Astrocytes in the Neuropathology of Bipolar Disorder: Review of Current Evidence
Abstract
1. Introduction
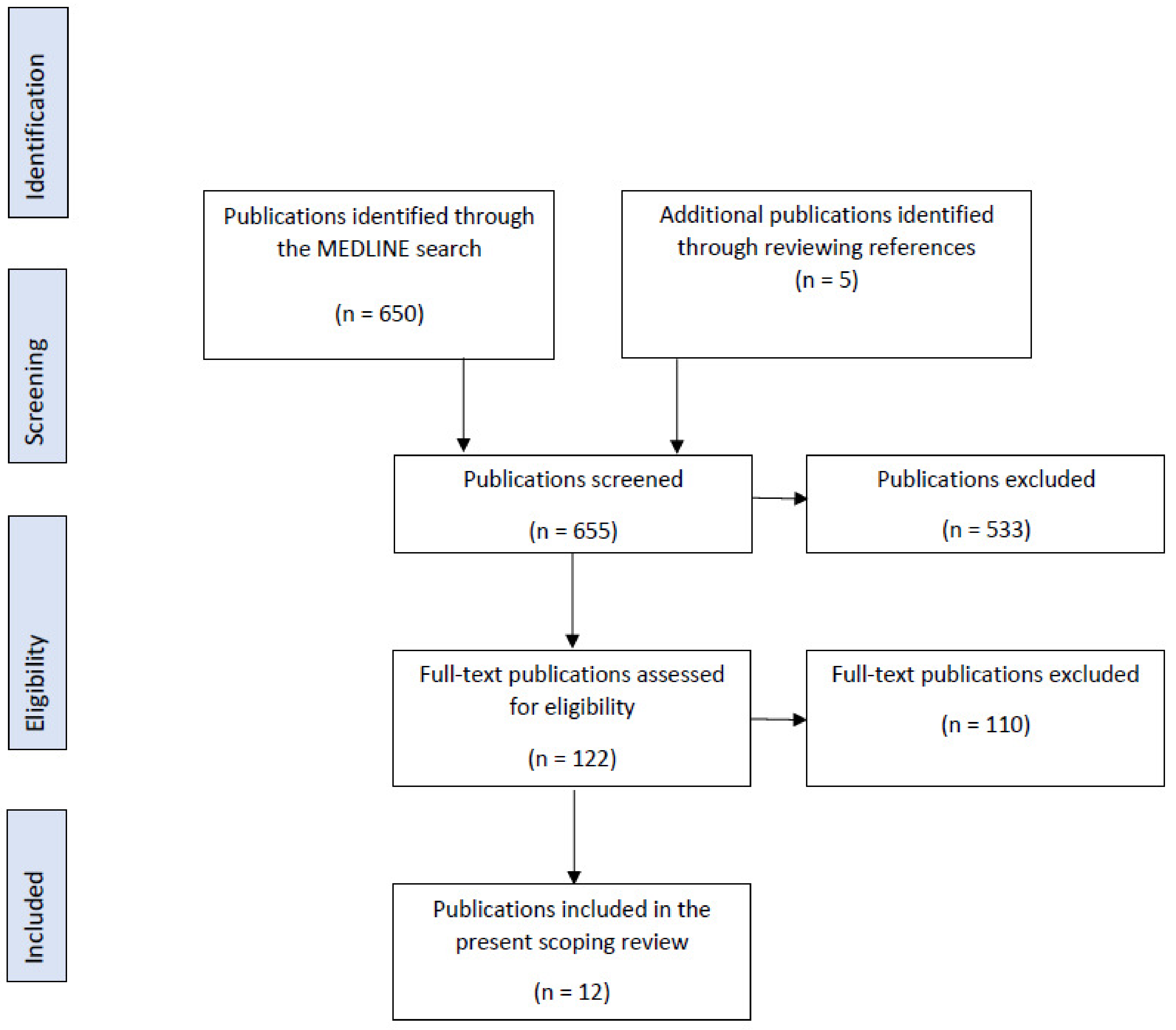
2. Materials and Methods
3. Results
3.1. In Vitro Studies
3.1.1. S100B
3.1.2. Glial Fibrillary Acidic Protein
3.2. Ex Vivo Studies
3.3. In Vivo and In Vitro Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Mental Disorders. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-disorders (accessed on 2 August 2022).
- National Institute of Mental Health (NIMH). Any Mood Disorder. Available online: https://www.nimh.nih.gov/health/statistics/any-mood-disorder (accessed on 27 May 2022).
