Contingent Social Interaction Does Not Prevent Habituation towards Playback of Pro-Social 50-kHz Calls: Behavioral Responses and Brain Activation Patterns
Abstract
1. Introduction
2. Subjects and Methods
2.1. Animals and Housing
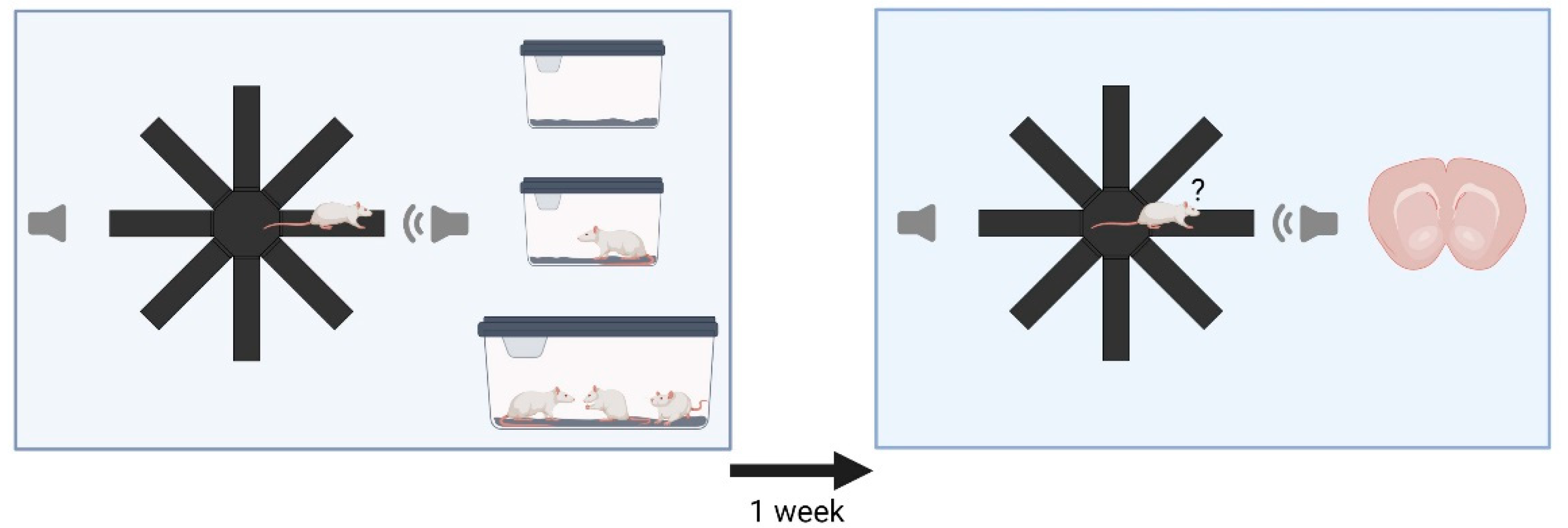
2.2. Experimental Design
2.3. Acoustic Stimuli and Experimental Setups
2.4. Radial Maze Playback Paradigm
2.5. Behavioral Analysis
2.6. Immunohistochemical Analysis
Double Immunofluorescence
2.7. Statistical Analysis
3. Results
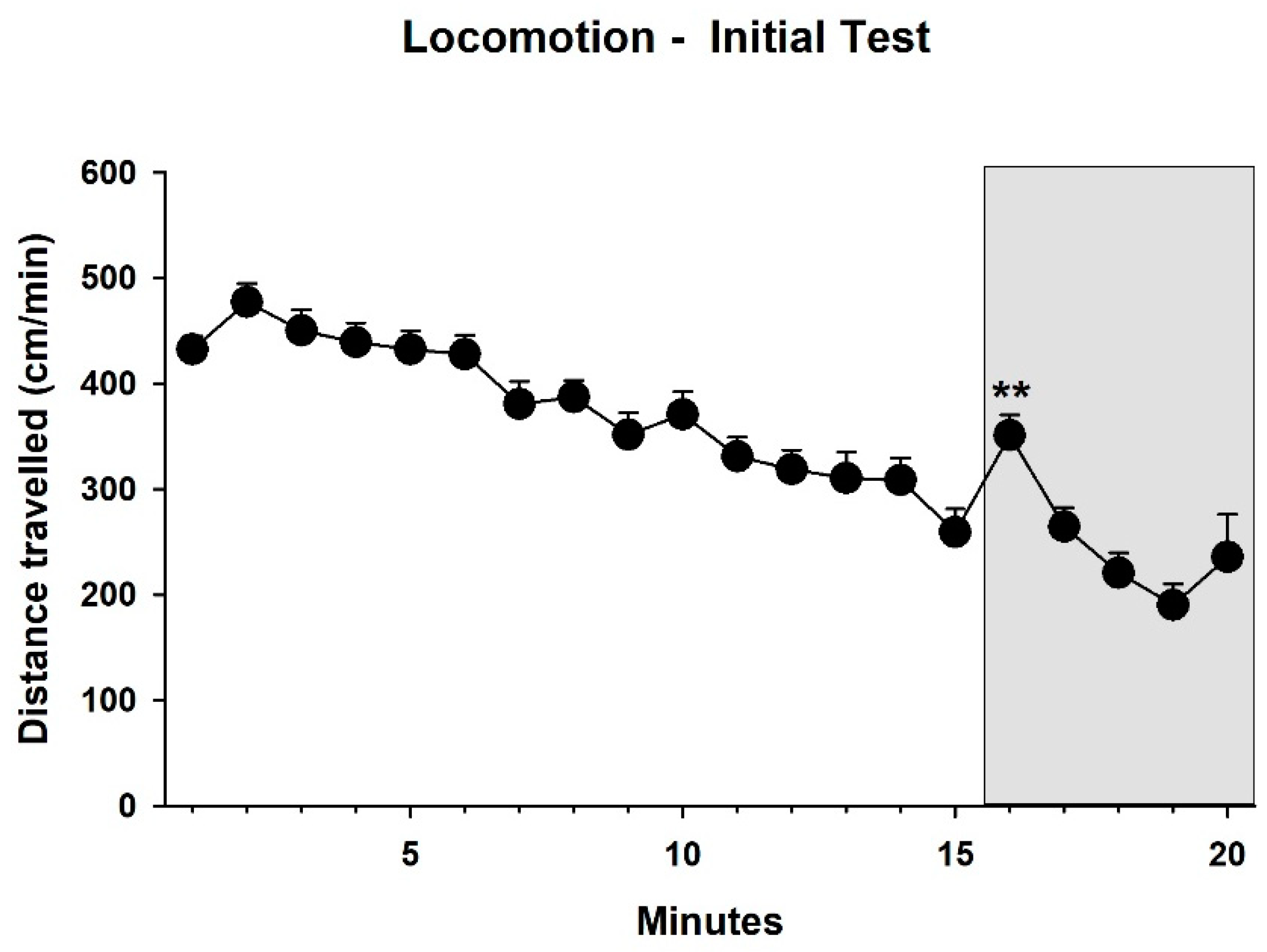
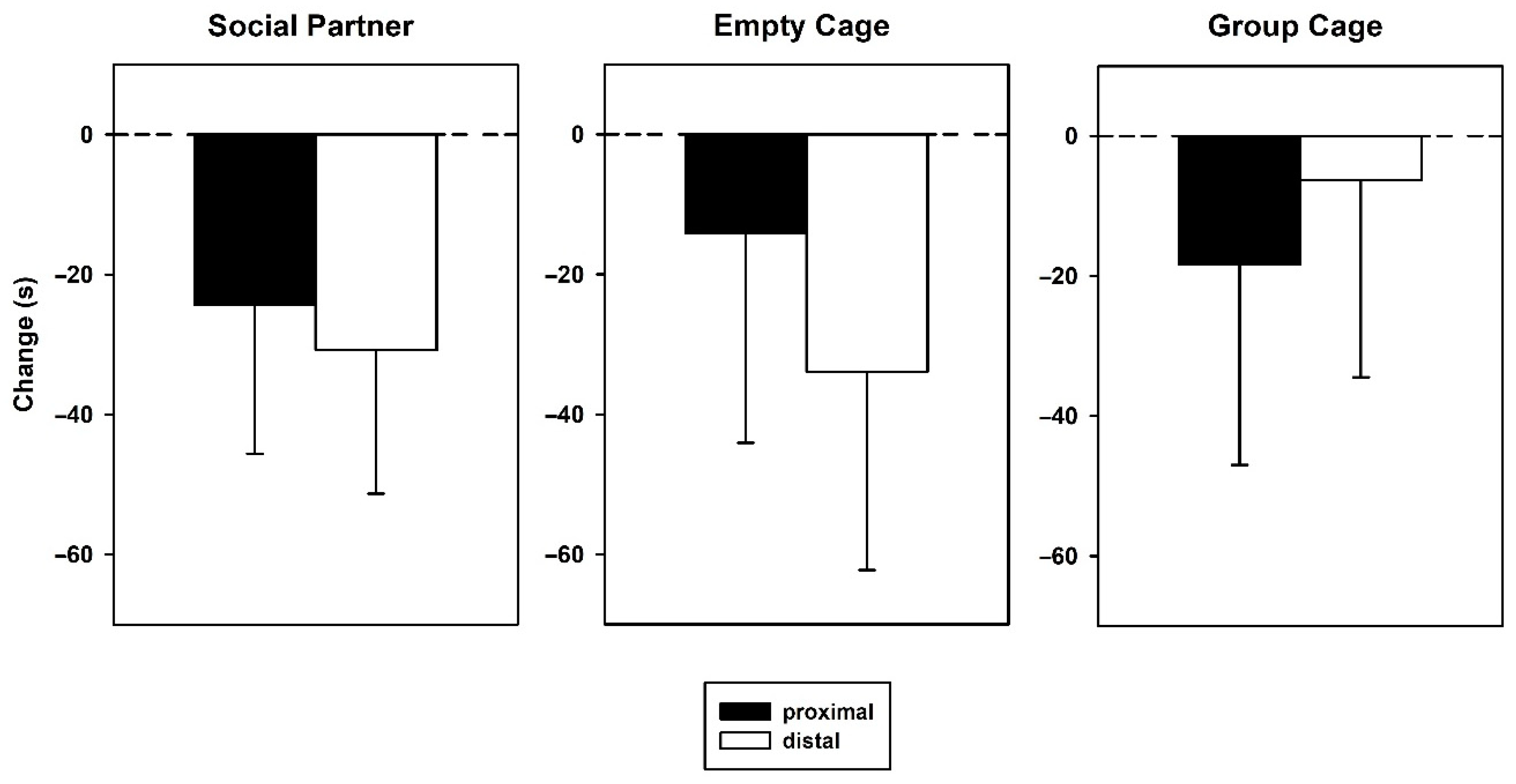
3.1. Behavior—Initial Test
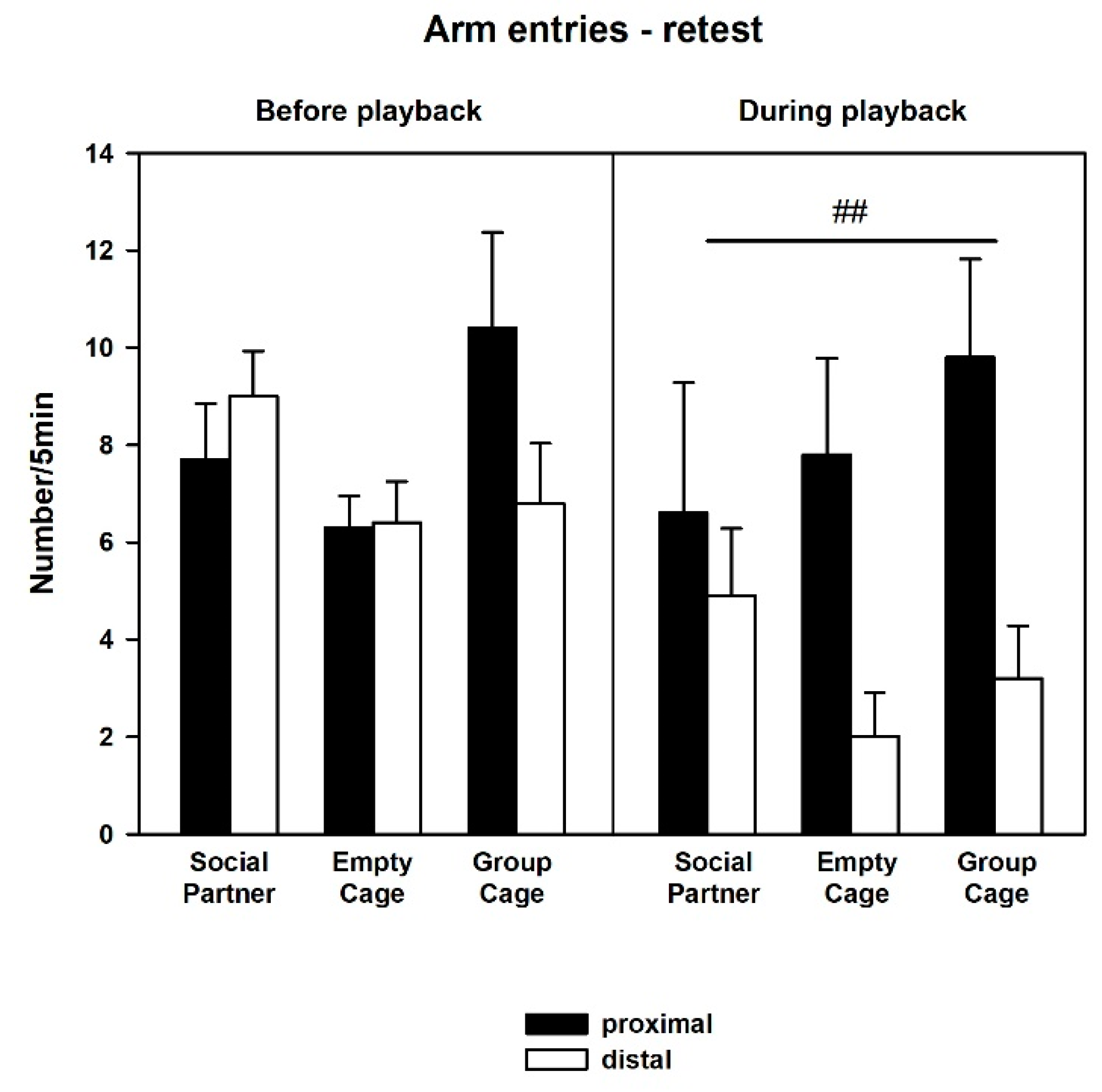
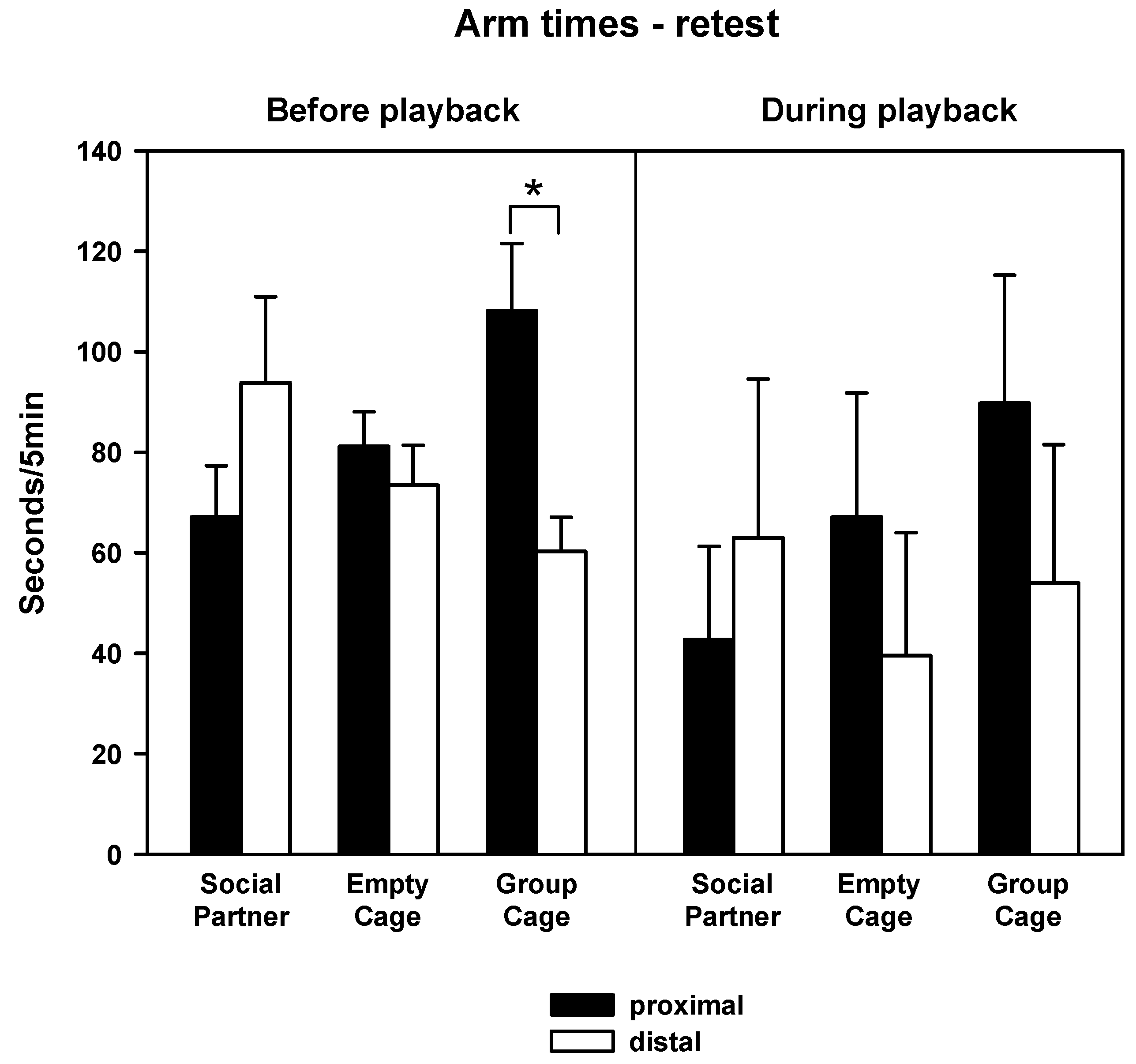
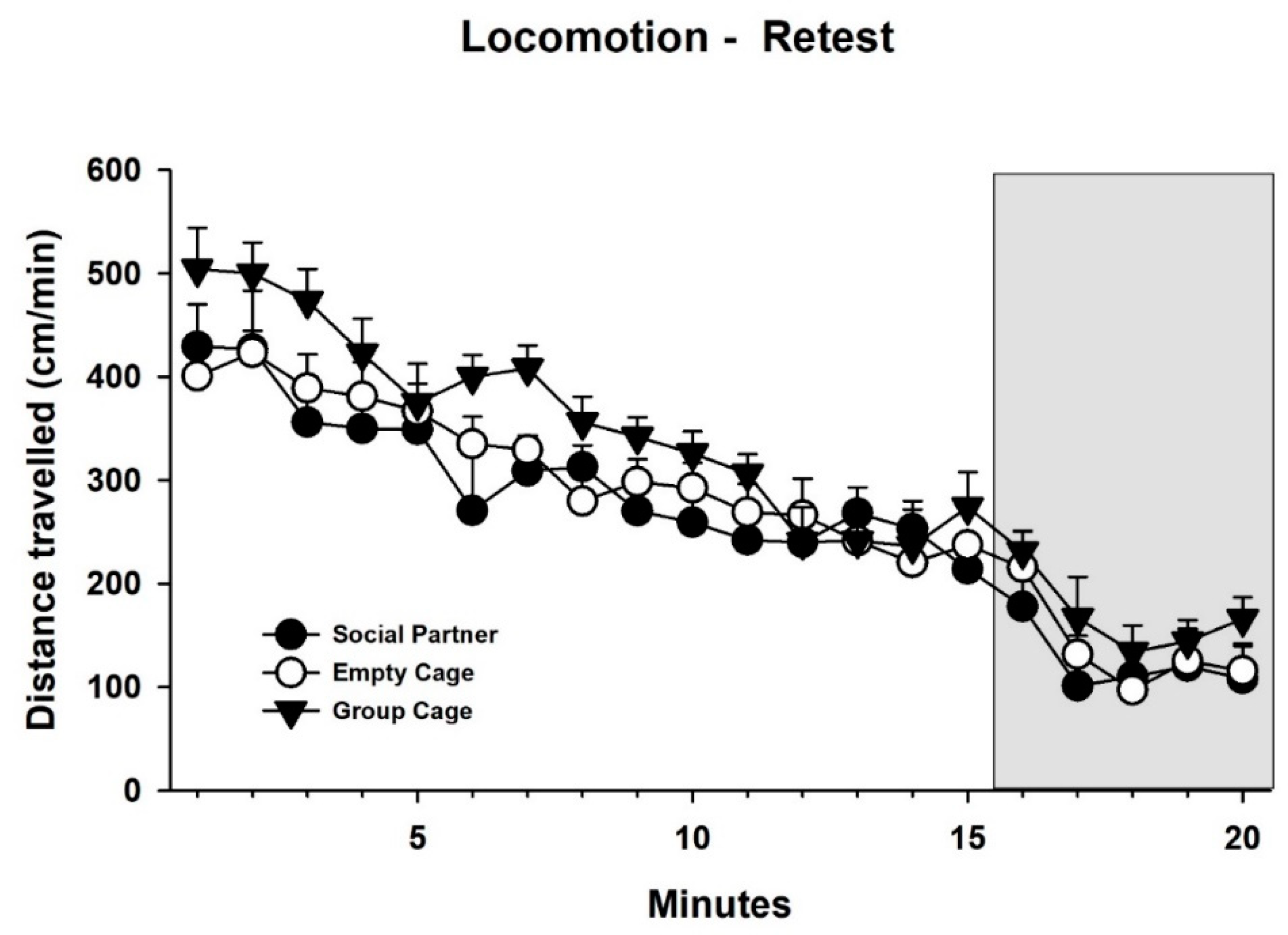
3.2. Behavior—Retest
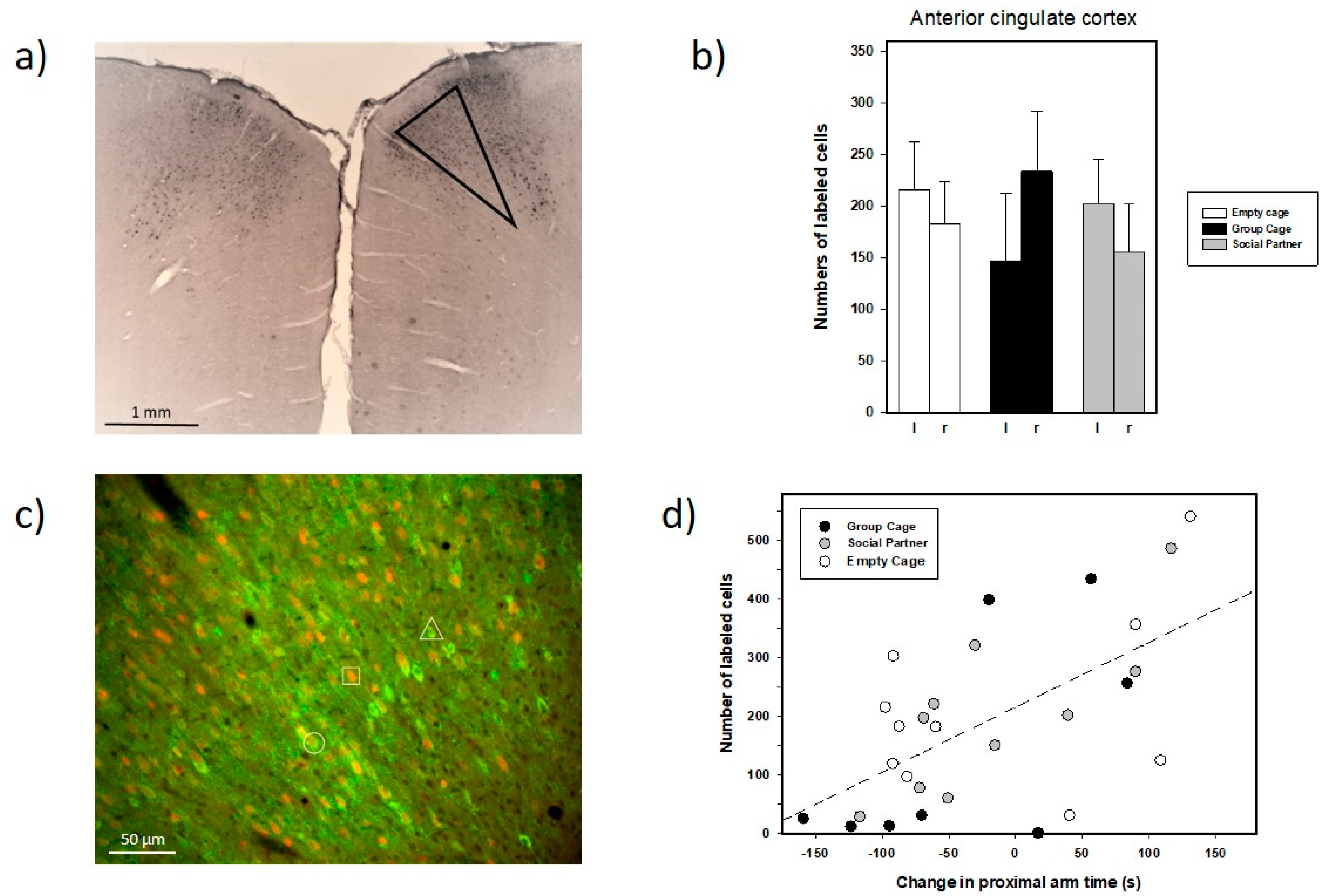
3.3. Immunohistochemistry
4. Discussion
4.1. Behavioral Findings
4.2. Anatomical Findings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brudzynski, S.M. Ethotransmission: Communication of emotional states through ultrasonic vocalization in rats. Curr. Opin. Neurobiol. 2013, 23, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Wöhr, M.; Schwarting, R.K.W. Affective communication in rodents: Ultrasonic vocalizations as a tool for research on emotion and motivation. Cell. Tiss. Res. 2013, 354, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, R.; Blanchard, D.; Agullana, R.; Weiss, S.M. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiol. Behav. 1991, 50, 967–972. [Google Scholar] [CrossRef]
- Fendt, M.; Brosch, M.; Wernecke, K.E.A.; Willadsen, M.; Wöhr, M. Predator odour but not TMT induces 22-kHz ultrasonic vocalizations in rats that lead to defensive behaviours in conspecifics upon replay. Sci. Rep. 2018, 8, 11041. [Google Scholar] [CrossRef]
- Knutson, B.; Burgdorf, J.; Panksepp, J. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J. Comp. Psychol. 1998, 112, 65–73. [Google Scholar] [CrossRef]
