Gomes, F.A.; Kapczinski, F. Brain-derived neurotrophic factor as a state-marker of mood episodes in bipolar disorders: A systematic review and meta-regression analysis. J. Psychiatr. Res. 2011, 45, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Lee, C.T.; Lo, Y.T.; Huang, T.L. Brain-derived neurotrophic factor protein and mRNA levels in patients with bipolar mania—A preliminary study. Biomed. J. 2016, 39, 272–276. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Valiengo, L.L.; Soeiro-De-Souza, M.G.; Marques, A.; Moreno, D.H.; Juruena, M.F.; Andreazza, A.C.; Gattaz, W.F.; Machado-Vieira, R. Plasma cortisol in first episode drug-naïve mania: Differential levels in euphoric versus irritable mood. J. Affect Disord. 2012, 38, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Boisoneau, D.; Mason, J.W.; Giller, E.L. Glucocorticoid receptor number and cortisol excretion in mood, anxiety, and psychotic disorders. Biol. Psychiatry 1993, 34, 18–25. [Google Scholar] [CrossRef]
- Jiang, H.-K.; Wang, J.-Y.; Lin, J.-C. The central mechanism of hypothalamic–pituitary– adrenocortical system hyperfunction in depressed patients. Psychiatry Clin. Neurosci. 2000, 54, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Juruena, M.F.; Cleare, A.J.; Papadopoulos, A.S.; Poon, L.; Lightman, S.; Pariante, C.M. Different responses to dexamethasone and prednisolone in the same depressed patients. Psychopharmacology 2006, 189, 225–235. [Google Scholar] [CrossRef]
- Feng, G.; Kang, C.; Yuan, J.; Zhang, Y.; Wei, Y.; Xu, L.; Zhou, F.; Fan, X.; Yang, J. Neuroendocrine abnormalities areassociated with untreated firstepisode patients with major depressive disorder and bipolar disorder. Psychoneuroendocrinology 2019, 107, 119–123. [Google Scholar] [CrossRef]
- Havermans, R.; Nicolson, N.A.; Berkhof, J.; deVries, M.W. Patterns of salivary cortisol secretion and responses to daily events in patients with remitted bipolar disorder. Psychoneuroendocrinology 2011, 36, 258–265. [Google Scholar] [CrossRef]
- Huang, M.C.; Chuang, S.C.; Tseng, M.M.; Chien, Y.L.; Liao, S.C.; Chen, H.C.; Kuo, P.H. Cortisol awakening response in patients with bipolar disorder during acute episodes and partial remission: A pilot study. Psychiatry Res. 2017, 258, 594–597. [Google Scholar] [CrossRef]
- Mazer, A.K.; Cleare, A.J.; Young, A.H.; Juruena, M.F. Bipolar affective disorder and borderline personality disorder: Differentiation based on the history of early life stress and psychoneuroendocrine measures. Behav. Brain Res. 2019, 357–358, 48–56. [Google Scholar] [CrossRef]