- Grande, I.; Berk, M.; Birmaher, B.; Vieta, E. Bipolar disorder. Lancet 2016, 387, 1561–1572. [Google Scholar] [CrossRef]
- Sekhon, S.; Gupta, V. Mood Disorder. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hashimoto, K. Brain-derived neurotrophic factor as a biomarker for mood disorders: An historical overview and future directions. Psychiatry Clin. Neurosci. 2010, 64, 341–357. [Google Scholar] [CrossRef]
- Post, R.M. The impact of bipolar depression. J. Clin. Psychiatry 2005, 66 (Suppl. 5), 5–10. [Google Scholar]
- Kritzer, M. Ketamine for treatment of mood disorders and suicidality: A narrative review of recent progress. Ann. Clin. Psychiatry 2021, 34, 33–43. [Google Scholar] [CrossRef]
- Geddes, J.R.; Miklowitz, D.J. Treatment of bipolar disorder. Lancet 2013, 381, 1672–1682. [Google Scholar] [CrossRef]
- Gitlin, M. Treatment-resistant bipolar disorder. Mol. Psychiatry 2006, 11, 227–240. [Google Scholar] [CrossRef]
- Rihmer, Z. Suicide risk in mood disorders. Curr. Opin. Psychiatry 2007, 20, 17–22. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; McIntyre, R.S. Bipolar Disorder and Immune Dysfunction: Epidemiological Findings, Proposed Pathophysiology and Clinical Implications. Brain Sci. 2017, 7, 144. [Google Scholar] [CrossRef]
- Rantala, M.J.; Luoto, S.; Borráz-León, J.I.; Krams, I. Bipolar disorder: An evolutionary psychoneuroimmunological approach. Neurosci. Biobehav. Rev. 2021, 122, 28–37. [Google Scholar] [CrossRef]
- Jones, B.D.M.; Daskalakis, Z.J.; Carvalho, A.F.; Strawbridge, R.; Young, A.H.; Mulsant, B.H.; Husain, M.I. Inflammation as a treatment target in mood disorders: Review. BJPsych Open 2020, 6, e60. [Google Scholar] [CrossRef]
- Garland, E.F.; Hartnell, I.J.; Boche, D. Microglia and Astrocyte Function and Communication: What Do We Know in Humans? Front. Neurosci. 2022, 16, 824888. [Google Scholar] [CrossRef] [PubMed]
- Hasel, P.; Liddelow, S.A. Astrocytes. Curr. Biol. 2021, 31, R326–R327. [Google Scholar] [CrossRef]
- Zhang, X.; Alnafisah, R.S.; Hamoud, A.-R.A.; Shukla, R.; Wen, Z.; McCullumsmith, R.E.; O’Donovan, S.M. Role of Astrocytes in Major Neuropsychiatric Disorders. Neurochem. Res. 2021, 46, 2715–2730. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Zhang, G.; Li, S. Astrogliosis and Axonal Regeneration. In Neural Regeneration; So, K.-F., Xu, X.-M., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 181–196. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, B.; Verkhratsky, A. Targeting astrocytes in bipolar disorder. Expert Rev. Neurother. 2016, 16, 649–657. [Google Scholar] [CrossRef][Green Version]
- Pinto, J.V.; Passos, I.C.; Librenza-Garcia, D.; Marcon, G.; Schneider, M.A.; Conte, J.H.; Da Silva, J.P.A.; Lima, L.P.; Quincozes-Santos, A.; Kauer-Sant’anna, M.; et al. Neuron-glia Interaction as a Possible Pathophysiological Mechanism of Bipolar Disorder. Curr. Neuropharmacol. 2018, 16, 519–532. [Google Scholar] [CrossRef]
- Fakhoury, M. Microglia and Astrocytes in Alzheimer’s Disease: Implications for Therapy. Curr. Neuropharmacol. 2018, 16, 508–518. [Google Scholar] [CrossRef]
- Isgren, A.; Sellgren, C.; Ekman, C.-J.; Holmén-Larsson, J.; Blennow, K.; Zetterberg, H.; Jakobsson, J.; Landén, M. Markers of neuroinflammation and neuronal injury in bipolar disorder: Relation to prospective clinical outcomes. Brain. Behav. Immun. 2017, 65, 195–201. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Straus, S.E. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Andreazza, A.C.; Cassini, C.; Rosa, A.; Leite, M.C.; de Almeida, L.M.; Nardin, P.; Cunha, A.B.; Ceresér, K.M.; Santin, A.; Gottfried, C.; et al. Serum S100B and antioxidant enzymes in bipolar patients. J. Psychiatr. Res. 2007, 41, 523–529. [Google Scholar] [CrossRef]
- Ferensztajn-Rochowiak, E.; Kucharska-Mazur, J.; Tarnowski, M.; Samochowiec, J.; Ratajczak, M.Z.; Rybakowski, J.K. Stem cells, pluripotency and glial cell markers in peripheral blood of bipolar patients on long-term lithium treatment. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 28–33. [Google Scholar] [CrossRef]