- Burgdorf, J.; Kroes, R.A.; Moskal, J.R.; Pfaus, J.G.; Brudzynski, S.M.; Panksepp, J.B. Ultrasonic Vocalizations of Rats (Rattus norvegicus) During Mating, Play, and Aggression: Behavioral Concomitants, Relationship to Reward, and Self-Administration of Playback. J. Comp. Psychol. 2008, 122, 357–367. [Google Scholar] [CrossRef]
- Panksepp, J. Affective consciousness: Core emotional feelings in animals and humans. Conscious. Cogn. 2005, 14, 30–80. [Google Scholar] [CrossRef]
- Siviy, S.M.; Panksepp, J. Sensory modulation of juvenile play in rats. Dev. Psychobiol. 1987, 20, 39–55. [Google Scholar] [CrossRef]
- Brudzynski, S.M.; Pniak, A. Social contacts and production of 50-kHz short ultrasonic calls in adult rats. J. Comp. Psychol. 2002, 116, 73–82. [Google Scholar] [CrossRef]
- Panksepp, J.; Burgdorf, J. “Laughing” rats and the evolutionary antecedents of human joy? Physiol. Behav. 2003, 79, 533–547. [Google Scholar] [CrossRef]
- Schwarting, R.K.; Jegan, N.; Wöhr, M. Situational factors, conditions and individual variables which can determine ultrasonic vocalizations in male adult Wistar rats. Behav. Brain Res. 2007, 182, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Wöhr, M.; Houx, B.; Schwarting, R.K.; Spruijt, B. Effects of experience and context on 50-kHz vocalizations in rats. Physiol. Behav. 2008, 93, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Łopuch, S.; Popik, P. Cooperative behavior of laboratory rats (Rattus norvegicus) in an instrumental task. J. Comp. Psychol. 2011, 125, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Kisko, T.M.; Himmler, B.T.; Himmler, S.M.; Euston, D.R.; Pellis, S.M. Are 50-kHz calls used as play signals in the playful interactions of rats? II. Evidence from the effects of devocalization. Behav. Process. 2015, 111, 25–33. [Google Scholar] [CrossRef]
- Heinla, I.; Chu, X.; Ågmo, A.; Snoeren, E. Rat ultrasonic vocalizations and novelty-induced social and non-social investigation behavior in a seminatural environment. Physiol. Behav. 2021, 237, 113450. [Google Scholar] [CrossRef]