- Aas, M.; Henry, C.; Andreassen, O.A.; Bellivier, F.; Melle, I.; Etain, B. The role of childhood trauma in bipolar disorders. Int. J. Bipolar. Disord. 2016, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Kumsta, R.; Entringer, S.; Koper, J.W.; van Rossum, E.F.; Hellhammer, D.H.; Wüst, S. Sex specific associations between common glucocorticoid receptor gene variants and hypothalamus-pituitary- adrenal axis responses to psychosocial stress. Biol. Psychiatry 2007, 62, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Wüst, S.; Van Rossum, E.F.; Federenko, I.S.; Koper, J.W.; Kumsta, R.; Hellhammer, D.H. Common polymorphisms in the glucocorticoid receptor gene are associated with adrenocortical responses to psychosocial stress. J. Clin. Endocrinol Metab. 2004, 89, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Velders, F.P.; Dieleman, G.; Cents, R.A.; Bakermans-Kranenburg, M.J.; Jaddoe, V.W.; Hofman, A.; Van Ijzendoorn, M.H.; Verhulst, F.C.; Tiemeier, H. Variation in the glucocorticoid receptor gene at rs41423247 moderates the effect of prenatal maternal psychological symptoms on child cortisol reactivity and behavior. Neuropsychopharmacology 2012, 37, 2541–2549. [Google Scholar] [CrossRef]
- Bet, P.M.; Penninx, B.W.J.H.; Bochdanovits, Z.; Uitterlinden, A.G.; Beekman, A.T.F.; Van Schoor, N.M.; Deeg, D.J.H.; Hoogendijk, W.J.G. Glucocorticoid receptor gene polymorphisms and childhood adversity are associated with depression: New evidence for a gene-environment interaction. Am. J. Med. Genet B Neuropsychiatr. Genet 2009, 150B, 660–669. [Google Scholar] [CrossRef]
- Gałecka, E.; Szemraj, J.; Bienkiewicz, M.; Majsterek, I.; Przybyłowska-Sygut, K.; Gałecki, P.; Lewiński, A. Single nucleotide polymorphisms of NR3C1 gene and recurrent depressive disorder in population of Poland. Mol. Biol. Rep. 2013, 40, 1693–1699. [Google Scholar] [CrossRef]
- van Rossum, E.F.C.; Binder, E.B.; Majer, M.; Koper, J.W.; Ising, M.; Modell, S.; Salyakina, D.; Lamberts, S.W.; Holsboer, F. Polymorphisms of the Glucocorticoid Receptor Gene and Major Depression. Biol. Psychiatry 2006, 59, 681–688. [Google Scholar] [CrossRef]
- Emanuele, E.; Geroldi, D.; Minoretti, P.; Coen, E.; Politi, P. Increased Plasma Aldosterone in Patients with Clinical Depression. Arch. Med. Res. 2005, 36, 544–548. [Google Scholar] [CrossRef]
- Hullin, R.P.; Lee, M.R.; Levell, M.J. Aldosterone and sweat potassium in manic-depression. Lancet 1977, 2, 617–618. [Google Scholar] [CrossRef]
- Murck, H.; Braunisch, M.C.; Konrad, C.; Jezova, D.; Kircher, T. Markers of mineralocorticoid receptor function: Changes over time and relationship to response in patients with major depression. Int. Clin. Psychopharmacol. 2019, 34, 18–26. [Google Scholar] [CrossRef]
- Büttner, M.; Jezova, D.; Greene, B.; Konrad, C.; Kircher, T.; Murck, H. Target-based biomarker selection e Mineralocorticoid receptor-related biomarkers and treatment outcome in major depression. J. Psychiatr. Res. 2015, 66–67, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Holsboer, F.; Dörr, H.G.; Sippell, W.G. Blunted aldosterone response to dexamethasone in female patients with endogenous depression. Psychoneuroendocrinology 1982, 7, 155–162. [Google Scholar] [CrossRef]
