In Vivo Measurements of Transcranial Electrical Stimulation in Lesioned Human Brain: A Case Report
Abstract
1. Introduction
2. Case Presentation
3. Materials and Methods
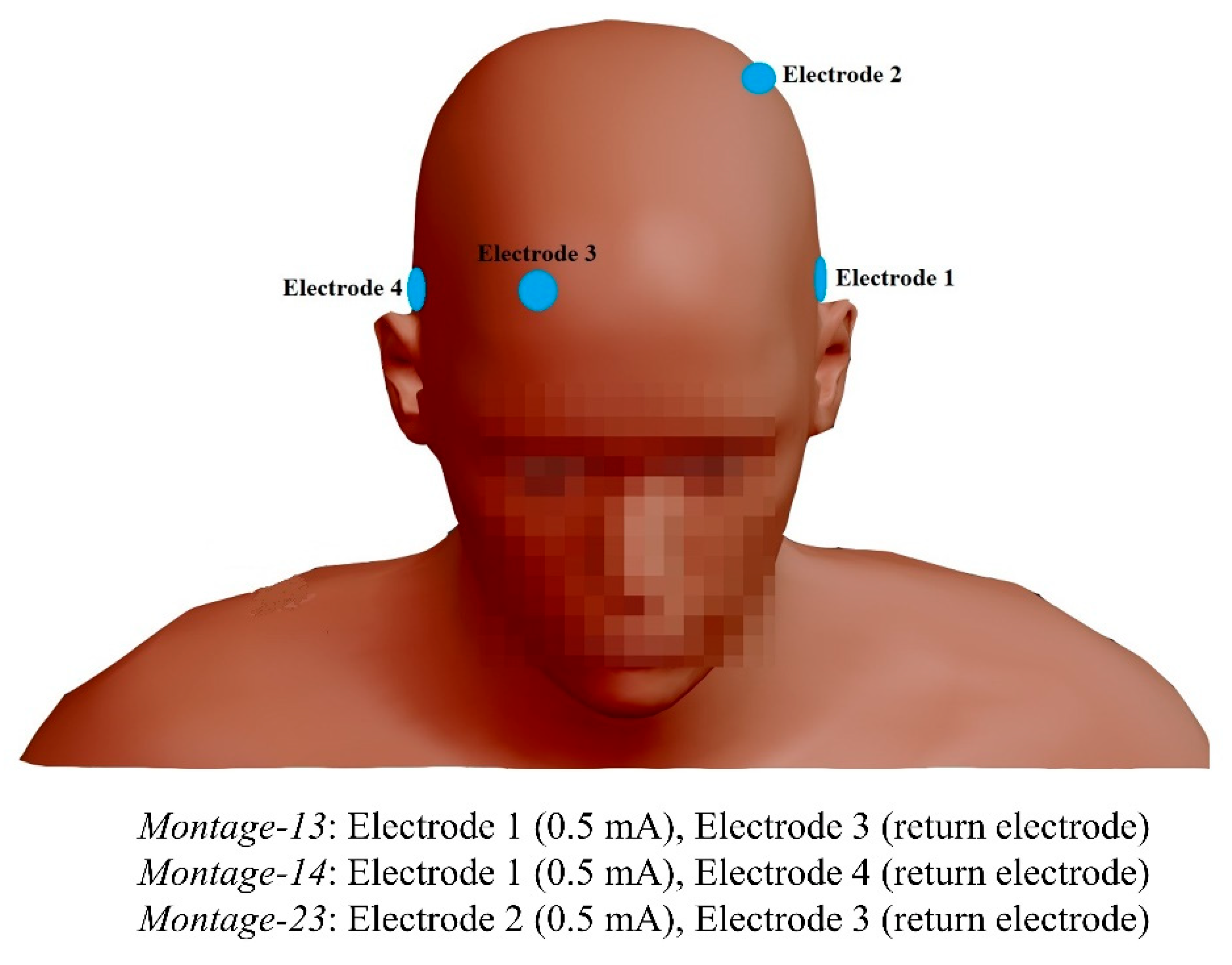
3.1. Transcranial Alternating Current Stimulation
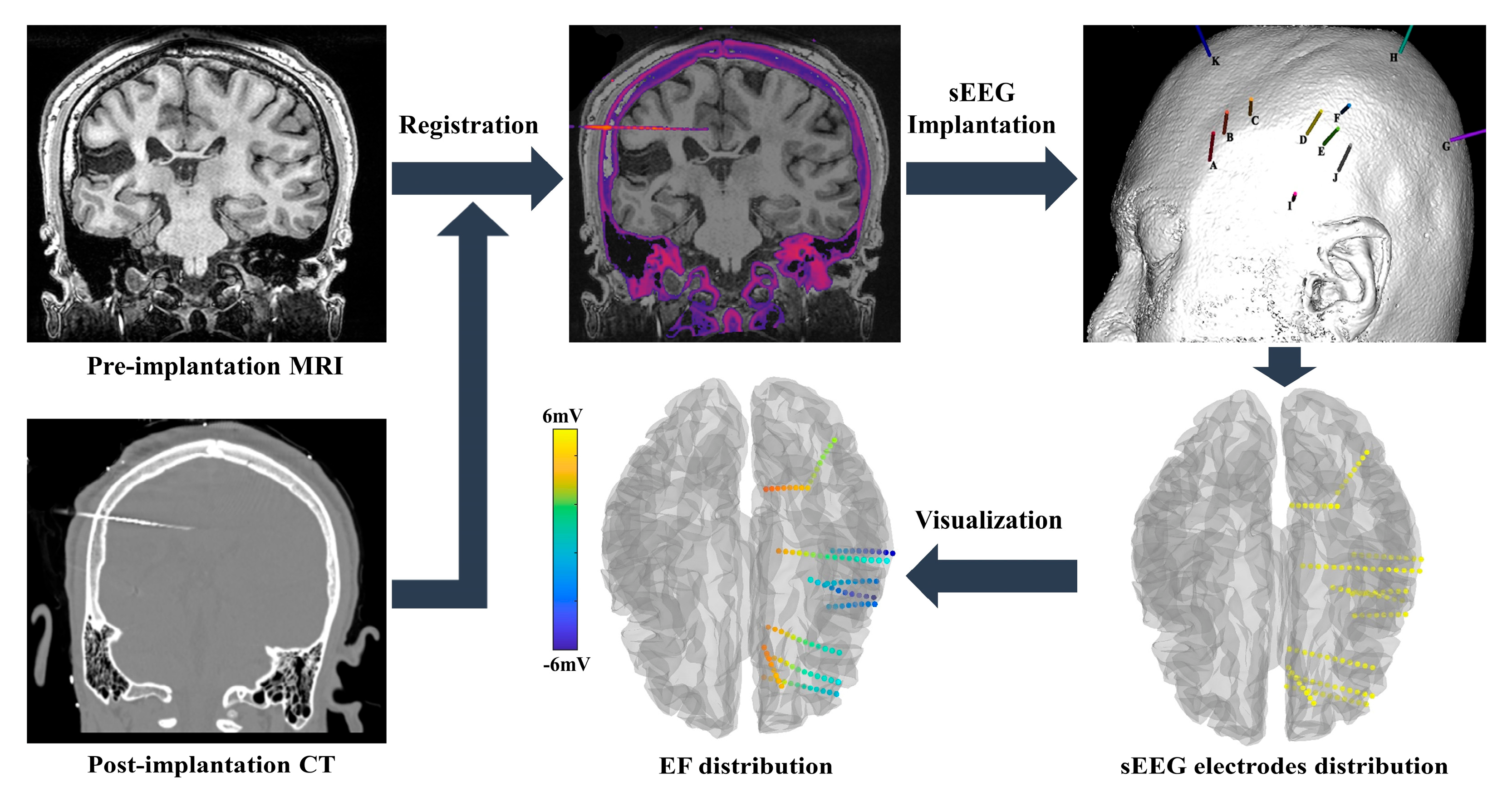
3.2. Intracranial Recording Setup
3.3. Model Simulations
3.4. Data Analysis
4. Measured and Simulated Electric Field of tES
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feil, S.; Eisenhut, P.; Strakeljahn, F.; Müller, S.; Nauer, C.; Bansi, J.; Weber, S.; Liebs, A.; Lefaucheur, J.-P.; Kesselring, J. Left Shifting of Language Related Activity Induced by Bihemispheric TDCS in Postacute Aphasia Following Stroke. Front. Neurosci. 2019, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Kim, D.Y.; Park, D.H. Enhancement of Cortical Excitability and Lower Limb Motor Function in Patients with Stroke by Transcranial Direct Current Stimulation. Brain Stimul. 2015, 8, 561–566. [Google Scholar] [CrossRef] [PubMed]
- San-Juan, D.; Morales-Quezada, L.; Garduño, A.J.O.; Alonso-Vanegas, M.; González-Aragón, M.F.; López, D.A.E.; Gregorio, R.V.; Anschel, D.J.; Fregni, F. Transcranial Direct Current Stimulation in Epilepsy. Brain Stimul. 2015, 8, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, Q.; Xu, C.; Fang, F.; Fan, J.; Li, L.; Du, Q.; Zhang, R.; Wang, Y.; Lin, Y.; et al. Transcranial Direct Current Stimulation Reduces Seizure Frequency in Patients with Refractory Focal Epilepsy: A Randomized, Double-Blind, Sham-Controlled, and Three-Arm Parallel Multicenter Study. Brain Stimul. 2020, 13, 109–116. [Google Scholar] [CrossRef]
- Regner, G.G.; Pereira, P.; Leffa, D.T.; De Oliveira, C.; Vercelino, R.; Fregni, F.; Torres, I.L. Preclinical to Clinical Translation of Studies of Transcranial Direct-Current Stimulation in the Treatment of Epilepsy: A Systematic Review. Front. Neurosci. 2018, 12, 189. [Google Scholar] [CrossRef]