- Wöhr, M.; Schwarting, R.K.W. Ultrasonic Communication in Rats: Can Playback of 50-kHz Calls Induce Approach Behavior? PLoS ONE 2007, 2, e1365. [Google Scholar] [CrossRef]
- Wöhr, M.; Schwarting, R.K.W. Ultrasonic communication in rats: Effects of morphine and naloxone on vocal and behavioral responses to playback of 50-kHz vocalizations. Pharmacol. Biochem. Behav. 2009, 94, 285–295. [Google Scholar] [CrossRef]
- Wöhr, M.; Schwarting, R.K.W. Testing social acoustic memory in rats: Effects of stimulus configuration and long-term memory on the induction of social approach behavior by appetitive 50-kHz ultrasonic vocalizations. Neurobiol. Learn. Mem. 2012, 98, 154–164. [Google Scholar] [CrossRef]
- Willadsen, M.; Seffer, D.; Schwarting, R.K.W.; Wöhr, M. Rodent ultrasonic communication: Male prosocial 50-kHz ultrasonic vocalizations elicit social approach behavior in female rats (Rattus norvegicus). J. Comp. Psychol. 2014, 128, 56–64. [Google Scholar] [CrossRef]
- Seffer, D.; Rippberger, H.; Schwarting, R.K.W.; Wöhr, M. Pro-social 50-kHz ultrasonic communication in rats: Post-weaning but not post-adolescent social isolation leads to social impairments—phenotypic rescue by re-socialization. Front. Behav. Neurosci. 2015, 9, 102. [Google Scholar] [CrossRef]
- Brenes, J.C.; Lackinger, M.; Höglinger, G.U.; Schratt, G.; Schwarting, R.K.W.; Wöhr, M. Differential effects of social and physical environmental enrichment on brain plasticity, cognition, and ultrasonic communication in rats. J. Comp. Neurol. 2016, 524, 1586–1607. [Google Scholar] [CrossRef] [PubMed]
- Wöhr, M.; Willadsen, M.; Kisko, T.M.; Schwarting, R.K.W.; Fendt, M. Sex-dependent effects of Cacna1c haploinsufficiency on behavioral inhibition evoked by conspecific alarm signals in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiat. 2020, 99, 109849. [Google Scholar] [CrossRef] [PubMed]