- Machado-Vieira, R.; Lara, D.; Portela, L.; Gonçalves, C.; Soares, J.; Kapczinski, F.; Souza, D. Elevated serum S100B protein in drug-free bipolar patients during first manic episode: A pilot study. Eur. Neuropsychopharmacol. 2002, 12, 269–272. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Huang, T.-L. Decreased S100B serum levels after treatment in bipolar patients in a manic phase. Compr. Psychiatry 2017, 74, 27–34. [Google Scholar] [CrossRef]
- Hol, E.M.; Pekny, M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr. Opin. Cell Biol. 2015, 32, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Ferensztajn-Rochowiak, E.; Tarnowski, M.; Samochowiec, J.; Michalak, M.; Ratajczak, M.Z.; Rybakowski, J.K. Increased mRNA expression of peripheral glial cell markers in bipolar disorder: The effect of long-term lithium treatment. Eur. Neuropsychopharmacol. 2016, 26, 1516–1521. [Google Scholar] [CrossRef]
- Feresten, A.H.; Barakauskas, V.; Ypsilanti, A.; Barr, A.M.; Beasley, C.L. Increased expression of glial fibrillary acidic protein in prefrontal cortex in psychotic illness. Schizophr. Res. 2013, 150, 252–257. [Google Scholar] [CrossRef]
- Hercher, C.; Chopra, V.; Beasley, C.L. Evidence for morphological alterations in prefrontal white matter glia in schizophrenia and bipolar disorder. J. Psychiatry Neurosci. 2014, 39, 376–385. [Google Scholar] [CrossRef]
- Zhang, L.; Verwer, R.W.; Lucassen, P.J.; Huitinga, I.; Swaab, D.F. Sex difference in glia gene expression in the dorsolateral prefrontal cortex in bipolar disorder: Relation to psychotic features. J. Psychiatr. Res. 2020, 125, 66–74. [Google Scholar] [CrossRef]
- Weis, S.; Llenos, I.C.; Dulay, J.R.; Verma, N.; Sabunciyan, S.; Yolken, R.H. Changes in region- and cell type-specific expression patterns of neutral amino acid transporter 1 (ASCT-1) in the anterior cingulate cortex and hippocampus in schizophrenia, bipolar disorder and major depression. J. Neural Transm. 2006, 114, 261–271. [Google Scholar] [CrossRef]
- Webster, M.; O’Grady, J.; Kleinman, J.; Weickert, C. Glial fibrillary acidic protein mRNA levels in the cingulate cortex of individuals with depression, bipolar disorder and schizophrenia. Neuroscience 2005, 133, 453–461. [Google Scholar] [CrossRef]
- Webster, M.; Knable, M.; Johnston-Wilson, N.; Nagata, K.; Inagaki, M.; Yolken, R. Immunohistochemical Localization of Phosphorylated Glial Fibrillary Acidic Protein in the Prefrontal Cortex and Hippocampus from Patients with Schizophrenia, Bipolar Disorder, and Depression. Brain Behav. Immun. 2001, 15, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Altshuler, L.L.; Abulseoud, O.A.; Foland-Ross, L.; Bartzokis, G.; Chang, S.; Mintz, J.; Hellemann, G.; Vinters, H.V. Amygdala astrocyte reduction in subjects with major depressive disorder but not bipolar disorder: Amygdala astrocyte reduction in MDD. Bipolar Disord. 2010, 12, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-Y.; Sajatovic, M.; Hsu, J.-L.; Chung, K.-H.; Chen, P.-H.; Huang, Y.-J. Peripheral inflammatory markers associated with brain volume reduction in patients with bipolar I disorder. Acta Neuropsychiatr. 2021, 34, 191–200. [Google Scholar] [CrossRef]
- Simon, M.J.; Murchison, C.; Iliff, J.J. A transcriptome-based assessment of the astrocytic dystrophin-associated complex in the developing human brain. J. Neurosci. Res. 2017, 96, 180–193. [Google Scholar] [CrossRef]
- Toker, L.; Mancarci, B.O.; Tripathy, S.; Pavlidis, P. Transcriptomic Evidence for Alterations in Astrocytes and Parvalbumin Interneurons in Subjects With Bipolar Disorder and Schizophrenia. Biol. Psychiatry 2018, 84, 787–796. [Google Scholar] [CrossRef]
- Samanta, S.; Rajasingh, S.; Drosos, N.; Zhou, Z.; Dawn, B.; Rajasingh, J. Exosomes: New molecular targets of diseases. Acta Pharmacol. Sin. 2017, 39, 501–513. [Google Scholar] [CrossRef]