- Dong, K.; Meng, S.; Guo, Z.; Zhang, R.; Xu, P.; Yuan, E.; Lian, T. The Effects of Transcranial Direct Current Stimulation on Balance and Gait in Stroke Patients: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 643. [Google Scholar] [CrossRef]
- Rawji, V.; Ciocca, M.; Zacharia, A.; Soares, D.; Truong, D.; Bikson, M.; Rothwell, J.; Bestmann, S. TDCS Changes in Motor Excitability Are Specific to Orientation of Current Flow. Brain Stimul. 2018, 11, 289–298. [Google Scholar] [CrossRef]
- Liu, A.; Vöröslakos, M.; Kronberg, G.; Henin, S.; Krause, M.R.; Huang, Y.; Opitz, A.; Mehta, A.; Pack, C.C.; Krekelberg, B. Immediate Neurophysiological Effects of Transcranial Electrical Stimulation. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- van der Cruijsen, J.; Piastra, M.C.; Selles, R.W.; Oostendorp, T.F. A Method to Experimentally Estimate the Conductivity of Chronic Stroke Lesions: A Tool to Individualize Transcranial Electric Stimulation. Front. Hum. Neurosci. 2021, 15, 738200. [Google Scholar] [CrossRef]
- Peterchev, A.V.; Wagner, T.A.; Miranda, P.C.; Nitsche, M.A.; Paulus, W.; Lisanby, S.H.; Pascual-Leone, A.; Bikson, M. Fundamentals of Transcranial Electric and Magnetic Stimulation Dose: Definition, Selection, and Reporting Practices. Brain Stimul. Basic Transl. Clin. Res. Neuromodul. 2012, 5, 435–453. [Google Scholar] [CrossRef]
- Puonti, O.; Van Leemput, K.; Saturnino, G.B.; Siebner, H.R.; Madsen, K.H.; Thielscher, A. Accurate and Robust Whole-Head Segmentation from Magnetic Resonance Images for Individualized Head Modeling. Neuroimage 2020, 219, 117044. [Google Scholar] [CrossRef] [PubMed]
- Piastra, M.C.; Van Der Cruijsen, J.; Piai, V.; Jeukens, F.E.; Manoochehri, M.; Schouten, A.C.; Selles, R.W.; Oostendorp, T. ASH: An Automatic Pipeline to Generate Realistic and Individualized Chronic Stroke Volume Conduction Head Models. J. Neural Eng. 2021, 18, 044001. [Google Scholar] [CrossRef] [PubMed]