- Häfner, S.; Baumert, J.; Emeny, R.T.; Lacruz, M.E.; Bidlingmaier, M.; Reincke, M.; Ladwig, K.H. Hypertension and depressed symptomatology: A cluster related to the activation of the renin–angiotensin–aldosterone system (RAAS). Findings from population based KORA F4 study. Psychoneuroendocrinology 2013, 38, 2065–2074. [Google Scholar] [CrossRef] [PubMed]
- Kuningas, M.; de Rijk, R.H.; Westendorp, R.G.; Jolles, J.; Slagboom, P.E.; van Heemst, D. Mental performance in old age dependent on cortisol and genetic variance in the mineralocorticoid and glucocorticoid receptors. Neuropsychopharmacology 2007, 32, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- ter Heegde, F.; De Rijk, R.H.; Vinkers, C.H. The brain mineralocorticoid receptor and stress resilience. Psychoneuroendocrinology 2015, 52, 92–110. [Google Scholar] [CrossRef]
- Taylor, M.K.; Beckerley, S.E.; Henniger, N.E.; Hernández, L.M.; Larson, G.E.; Granger, D.A. A genetic risk factor for major depression and suicidal ideation is mitigated by physical activity. Psychiatry Res. 2017, 249, 304–306. [Google Scholar] [CrossRef]
- Martins-Monteverde, C.M.S.; Baes, C.v.W.; Reisdorfer, E.; Padovan, T.; Tofoli, S.M.C.; Juruena, M.F. Relationship between Depression and Subtypes of Early Life Stress in Adult Psychiatric Patients. Front. Psychiatry 2019, 10, 19. [Google Scholar] [CrossRef]

| Genetic Sample | Statistics | Cortisol Sample | Statistics | Aldosterone Sample | Statistics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HC | MDD | BD | HC | MDD | BD | HC | MDD | BD | ||||
| AGE (mean ± sd) | 37.20 (15.27) | 41.17 (12.34) | 40.18 (12.59) | F = 2.098; p = 0.619 | 35.98 (14.46) | 40.90 (12.62) | 39.73 (12.67) | F = 3.083; p = 0.048 a,b | 35.47 (14.19) | 40.61 (12.33) | 40.0 (12.36) | F = 3.939; p = 0.021 a,b |
| SEX (F/M) (%) | 69.91/ 30.11 | 79.49/ 20.51 | 80.49/19.51 | X2 = 3.699; p = 0.157 | 70.1 | 79.7 | 80.6 | X2 = 3.264; p = 0.207 | 70.9 | 80.6 | 80.0 | X2 = 2.840; p = 0.258 |
| BMI (Kg/m2) | 26.52 (5.80) | 28.33 (6.42) | 27.92 (6.33) | F = 1.985; p = 0.142 | 26.37 | 27.90 | 27.98 | F = 1.521; p = 0.221 | 26.27 (5.83) | 27.55 (6.16) | 27.77 (6.50) | F = 1.307; p = 0.273 |
| GRID-HAM-D21 | 0.5 (0.907) | 17.78 (9.76) | 19.03 (9.88) | X2 = 257.839; p < 0.001 a,b | 0.51 (0.96) | 17.84 (9.92) | 19.05 (9.61) | X2 = 223.576; p < 0.001 a,b | 0.52 (0.94) | 18.46 (9.90) | 19.85 (9.36) | F = 87.694; p < 0.001 a,b |
| CTQ | 30.55 (8.64) | 45.32 (16.60) | 50.68 (24.12) | X2 = 31.147; p < 0.001 a,b | 30.31 (8.73) | 46.07 (16.85) | 50.58 (24.40) | X2 = 31.147; p < 0.001 a,b | 30.03 (8.40) | 46.01 (17.37) | 49.63 (24.08) | F = 32.89; p < 0.001 a.b |