- Attili, D.; Schill, D.J.; DeLong, C.J.; Lim, K.C.; Jiang, G.; Campbell, K.F.; Walker, K.; Laszczyk, A.; McInnis, M.G.; O’Shea, K.S. Astrocyte-Derived Exosomes in an iPSC Model of Bipolar Disorder. Adv. Neurobiol. 2020, 25, 219–235. [Google Scholar] [CrossRef]
- Lee, Y.; EL Andaloussi, S.; Wood, M.J. Exosomes and microvesicles: Extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 2012, 21, R125–R134. [Google Scholar] [CrossRef]
- Rouillard, M.E.; Sutter, P.A.; Durham, O.R.; Willis, C.M.; Crocker, S.J. Astrocyte-Derived Extracellular Vesicles (ADEVs): Deciphering their Influences in Aging. Aging Dis. 2021, 12, 1462–1475. [Google Scholar] [CrossRef]
- Keshavarz, M. Glial cells as key elements in the pathophysiology and treatment of bipolar disorder. Acta Neuropsychiatr. 2016, 29, 140–152. [Google Scholar] [CrossRef]
- Gittins, R.A.; Harrison, P.J. A morphometric study of glia and neurons in the anterior cingulate cortex in mood disorder. J. Affect. Disord. 2011, 133, 328–332. [Google Scholar] [CrossRef]
- Johnston-Wilson, N.L.; Sims, C.D.; Hofmann, J.P.; Anderson, L.; Shore, A.D.; Torrey, E.F.; Yolken, R.H. Disease-specific alterations in frontal cortex brain proteins in schizophrenia, bipolar disorder, and major depressive disorder. Mol. Psychiatry 2000, 5, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Gos, T.; Schroeter, M.L.; Lessel, W.; Bernstein, H.-G.; Dobrowolny, H.; Schiltz, K.; Bogerts, B.; Steiner, J. S100B-immunopositive astrocytes and oligodendrocytes in the hippocampus are differentially afflicted in unipolar and bipolar depression: A postmortem study. J. Psychiatr. Res. 2013, 47, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Toro, C.T.; Hallak, J.E.; Dunham, J.S.; Deakin, J.F. Glial fibrillary acidic protein and glutamine synthetase in subregions of prefrontal cortex in schizophrenia and mood disorder. Neurosci. Lett. 2006, 404, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Tkachev, D.; Mimmack, M.L.; Ryan, M.M.; Wayland, M.; Freeman, T.; Jones, P.B.; Starkey, M.; Webster, M.J.; Yolken, R.H.; Bahn, S. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet 2003, 362, 798–805. [Google Scholar] [CrossRef]
- Ochoa, E.L.M. Lithium as a Neuroprotective Agent for Bipolar Disorder: An Overview. Cell. Mol. Neurobiol. 2021, 42, 85–97. [Google Scholar] [CrossRef]
- Rivera, A.D.; Butt, A.M. Astrocytes are direct cellular targets of lithium treatment: Novel roles for lysyl oxidase and peroxisome-proliferator activated receptor-γ as astroglial targets of lithium. Transl. Psychiatry 2019, 9, 14. [Google Scholar] [CrossRef]
- Nierenberg, A.A.; Ghaznavi, S.A.; Mathias, I.S.; Ellard, K.K.; Janos, J.A.; Sylvia, L.G. Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1 Alpha as a Novel Target for Bipolar Disorder and Other Neuropsychiatric Disorders. Biol. Psychiatry 2018, 83, 761–769. [Google Scholar] [CrossRef]
- Brusotti, G.; Montanari, R.; Capelli, D.; Cattaneo, G.; Laghezza, A.; Tortorella, P.; Pochetti, G. Betulinic acid is a PPARγ antagonist that improves glucose uptake, promotes osteogenesis and inhibits adipogenesis. Sci. Rep. 2017, 7, 5777. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kadluczka, J.; Kuter, K.Z. Beyond the GFAP-Astrocyte Protein Markers in the Brain. Biomolecules 2021, 11, 1361. [Google Scholar] [CrossRef]
- Moriguchi, S.; Wilson, A.A.; Miler, L.; Rusjan, P.M.; Vasdev, N.; Kish, S.J.; Meyer, J.H. Monoamine Oxidase B Total Distribution Volume in the Prefrontal Cortex of Major Depressive Disorder: An [11C]SL25.1188 Positron Emission Tomography Study. JAMA Psychiatry 2019, 76, 634–641. [Google Scholar] [CrossRef]
- Hostenbach, S.; Cambron, M.; D’Haeseleer, M.; Kooijman, R.; De Keyser, J. Astrocyte loss and astrogliosis in neuroinflammatory disorders. Neurosci. Lett. 2013, 565, 39–41. [Google Scholar] [CrossRef]