- Kisko, T.M.; Braun, M.D.; Michels, S.; Witt, S.H.; Rietschel, M.; Culmsee, C.; Schwarting, R.K.W.; Wöhr, M. Sex-dependent effects of Cacna1c haploinsufficiency on juvenile social play behavior and pro-social 50-kHz ultrasonic communication in rats. Genes. Brain. Behav. 2020, 19, e12552. [Google Scholar] [CrossRef]
- Berz, A.; de Souza, C.P.; Wöhr, M.; Schwarting, R.K. Limited generalizability, pharmacological modulation, and state-dependency of habituation towards pro-social 50-kHz calls in rats. iScience 2021, 24, 102426. [Google Scholar] [CrossRef] [PubMed]
- Wöhr, M.; Seffer, D.; Schwarting, R.K.W. Studying socio-affective communication in rats through playback of ultrasonic vocalizations. Curr. Protoc. Neurosci. 2016, 75, 8.35.1–8.35.17. [Google Scholar]
- Schwarting, R.K.W.; Kisko, T.; Wöhr, M. Playback of ultrasonic vocalizations to juvenile and adult rats: Behavioral and neuronal effects. In Handbook of Ultrasonic Vocalization; Brudzynski, S.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 357–369. [Google Scholar]
- Thor, D.H.; Holloway, W.R., Jr. Sex and social play in juvenile rats (Rattus novegicus). J. Comp. Psychol. 1984, 98, 276–284. [Google Scholar] [CrossRef]
- Kisko, T.M.; Braun, M.D.; Michels, S.; Witt, S.H.; Rietschel, M.; Culmsee, C.; Schwarting, R.K.W.; Wöhr, M. Cacna1c haploinsufficiency leads to pro-social 50-kHz ultrasonic communication deficits in rats. Dis. Model. Mech. 2018, 11, dmm034116. [Google Scholar] [CrossRef]
- Pultorak, J.D.; Kelm-Nelson, C.A.; Holt, L.R.; Blue, K.V.; Ciucci, M.R.; Johnson, A.M. Decreased approach behavior and nucleus accumbens immediate early gene expression in response to Parkinsonian ultrasonic vocalizations in rats. Soc. Neurosci. 2016, 11, 365–379. [Google Scholar] [CrossRef]
- Berg, E.L.; Copping, N.A.; Rivera, J.K.; Pride, M.C.; Careaga, M.; Bauman, M.D.; Berman, R.F.; Lein, P.J.; Harony-Nicolas, H.; Buxbaum, J.D.; et al. Developmental social communication deficits in the Shank3 rat model of phelan-mcdermid syndrome and autism spectrum disorder. Autism Res. 2018, 11, 587–601. [Google Scholar] [CrossRef]