| ELS (%) | 7.24 | 56.84 | 59.49 | X2 = 71.394; p < 0.001 a,b | 4.3 | 58.8 | 59.1 | X2 = 71.400; p < 0.001 a,b | 5.0 | 56.7 | 59.4 | X2 = 70.103; p < 0.001 a,b |
| MR MI180V AA/AG/GG | 79.65/ 18.58/ 1.77 | 79.79/ 19.23/ 1.28 | 85.37/ 14.63/ 0.0 | X2 = 2.215; p = 0.757 | 78.4/ 19.6/ 2.1 | 79.7/ 18.8/ 1.4 | 85.3/ 14.7/ 0.0 | X2 = 2.173; p = 0.765 | 78.6/ 19.4/ 1.9 | 77.6/ 20.9/ 1.5 | 86.2/ 13.8/ 0.0 | X2 = 2.605; p = 0.693 |
| MR-2 G/C CC/CG/GG | 28.32/ 45.13/ 26.55 | 21.79/ 48.72/ 29.49 | 20.73/ 50.0/ 29.27 | X2 = 1.840; p = 0.768 | 26.8/ 47.4/ 25.8 | 24.6/ 46.4/ 29.0 | 22.1/ 51.5/ 26.5 | X2 = 0.736; p = 0.948 | 28.2/ 45.6/ 26.2 | 20.9/ 49.3/ 29.9 | 21.5/ 50.8/ 27.7 | X2 = 1.595; p = 0.815 |
| GR N363S TT/TC/CC | 95.59/ 4.04/ 0.0 | 93.59/ 5.41/ 0.0 | 93.92/ 5.08/ 0.0 | X2 = 6.251; p = 0.131 | 98.0/ 1.0/ 1.0 | 92.8/ 7.2/ 0.0 | 92.6/ 7.4/ 0.0 | X2 = 6.324; p = 0.124 | 99.0/ 1.0/ 0.0 | 92.5/ 7.5 /0.0 | 93.8/ 6.2/ 0.0 | X2 = 4.932; p = 0.103 |
| GR R22/23K CC/CT/TT | 97.32/ 2.68/ 0.0 | 98.72/ 1.28/ 0.0 | 98.78/ 1.22/ 0.0 | X2 = 0.743; p = 0.737 | 99.0/ 1.0/ 0.0 | 98.6/ 1.4/ 0.0 | 98.5/ 1.5/ 0.0 | X2 = 0.083; p = 1.0 | 98.0/ 2.0/ 0.0 | 98.5/ 1.5/ 0.0 | 98.5/ 1.5/ 0.0 | X2 = 0.068; p = 1.0 |
| GR BclI GG/GC/CC | 45.79/ 41.59/ 10.62 | 37.18/ 55.13/ 7.69 | 51.22/ 37.81/ 10.97 | X2 = 5.460; p = 0.245 | 45.4/ 43.3/ 9.1 | 31.8/ 30.7/ 6.5 | 52.9/ 38.2/ 8.8 | X2 = 3.455; p = 0.492 | 46.6/ 41.7/ 11.7 | 37.3/ 56.7/ 6.0 | 53.8/ 38.5/ 7.7 | X2 = 6.496; p = 0.166 |
| HC | UNIPOLAR | BIPOLAR | |||||
|---|---|---|---|---|---|---|---|
| Without ELS | With ELS | Without ELS | With ELS | Without ELS | With ELS | ||
| (Mean ± Standard Error) | STATISTICS | ||||||
| MR MI180V AA | 348.34 (1.10) | 244.34 (1.46) | 318.42 (1.13) | 311.17 (1.13) | 207.97 (1.15) | 322.11 (1.13) | G: F = 2.295; p = 0.104; ELS: F = 0.389; p = 0.534; SNP: F = 0.975; p = 0.379; GxELS: F = 1.867; p = 0.158 BD (AA: without ELS < with ELS) *; GxSNP: F = 0.620; p = 0.603; ELSxSNP: F = 0.256; p = 0.614 Without ELS (AA: BD < HC) *; GxSNPxELS: F = 0.659; p = 0.519. |
| MR MI180V AG | 322.66 (1.20) | 465.59 (1.71) | 430.53 (1.32) | 337.29 (1.28) | 223.87 (1.36) | 342.77 (1.32) | |
| MR MI180V GG | -- | 244.91 (1.71) | -- | 663.74 (1.71) | -- | -- | |
| MR-2 G/C CC | 322.85 (1.14) | 138.68 (1.71) | 339.62 (1.20) | 333.43 (1.27) | 221.31 (1.37) | 322.85 (1.26) | G: F = 1.674; p = 0.191; ELS: F = 0.000; p = 0.995 BD (without ELS < with ELS) *; SNP: F = 2.181; p = 0.117; GxELS: F = 2.846; p = 0.061 (Without ELS: BD < MDD) *; GxSNP: F = 0.726; p = 0.576; ELSxSNP: F = 1.102; p = 0.335; GxSNPxELS: F = 0.427; p = 0.789 BD (CG: without ELS < with ELS). |
| MR-2 G/C CG | 379.31 (1.13) | 437.52 (1.46) | 343.56 (1.23) | 373.25 (1.16) | 215.28 (1.22) | 347.57 (1.16) | |
| MR-2 G/C GG | 309.03 (1.17) | 235.50 (1.71) | 333.43 (1.25) | 254.10 (1.20) | 204.17 (1.21) | 2.45 (1.26) | |