- Akkouh, I.A.; Skrede, S.; Holmgren, A.; Ersland, K.M.; Hansson, L.; Bahrami, S.; Andreassen, O.A.; Steen, V.M.; Djurovic, S.; Hughes, T. Exploring lithium’s transcriptional mechanisms of action in bipolar disorder: A multi-step study. Neuropsychopharmacology 2019, 45, 947–955. [Google Scholar] [CrossRef]
| Publication Name, Authors | Year of Publication | Type of Study | Purpose | Number of Participants (Total/Subjects/Healthy Controls) | Results |
|---|---|---|---|---|---|
| Serum S100B and antioxidant enzymes in bipolar patients, Andreazza et al. [24] | 2007 | In vitro | To evaluate brain injury using serum S100B content and oxidative stress in BD patients. | 84/52/32 | Significant increment of serum S100B in hypomanic (p = 0.004) and manic (p = 0.011) and no changes in euthymic patients (p = 0.263). |
| Stem cells, pluripotency and glial cell markers in peripheral blood of bipolar patients on long-term lithium treatment, Ferensztajn-Rochowiak et al. [25] | 2018 | In vitro | To investigate the effect of long-term lithium treatment on very-small embryonic-like stem cells (VSELs) and the mRNA expression of pluripotency and glial markers in peripheral blood in patients with bipolar disorder (BD). | 45/30/15 | BD lithium-negative patients exhibited significantly higher numbers of VSELs in comparison to HC. |
| Elevated serum S100B protein in drug-free bipolar patients during first manic episode: a pilot study, Machado-Vieira et al. [26] | 2002 | In vitro | To examine the possible effects of mania on S100B turnover in serum. | 40/20/20 | Statistically significant increased levels of S100B in bipolar mania (Wilcoxon signed-rank test, Z522.45, P50.01). |
| Decreased S100B serum levels after treatment in bipolar patients in a manic phase, Tsai et al. [27] | 2017 | In vitro | To investigate the serum levels of S100A10 and brain injury-related biomarkers, including S100B, NSE, and HSP70, in BD patients experiencing a manic phase compared to HC. In addition, to assess the relationship between these markers and Young Mania Rating Scale (YMRS) scores in BD patients during manic episodes and investigate the changes in these markers in BD patients after treatment. | 47/17/30 | Significantly decreased S100B levels only in bipolar manic patients after treatment (p = 0.002), but S100B levels were not significantly different from those in healthy controls (p > 0.05). |
| Publication Name, Authors | Year of Publication | Type of Study | Purpose | Number of Participants (Total/Subjects/Healthy Controls | Results |
|---|---|---|---|---|---|
| Increased expression of glial fibrillary acidic protein in prefrontal cortex in psychotic illness, Feresten et al. [30] | 2013 | Ex vivo | To investigate if levels of four astrocyte-specific proteins, i.e., glial fibrillary acidic protein (GFAP), aldehyde dehydrogenase\1 L1 (ALDH1L1), vimentin, and excitatory amino acid transporter 1 (EAAT1), are altered in SCZ and BPD. | 104/69/35 | High levels of GFAP in SCZ and BD when compared to controls and when comparing individuals with psychotic symptoms against those without. |
| Evidence for morphological alterations in prefrontal white matter glia in schizophrenia and bipolar disorder, Hercher et al. [31] | 2014 | Ex vivo | To assess oligodendrocyte, astrocyte, and microglial populations in post-mortem white matter from SCZ, BD, and HC samples. | 60/40/20 | Significant increase in oligodendrocyte density (p = 0.012) and CNPase protein levels (p = 0.038) in BD patient groups compared with control samples. |
| Sex difference in glia gene expression in the dorsolateral prefrontal cortex in bipolar disorder: Relation to psychotic features, Zhang et al. [32] | 2020 | Ex vivo | To investigate on transcriptional changes of markers of astrocytes, microglia, and oligodendrocytes in BD in relation to suicide, psychotic features, and sex in the DLPFC and ACC. | 64/30/34 | Significant sex difference of the glia-related gene expression in individuals with BD in the DLPFC. Males compared to females have a higher expression of most detected genes. |