- Berg, E.L.; Pride, M.C.; Petkova, S.P.; Lee, R.D.; Copping, N.A.; Shen, Y.; Adhikari, A.; Fenton, T.A.; Pedersen, L.R.; Noakes, L.S.; et al. Translational outcomes in a full gene deletion of ubiquitin protein ligase E3A rat model of Angelman syndrome. Transl. Psychiatry 2020, 10, 39. [Google Scholar] [CrossRef]
- Tonelli, L.C.; Wöhr, M.; Schwarting, R.K.W.; Melo-Thomas, L. Paradoxical kinesia induced by appetitive 50-kHz ultrasonic vocalizations in rats depends on glutamatergic mechanisms in the inferior colliculus. Neuropharmacology 2018, 135, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Bigelow, L.J.; Fiset, C.; Jarvis, J.H.; Macleod, S.; Wöhr, M.; Benke, T.A.; Bernard, P.B. Early-life seizures modify behavioral response to ultrasonic vocalization playback in adult rats. Epilepsy Behav. 2022, 127, 108494. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, K.-A.; Fuchs, E.; Schwarting, R.K.; Wöhr, M. Effects of amphetamine on pro-social ultrasonic communication in juvenile rats: Implications for mania models. Eur. Neuropsychopharmacol. 2017, 27, 261–273. [Google Scholar] [CrossRef]
- Engelhardt, K.A.; Schwarting, R.K.W.; Wöhr, M. Mapping trait-like socio-affective phenotypes in rats through 50-kHz ultrasonic vocalizations. Psychopharmacology 2018, 235, 83–98. [Google Scholar] [CrossRef]
- Willuhn, I.; Tose, A.; Wanat, M.; Hart, A.S.; Hollon, N.G.; Phillips, P.; Schwarting, R.K.W.; Wöhr, M. Phasic Dopamine Release in the Nucleus Accumbens in Response to Pro-Social 50 kHz Ultrasonic Vocalizations in Rats. J. Neurosci. 2014, 34, 10616–10623. [Google Scholar] [CrossRef] [PubMed]
- Mogenson, G.J.; Jones, D.L.; Yim, C.Y. From motivation to action: Functional interface between the limbic system and the motor system. Prog. Neurobiol. 1980, 14, 69–97. [Google Scholar] [CrossRef]