| GR N363S TT | 343.56 (1.10) | 286.42 (1.31) | 322.11 (1.13) | 326.89 (1.12) | 213.30 (1.14) | 324.34 (1.12) | G: F = 1.261; p = 0.286; ELS: F = 0.076; p = 0.783; SNP: F = 0.593; p = 0.554; GxELS: F = 2.743; p = 0.068; GxSNP: F = 0.264; p = 0.769; SNPXELS: F = 0.739; p = 0.391; GxSNPXELS: F = 0.935; p = 0.335 Without ELS (TT: BD < HC) * BD (TT: without ELS < with ELS) *. |
| GR N363S TC | 588.84 (1.71) | -- | 506.99 (1.45) | 237.14 (1.37) | 180.30 (1.73) | 286.42 (1.48) | |
| GR N363S CC | 238.23 (1.70) | -- | -- | -- | -- | -- | |
| GR R22/23 K CC | 349.14 (1.10) | 287.08 (1.31) | 344.35 (1.12) | 319.15 (1.12) | 195.88 (1.14) | 324.34 (1.12) | G: F = 0.821; p = 0.442; ELS: F = 0.424; 0.516; GxELS: F = 4.347; p = 0.015 * (BD < HC*; BD < MDD *) SNP: F = 0.403; p = 0.526; GxSNP: F = 3.179; p = 0.044 * BD (CC: without ELS < with ELS) * BD (without ELS: CC < CT) * Without ELS (CC: BD < MDD) Without ELS (BD < MDD) *. |
| GR R22/23 K CT | 234.42 (1.69) | -- | 250.61 (1.69) | -- | 722.770 (1.70) | -- | |
| GR R22/23 K TT | -- | -- | -- | -- | -- | -- | |
| GR BclI GG | 406.44 (1.12) | 422.67 (1.69) | 350.75 (1.19) | 328.85 (1.67) | 244.34 (1.18) | 311.89 (1.16) | G: F = 0.927; p = 0.398; ELS: F = 0.038; p = 0.845 without ELS (HC > BD) * without ELS (MDD > BD) * BD (without ELS< with ELS) *; SNP: F = 3.346; p = 0.038 * (CC > GC) * BD (CC > GC) *; GxELS: F = 1.530; p = 0.220; GxSNP: F = 0.432; p = 0.785 ELSxSNP: F = 0.051; p = 0.950; GxSNPxELS: F = 0.151; p = 0.860 |
| GR BclI GC | 301.30 (1.12) | 257.63 (1.36) | 307.61 (1.18) | 305.50 (1.16) | 175.79 (1.20) | 271.02 (1.208) | |
| GR BclI CC | 380.19 (1.25) | -- | 442.59 (1.445) | 358.92 (1.48) | -- | 477.53 (1.28) | |
| HC | UNIPOLAR | BIPOLAR | |||||
|---|---|---|---|---|---|---|---|
| Without ELS | With ELS | Without ELS | With ELS | Without ELS | With ELS | ||
| (Mean ± Standard Error) | STATISTICS | ||||||
| MR MI180V AA | 329.61 (1.14) | 402.72 (1.50) | 317.69 (1.13) | 286.42 (1.19) | 273.53 (1.20) | 203.24 (1.18) | G: F = 3.104; p = 0.048 * (BD < MDD) * ELS: F = 0.342; p = 0.560; SNP: F = 1.138; p = 0.323; GxELS: F = 0.151; p = 0.860; GxSNP: F = 0.596; p = 0.619 SNPxELS: F = 0.063; p = 0.803; GxSNPxELS: F = 1.144; p = 0.321 Without ELS (AG:BD<HC) *. |
| MR MI180V AG | 391.74 (1.26) | 181.97 (2.01) | 299.22 (1.38) | 300.61 (1.37) | 123.59 (1.50) | 162.93 (1.50) | |
| MR MI180V GG | -- | 222.33 (2.00) | -- | 291.74 (1.99) | -- | -- | |
| MR-2 G/C CC | 279.25 (1.18) | 313.32 (1.97) | 295.12 (1.27) | 375.84 (1.48) | 216.77 (1.49) | 272.27 (1.34) | G: F = 2.102; p = 0.126; ELS: F = 0.575; p = 0.450; SNP: F = 0.867; p = 0.422; GxELS: F = 0.099; p = 0.906; GxSNP: F = 1.297; p = 0.274 CG (BD < MDD)*/MDD (GG < CG) *; SNPXELS: F = 1.064; p = 0.348; GxSNPxELS: F = 0.333; p = 0.855 With ELS (CG:U > B) *. |