| Changes in region- and cell type-specific expression patterns of neutral amino acid transporter 1 (ASCT-1) in the anterior cingulate cortex and hippocampus in schizophrenia, bipolar disorder and major depression, Weis et al. [33] | 2007 | Ex vivo | To characterize the expression pattern and cellular localization of the ASCT-1 protein in two brain regions (ACC and hippocampus) and to determine if this expression pattern is altered in SCZ, BD, and MDD using immunohistochemical techniques. | 60/45/15 | Significant loss of immunoreactivity on astrocytes, neurons, and interneurons in multiple regions for SCZ and BD patient groups. |
| Glial fibrillary acidic protein mRNA levels in the cingulate cortex of individuals with depression, bipolar disorder and schizophrenia, Webster et al. [34] | 2005 | Ex vivo | To evaluate levels of GFAP mRNA for potential reduction in astrocyte density and if it contributes to the decrease in glial density reported in the ACC in major mental illnesses. | 60/45/15 | Significant decrease in the levels of GFAP mRNA in the white matter underlying the cingulate cortex in subjects with BD and SCZ. |
| Immunohistochemical Localization of Phosphorylated GlialFibrillary Acidic Protein in the Prefrontal Cortex and Hippocampus from Patients with Schizophrenia, Bipolar Disorder, and Depression, Webster et al. [35] | 2001 | Ex vivo | To determine the immunohistochemical localization of phosphorylated GFAP (pGFAP) in the PFC and hippocampus and to investigate potential disease-related changes in distribution of pGFAP containing astrocytes. | 60/45/15 | pGFAP astrocytes found to be adjacent to blood vessels in the white matter of the gyrus and the polymorphic layer of the dentate gyrus. No significant differences between the patient and control groups. |
| Amygdala astrocyte reduction in subjects with major depressive disorder but not bipolar disorder, Altshuler et al. [36] | 2010 | Ex vivo | To evaluate the diagnostic tissue samples from subjects with BD, MDD, and SCZ. | 44/30/14 | No significant differences in neuronal densities were foundbetween patient and HC groups. |
| Publication Name, Authors | Year of Publication | Type of Study | Purpose | Number of Participants (Total/Subjects/Healthy Controls) | Results |
|---|---|---|---|---|---|
| Peripheral inflammatory markers associated with brain volume reduction in patients with bipolar I disorder, Tsai et al. [37] | 2021 | In vivo and in vitro | To investigate plasma levels of sTNF-R1, IL-1β, TGF-β1, MCP-1, YKL-40, and FKN to assess if peripheral inflammatory markers and illness severity may be associated with volume abnormalities in subregions of limbic, frontal, and temporal lobes in BD. | 31/16/15 | Significant increase of YKL-40 and sTNF-R1 levels associated with lower volumes of the left anterior cingulum, left frontal lobe, right superior temporal gyrus, and supramarginal gyrus. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, N.; Jones, B.D.M.; Husain, M.I. Astrocytes in the Neuropathology of Bipolar Disorder: Review of Current Evidence. Brain Sci. 2022, 12, 1513. https://doi.org/10.3390/brainsci12111513
Dai N, Jones BDM, Husain MI. Astrocytes in the Neuropathology of Bipolar Disorder: Review of Current Evidence. Brain Sciences. 2022; 12(11):1513. https://doi.org/10.3390/brainsci12111513
Chicago/Turabian StyleDai, Nasia, Brett D. M. Jones, and Muhammad Ishrat Husain. 2022. "Astrocytes in the Neuropathology of Bipolar Disorder: Review of Current Evidence" Brain Sciences 12, no. 11: 1513. https://doi.org/10.3390/brainsci12111513
APA StyleDai, N., Jones, B. D. M., & Husain, M. I. (2022). Astrocytes in the Neuropathology of Bipolar Disorder: Review of Current Evidence. Brain Sciences, 12(11), 1513. https://doi.org/10.3390/brainsci12111513