- Ti, C.H.E.; Yuan, K.; Tong, R.K. TDCS Inter-Individual Variability in Electric Field Distribution for Chronic Stroke: A Simulation Study. In Proceedings of the 2021 10th International IEEE/EMBS Conference on Neural Engineering (NER), Online, 4–6 May 2021; pp. 1048–1051. [Google Scholar] [CrossRef]
- Dmochowski, J.P.; Datta, A.; Huang, Y.; Richardson, J.D.; Bikson, M.; Fridriksson, J.; Parra, L.C. Targeted Transcranial Direct Current Stimulation for Rehabilitation after Stroke. Neuroimage 2013, 75, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Opitz, A.; Falchier, A.; Yan, C.-G.; Yeagle, E.M.; Linn, G.S.; Megevand, P.; Thielscher, A.; Deborah, A.R.; Milham, M.P.; Mehta, A.D. Spatiotemporal Structure of Intracranial Electric Fields Induced by Transcranial Electric Stimulation in Humans and Nonhuman Primates. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, A.A.; Lafon, B.; Friedman, D.; Dayan, M.; Wang, X.; Bikson, M.; Doyle, W.K.; Devinsky, O.; Parra, L.C. Measurements and Models of Electric Fields in the in Vivo Human Brain during Transcranial Electric Stimulation. eLife 2017, 6, e18834. [Google Scholar] [CrossRef]
- Lafon, B.; Henin, S.; Huang, Y.; Friedman, D.; Melloni, L.; Thesen, T.; Doyle, W.; Buzsáki, G.; Devinsky, O.; Parra, L.C. Low Frequency Transcranial Electrical Stimulation Does Not Entrain Sleep Rhythms Measured by Human Intracranial Recordings. Nat. Commun. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Vöröslakos, M.; Takeuchi, Y.; Brinyiczki, K.; Zombori, T.; Oliva, A.; Fernández-Ruiz, A.; Kozák, G.; Kincses, Z.T.; Iványi, B.; Buzsáki, G. Direct Effects of Transcranial Electric Stimulation on Brain Circuits in Rats and Humans. Nat. Commun. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Louviot, S.; Tyvaert, L.; Maillard, L.G.; Colnat-Coulbois, S.; Dmochowski, J.; Koessler, L. Transcranial Electrical Stimulation Generates Electric Fields in Deep Human Brain Structures. Brain Stimul. 2022, 15, 1–12. [Google Scholar] [CrossRef]