- Sadananda, M.; Wöhr, M.; Schwarting, R.K. Playback of 22-kHz and 50-kHz ultrasonic vocalizations induces differential c-fos expression in rat brain. Neurosci. Lett. 2008, 435, 17–23. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 3rd ed.; Academic Press: San Diego, CA, USA; London, UK, 1997. [Google Scholar]
- Schönfeld, L.-M.; Zech, M.-P.; Schäble, S.; Wöhr, M.; Kalenscher, T. Lesions of the rat basolateral amygdala reduce the behavioral response to ultrasonic vocalizations. Behav. Brain Res. 2020, 378, 112274. [Google Scholar] [CrossRef]
- Seffer, D.; Schwarting, R.K.; Wöhr, M. Pro-social ultrasonic communication in rats: Insights from playback studies. J. Neurosci. Methods 2014, 234, 73–81. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: San Diego, CA, USA; London, UK, 2006. [Google Scholar]
- Laubach, M.; Amarante, L.M.; Swanson, K.; White, S.R. What, If Anything, Is Rodent Prefrontal Cortex? eNeuro 2018, 5, e0315. [Google Scholar] [CrossRef]
- An Heukelum, S.; Mars, R.B.; Guthrie, M.; Buitelaar, J.K.; Beckmann, C.F.; Tiesinga, P.H.; Vogt, B.A.; Glennon, J.C.; Havenith, M.N. Where is Cingulate Cortex? A Cross-Species View. Trends Neurosci. 2020, 43, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Rudy, B.; Fishell, G.; Lee, S.; Hjerling-Leffler, J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev. Neurobiol. 2011, 71, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Kabbaj, M.; Akil, H. Individual differences in novelty-seeking behavior in rats: A c-fos study. Neuroscience 2001, 106, 535–545. [Google Scholar] [CrossRef]
- Sullivan, R.M.; Gratton, A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J. Neurosci. 1999, 19, 2834–2840. [Google Scholar] [CrossRef]
- Davidson, R.J. Cerebral asymmetry, emotions and affective style. In Brain Asymmetry; Davidson, R.J., Hughdahl, K., Eds.; MIT Press: Cambridge, MA, USA, 1998; pp. 361–387. [Google Scholar]
- Karolis, V.R.; Corbetta, M.; de Schotten, M.T. The architecture of functional lateralisation and its relationship to callosal connectivity in the human brain. Nat. Commun. 2019, 10, 1417. [Google Scholar] [CrossRef]