| MR-2 G/C CG | 367.28 (1.17) | 366.43 (1.50) | 445.66 (1.28) | 345.14 (1.22) | 208.93 (1.27) | 184.08 (1.22) | |
| MR-2 G/C GG | 412.10 (1.22) | 221.31 (1.98) | 221.31 (1.33) | 202.77 (1.25) | 298.54 (1.39) | 165.20 (1.35) | |
| GR N363S TT | 333.43 (1.13) | 307.61 (1.37) | 342.77 (1.17) | 293.76 (1.17) | 255.86 (1.19) | 195.43 (1.17) | G: F = 0.812; p = 0.446; ELS: F = 2.205; p = 0.140; SNP: F = 1.024; p = 0.313; GxELS: F = 0.178; p = 0.837; GxSNP: F = 0.174; p = 0.840; SNPxELS: F = 5.188; p = 0.024 * Without ELS (TC < TT) *; GxSNPxELS: F = 0.489; p = 0.485. |
| GR N363S TC | 198.15 (1.99) | -- | 153.10 (1.62) | 312.60 (1.49) | 86.70 (2.03) | 331.13 (1.66) | |
| GR N363S CC | -- | -- | -- | -- | -- | -- | |
| GR R22/23 K CC | 341.98 (1.13) | 303.39 (1.37) | 336.51 (1.16) | 293.09 (1.16) | 247.17 (1.19) | 197.70 (1.16) | G: F = 1.069; p = 0.346; ELS: F = 1.275; p = 0.260; SNP: F = 2.839; p = 0.094; GxELS: F = 0.045; p = 0.956; GxSNP: F = 1.267; p = 0.285 MDD (CT < CC) * MDD (without ELS: CT < CC) *. |
| GR R22/23 K CT | 308.32 (1.98) | -- | 69.66 (1.99) | -- | 173.38 (1.99) | -- | |
| GR R22/23 K TT | -- | -- | -- | -- | -- | -- | |
| GR BclI GG | 354.81 (1.17) | 516.42 (1.99) | 277.33 (1.28) | 314.05 (1.24) | 277.33 (1.24) | 165.20 (1.24) | G: F = 2.042; p = 0.134; ELS: F = 0.394; p = 0.531; SNP: F = 1.274; p = 0.283; GxELS: F = 0.323; p = 0.725; GxSNP: F = 0.282; p = 0.889; SNPXELS: F = 0.650; p = 0.523; GxSNPXELS: F = 1.237; p = 0.298. |
| GR BclI GC | 324.34 (1.17) | 285.76 (1.43) | 360.58 (1.22) | 255.86 (1.23) | 170.22 (1.34) | 205.59 (1.27) | |
| GR BclI CC | 375.84 (1.34) | -- | 225.94 (2.02) | 511.68 (1.68) | 272.90 (2.02) | 346.74 (1.49) | |
| Unipolar | Bipolar | ||||
|---|---|---|---|---|---|
| (Mean ± Standard Error) | (Mean ± Standard Error) | ||||
| Without ELS | With ELS | Without ELS | With ELS | ||
| MR MI180V AA | 7.97 (0.15) | 18.27 (0.13) | 11.53 (0.11) | 17.97 (0.12) | G: F = 0.273; p = 0.602; SNP: F = 2.673; p = 0.073 ELS: F = 6.855; p = 0.010 *; GxSNP: F = 0.212; p = 0.646; GxELS: F = 0.105; p = 0.747; SNPxELS: F = 1.307; p = 0.255; GxSNPxELS: F = 2.095; p = 0.150 (MDD without ELS: AA<AG) * |
| MR MI180V AG | 19.18 (0.29) | 18.27 (0.12) | 14.52 (0.38) | 24.82 (0.34) | |
| MR MI180V GG | -- | 8.04 (1.05) | -- | -- | |
| MR-2 G/C CC | 6.98 (0.26) | 9.59 (0.31) | 10.01 (0.31) | 14.15 (0.25) | G: F = 1.490; p = 0.224; SNP: F = 4.769; p = 0.010 * (CC < CG*; CC < GG*); ELS: F = 12.425; p = 0.001 *; GxSNP: F = 1.489; p = 0.229 MDD (CC < CG) *; GxELS: F = 0.004; p = 0.948; SNPxELS: F = 0.253; p = 0.777; GxSNPxELS: F = 2.118; p = 0.124 MDD(CG: without ELS < with ELS)* MDD (without ELS: CC < GG) * BD (CG without ELS < with ELS) *. |
| MR-2 G/C CG | 8.25 (0.21) | 19.70 (0.16) | 15.14 (0.22) | 19.61 (0.16) | |
| MR-2 G/C GG | 16.46 (0.10) | 19.80 (0.22) | 10.53 (0.24) | 23.04 (0.24) | |