- Esmaeilpour, Z.; Milosevic, M.; Azevedo, K.; Khadka, N.; Navarro, J.; Brunoni, A.; Popovic, M.R.; Bikson, M.; Fonoff, E.T. Proceedings# 21. Intracranial Voltage Recording during Transcranial Direct Current Stimulation (TDCS) in Human Subjects with Validation of a Standard Model. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 2017, 10, e72–e75. [Google Scholar]
- Chhatbar, P.Y.; Kautz, S.A.; Takacs, I.; Rowland, N.C.; Revuelta, G.J.; George, M.S.; Bikson, M.; Feng, W. Evidence of Transcranial Direct Current Stimulation-Generated Electric Fields at Subthalamic Level in Human Brain in Vivo. Brain Stimul. 2018, 11, 727–733. [Google Scholar] [CrossRef]
- Datta, A.; Bikson, M.; Fregni, F. Transcranial Direct Current Stimulation in Patients with Skull Defects and Skull Plates: High-Resolution Computational FEM Study of Factors Altering Cortical Current Flow. Neuroimage 2010, 52, 1268–1278. [Google Scholar] [CrossRef]
- Guidetti, M.; Arlotti, M.; Bocci, T.; Bianchi, A.M.; Parazzini, M.; Ferrucci, R.; Priori, A. Electric Fields Induced in the Brain by Transcranial Electric Stimulation: A Review of In Vivo Recordings. Biomedicines 2022, 10, 2333. [Google Scholar] [CrossRef]
- Huang, Y.; Datta, A.; Bikson, M.; Parra, L.C. Realistic Volumetric-Approach to Simulate Transcranial Electric Stimulation—ROAST—A Fully Automated Open-Source Pipeline. J. Neural Eng. 2019, 16, 056006. [Google Scholar] [CrossRef] [PubMed]
- McCann, H.; Pisano, G.; Beltrachini, L. Variation in Reported Human Head Tissue Electrical Conductivity Values. Brain Topogr. 2019, 32, 825–858. [Google Scholar] [CrossRef] [PubMed]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A User-Friendly Application for MEG/EEG Analysis. Comput. Intell. Neurosci. 2011, 2011, e879716. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Feng, T.; Jiang, H.; Zhu, J.; Feng, W.; Chhatbar, P.Y.; Zhang, J.; Zhang, S. In Vivo Measurements of Electric Fields during Cranial Electrical Stimulation in the Human Brain. Front. Hum. Neurosci. 2022, 16. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Baker, J.M.; Bikson, M.; Fridriksson, J. Individualized Model Predicts Brain Current Flow during Transcranial Direct-Current Stimulation Treatment in Responsive Stroke Patient. Brain Stimul. 2011, 4, 169–174. [Google Scholar] [CrossRef]
- Minjoli, S.; Saturnino, G.B.; Blicher, J.U.; Stagg, C.J.; Siebner, H.R.; Antunes, A.; Thielscher, A. The Impact of Large Structural Brain Changes in Chronic Stroke Patients on the Electric Field Caused by Transcranial Brain Stimulation. NeuroImage Clin. 2017, 15, 106–117. [Google Scholar] [CrossRef]
- Johnstone, A.; Zich, C.; Evans, C.; Lee, J.; Ward, N.; Bestmann, S. The Impact of Brain Lesions on TDCS-Induced Electric Field Magnitude. bioRxiv 2021. bioRxiv:19-436124. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Wang, M.; Wu, D.; Zhang, J.; Zhang, S. In Vivo Measurements of Transcranial Electrical Stimulation in Lesioned Human Brain: A Case Report. Brain Sci. 2022, 12, 1455. https://doi.org/10.3390/brainsci12111455
Jiang H, Wang M, Wu D, Zhang J, Zhang S. In Vivo Measurements of Transcranial Electrical Stimulation in Lesioned Human Brain: A Case Report. Brain Sciences. 2022; 12(11):1455. https://doi.org/10.3390/brainsci12111455
Chicago/Turabian StyleJiang, Hongjie, Minmin Wang, Dan Wu, Jianmin Zhang, and Shaomin Zhang. 2022. "In Vivo Measurements of Transcranial Electrical Stimulation in Lesioned Human Brain: A Case Report" Brain Sciences 12, no. 11: 1455. https://doi.org/10.3390/brainsci12111455
APA StyleJiang, H., Wang, M., Wu, D., Zhang, J., & Zhang, S. (2022). In Vivo Measurements of Transcranial Electrical Stimulation in Lesioned Human Brain: A Case Report. Brain Sciences, 12(11), 1455. https://doi.org/10.3390/brainsci12111455