- Thiel, C.; Schwarting, R. Dopaminergic Lateralisation in the Forebrain: Relations to Behavioural Asymmetries and Anxiety in Male Wistar Rats. Neuropsychobiology 2001, 43, 192–199. [Google Scholar] [CrossRef]
- Cerqueira, J.J.; Almeida, O.F.; Sousa, N. The stressed prefrontal cortex. Left? Right! Brain Behav. Immun. 2008, 22, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Czéh, B.; Perez-Cruz, C.; Fuchs, E.; Flügge, G. Chronic stress-induced cellular changes in the medial prefrontal cortex and their potential clinical implications: Does hemisphere location matter? Behav. Brain Res. 2008, 190, 1–13. [Google Scholar] [CrossRef]
- Costa, N.S.; Vicente, M.A.; Cipriano, A.C.; Miguel, T.T.; Nunes-de-Souza, R.L. Functional lateralization of the medial prefrontal cortex in the modulation of anxiety in mice: Left or right? Neuropharmacology 2016, 108, 82–90. [Google Scholar] [CrossRef]
- Monosov, I.E.; Haber, S.N.; Leuthardt, E.C.; Jezzini, A. Anterior Cingulate Cortex and the Control of Dynamic Behavior in Primates. Curr. Biol. 2020, 30, R1442–R1454. [Google Scholar] [CrossRef]
- Burgos-Robles, A.; Gothard, K.M.; Monfils, M.H.; Morozov, A.; Vicentic, A. Conserved features of anterior cingulate networks support observational learning across species. Neurosci. Biobehav. Rev. 2019, 107, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Tang, P.; Liang, Y.; Li, J.; Feng, J.; Zhang, N.; Lu, D.; He, J.; Chen, X. The anterior cingulate cortex directly enhances auditory cortical responses in air-puffing-facilitated flight behavior. Cell Rep. 2022, 38, 110506. [Google Scholar] [CrossRef] [PubMed]
- Apps, M.A.; Rushworth, M.F.; Chang, S.W. The Anterior Cingulate Gyrus and Social Cognition: Tracking the Motivation of Others. Neuron 2016, 90, 692–707. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.J.G.; Maier, E.; Brecht, M. Involvement of rat posterior prelimbic and cingulate area 2 in vocalization control. Eur. J. Neurosci. 2019, 50, 3164–3180. [Google Scholar] [CrossRef]