| GR N363S TT | 10.17 (0.14) | 17.75 (0.12) | 11.79 (0.15) | 18.45 (0.12) | G: F = 1.627; p = 0.204; SNP: F = 0.034; p = 0.854; ELS: F = 8.031; p = 0.005 *; GxSNP: F = 0.897; p = 0.345; GxELS: F = 0.397; p = 0.530; SNPxELS: F = 0.834; p = 0.363; GxSNPxELS: F = 0.186; p = 0.667; |
| GR N363S TC | 5.67 (0.68) | 20.58 (0.53) | 14.03 (0.53) | 27.91 (0.68) | |
| GR N363S CC | -- | -- | -- | -- | |
| GR R22/23 K CC | 9.59 (0.14) | 17.92 (0.12) | 11.94 (0.14) | 13.89 (1.08) | G: F = 0.071; p = 0.790; SNP: F = 0.755; p = 0.386; ELS: F = 17.660; p < 0.001 *; GxSNP: F = 0.357; p = 0.551 GxELS: F = 0.413; p = 0.522; |
| GR R22/23 K CT | 21.86 (1.07) | -- | 18.81 (0.12) | -- | |
| GR R22/23 K TT | - | - | - | - | |
| GR BclI GG | 7.79 (0.22) | 15.83 (0.21) | 11.22 (0.20) | 17.32 (0.18) | G: F = 0.016; p = 0.900; SNP: F = 1.462; p = 0.235; ELS: F = 10.651; p = 0.001 *; GxSNP: F = 0.936; p = 0.395; GxELS: F = 0.020; p = 0.889; SNPxELS: F = 0.121; p = 0.886; GxSNPxELS: F = 0.130; p = 0.878. |
| GR BclI GC | 11.19 (0.20) | 18.54 (0.16) | 13.55 (0.23) | 20.98 (0.21) | |
| GR BclI CC | 14.59 (0.53) | 26.35 (0.53) | 8.42 (0.71) | 18.50 (0.32) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menezes, I.C.; von Werne Baes, C.; Fígaro-Drumond, F.V.; Dias Macedo, B.B.; Bueno, A.C.; Lacchini, R.; Feijó de Mello, M.; de Castro, M.; Juruena, M.F. Differential Diagnosis of Major Depressive Disorder and Bipolar Disorder: Genetic and Hormonal Assessment and the Influence of Early-Life Stress. Brain Sci. 2022, 12, 1476. https://doi.org/10.3390/brainsci12111476
Menezes IC, von Werne Baes C, Fígaro-Drumond FV, Dias Macedo BB, Bueno AC, Lacchini R, Feijó de Mello M, de Castro M, Juruena MF. Differential Diagnosis of Major Depressive Disorder and Bipolar Disorder: Genetic and Hormonal Assessment and the Influence of Early-Life Stress. Brain Sciences. 2022; 12(11):1476. https://doi.org/10.3390/brainsci12111476
Chicago/Turabian StyleMenezes, Itiana Castro, Cristiane von Werne Baes, Fernanda Viana Fígaro-Drumond, Brisa Burgos Dias Macedo, Ana Carolina Bueno, Riccardo Lacchini, Marcelo Feijó de Mello, Margaret de Castro, and Mario Francisco Juruena. 2022. "Differential Diagnosis of Major Depressive Disorder and Bipolar Disorder: Genetic and Hormonal Assessment and the Influence of Early-Life Stress" Brain Sciences 12, no. 11: 1476. https://doi.org/10.3390/brainsci12111476
APA StyleMenezes, I. C., von Werne Baes, C., Fígaro-Drumond, F. V., Dias Macedo, B. B., Bueno, A. C., Lacchini, R., Feijó de Mello, M., de Castro, M., & Juruena, M. F. (2022). Differential Diagnosis of Major Depressive Disorder and Bipolar Disorder: Genetic and Hormonal Assessment and the Influence of Early-Life Stress. Brain Sciences, 12(11), 1476. https://doi.org/10.3390/brainsci12111476