- Burgdorf, J.; Wood, P.L.; Kroes, R.A.; Moskal, J.R.; Panksepp, J. Neurobiology of 50-kHz ultrasonic vocalizations in rats: Electrode mapping, lesion, and pharmacology studies. Behav. Brain Res. 2007, 182, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Okanoya, K. Response characteristics of the rat anterior cingulate cortex to ultrasonic communicative vocalizations. NeuroReport 2017, 28, 479–484. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berz, A.; Pasquini de Souza, C.; Wöhr, M.; Steinmüller, S.; Bruntsch, M.; Schäfer, M.K.-H.; Schwarting, R.K.W. Contingent Social Interaction Does Not Prevent Habituation towards Playback of Pro-Social 50-kHz Calls: Behavioral Responses and Brain Activation Patterns. Brain Sci. 2022, 12, 1474. https://doi.org/10.3390/brainsci12111474
Berz A, Pasquini de Souza C, Wöhr M, Steinmüller S, Bruntsch M, Schäfer MK-H, Schwarting RKW. Contingent Social Interaction Does Not Prevent Habituation towards Playback of Pro-Social 50-kHz Calls: Behavioral Responses and Brain Activation Patterns. Brain Sciences. 2022; 12(11):1474. https://doi.org/10.3390/brainsci12111474
Chicago/Turabian StyleBerz, Annuska, Camila Pasquini de Souza, Markus Wöhr, Sebastian Steinmüller, Maria Bruntsch, Martin K.-H. Schäfer, and Rainer K. W. Schwarting. 2022. "Contingent Social Interaction Does Not Prevent Habituation towards Playback of Pro-Social 50-kHz Calls: Behavioral Responses and Brain Activation Patterns" Brain Sciences 12, no. 11: 1474. https://doi.org/10.3390/brainsci12111474
APA StyleBerz, A., Pasquini de Souza, C., Wöhr, M., Steinmüller, S., Bruntsch, M., Schäfer, M. K.-H., & Schwarting, R. K. W. (2022). Contingent Social Interaction Does Not Prevent Habituation towards Playback of Pro-Social 50-kHz Calls: Behavioral Responses and Brain Activation Patterns. Brain Sciences, 12(11), 1474. https://doi.org/10.3390/brainsci12111474




