Ventriculoperitoneal Shunt Treatment Increases 7 Alpha Hy-Droxy-3-Oxo-4-Cholestenoic Acid and 24-Hydroxycholesterol Concentrations in Idiopathic Normal Pressure Hydrocephalus
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Study Setting
2.2. Laboratory Analyses
2.3. Statistical Analysis
2.4. Ethical Aspects
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 24-OH | 24S-hydroxycholesterol |
| 27-OH | 27-hydroxycholesterol |
| 7HOCA | 7 alpha hydroxy-3-oxo-4-cholestenoic acid |
| DHEA | Cortisone dehydroepiandrosterone |
| 7DHEA | 7 alpha hydroxy dehydroepiandrosterone |
| CSF | Cerebrospinal fluid |
| CYP7B1 | Cytochrome P450 family 7 subfamily B member 1 |
| CYP46 | Cytochrome P450 family 46 |
| iNPH | Idiopathic normal pressure hydrocephalus |
| VP | ventriculoperitoneal |
References
- Adams, R.D.; Fisher, C.M.; Hakim, S.; Ojemann, R.G.; Sweet, W.H. Symptomatic Occult Hydrocephalus with Normal Cerebrospinal-Fluid Pressure. N. Engl. J. Med. 2010, 97, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.A.; Malm, J. Diagnosis and Treatment of Idiopathic Normal Pressure Hydrocephalus. Continuum 2016, 22, 579–599. [Google Scholar] [CrossRef] [PubMed]
- Picascia, M.; Zangaglia, R.; Bernini, S.; Minafra, B.; Sinforiani, E.; Pacchetti, C. A Review of Cognitive Impairment and Differential Diagnosis in Idiopathic Normal Pressure Hydrocephalus. Funct. Neurol. 2015, 30, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. Editorial of Special Issue “Dissecting Neurological and Neuropsychiatric Diseases: Neurodegeneration and Neuroprotection”. Int. J. Mol. Sci. 2022, 23, 6991. [Google Scholar] [CrossRef]
- Battaglia, S.; Harrison, B.J.; Fullana, M.A. Does the Human Ventromedial Prefrontal Cortex Support Fear Learning, Fear Extinction or Both? A Commentary on Subregional Contributions. Mol. Psychiatry 2021, 27, 784–786. [Google Scholar] [CrossRef]
- Larsson, A.; Ärlig, A.; Bergh, A.C.; Bilting, M.; Jacobsson, L.; Stephensen, H.; Wikkelsö, C. Quantitative SPECT Cisternography in Normal Pressure Hydrocephalus. Acta Neurol. Scand. 1994, 90, 190–196. [Google Scholar] [CrossRef]
- Kristensen, B.; Malm, J.; Fagerlund, M.; Hietala, S.O.; Johansson, B.; Ekstedt, J.; Karlsson, T. Regional Cerebral Blood Flow, White Matter Abnormalities, and Cerebrospinal Fluid Hydrodynamics in Patients with Idiopathic Adult Hydrocephalus Syndrome. J. Neurol. Neurosurg. Psychiatry 1996, 60, 282–288. [Google Scholar] [CrossRef]
- Momjian, S.; Owler, B.K.; Czosnyka, Z.; Czosnyka, M.; Pena, A.; Pickard, J.D. Pattern of White Matter Regional Cerebral Blood Flow and Autoregulation in Normal Pressure Hydrocephalus. Brain 2004, 127, 965–972. [Google Scholar] [CrossRef]
- Lenfeldt, N.; Hansson, W.; Larsson, A.; Birgander, R.; Eklund, A.; Malm, J. Three-Day CSF Drainage Barely Reduces Ventricular Size in Normal Pressure Hydrocephalus. Neurology 2012, 79, 237–242. [Google Scholar] [CrossRef]
- Sosvorova, L.; Hill, M.; Mohapl, M.; Vitku, J.; Hampl, R. Steroid Hormones in Prediction of Normal Pressure Hydrocephalus. J. Steroid Biochem. Mol. Biol. 2015, 152, 124–132. [Google Scholar] [CrossRef]
- Meaney, S.; Heverin, M.; Panzenboeck, U.; Ekström, L.; Axelsson, M.; Andersson, U.; Diczfalusy, U.; Pikuleva, I.; Wahren, J.; Sattler, W.; et al. Novel Route for Elimination of Brain Oxysterols across the Blood-Brain Barrier: Conversion into 7alpha-Hydroxy-3-Oxo-4-Cholestenoic Acid. J. Lipid Res. 2007, 48, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Axelson, M.; Mork, B.; Sjovallt, J. Occurrence of 3 Beta-Hydroxy-5-Cholestenoic Acid, 3 Beta,7 Alpha-Dihydroxy-5-Cholestenoic Acid, and 7 Alpha-Hydroxy-3-Oxo-4-Cholestenoic Acid as Normal Constituents in Human Blood. J. Lipid Res. 1988, 29, 629–641. [Google Scholar] [CrossRef]
- Lund, E.; Andersson, O.; Zhang, J.; Babiker, A.; Ahlborg, G.; Diczfalusy, U.; Einarsson, K.; Sjövall, J.; Björkhem, I. Importance of a Novel Oxidative Mechanism for Elimination of Intracellular Cholesterol in Humans. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Axelson, M.; Bjoerkhem, I.; Matsutani, M.; Takakura, K. Significance of Cholesterol Metabolites in Chronic Subdural Hematoma. Recent Adv. Neurotraumatol. 1993, 49–52. [Google Scholar] [CrossRef]
- Ogundare, M.; Theofilopoulos, S.; Lockhart, A.; Hall, L.J.; Arenas, E.; Sjövall, J.; Brenton, A.G.; Wang, Y.; Griffiths, W.J. Cerebrospinal Fluid Steroidomics: Are Bioactive Bile Acids Present in Brain? J. Biol. Chem. 2010, 285, 4666–4679. [Google Scholar] [CrossRef]
- Saeed, A.; Floris, F.; Andersson, U.; Pikuleva, I.; LÖvgren-Sandblom, A.; Bjerke, M.; Paucar, M.; Wallin, A.; Svenningsson, P.; BjÖrkhem, I. 7α-Hydroxy-3-Oxo-4-Cholestenoic Acid in Cerebrospinal Fluid Reflects the Integrity of the Blood-Brain Barrier. J. Lipid Res. 2014, 55, 313–318. [Google Scholar] [CrossRef]
- Björkhem, I.; Leoni, V.; Svenningsson, P. On the Fluxes of Side-Chain Oxidized Oxysterols across Blood-Brain and Blood-CSF Barriers and Origin of These Steroids in CSF (Review). J. Steroid Biochem. Mol. Biol. 2019, 188, 86–89. [Google Scholar] [CrossRef]
- Leoni, V.; Masterman, T.; Patel, P.; Meaney, S.; Diczfalusy, U.; Björkhem, I. Side Chain Oxidized Oxysterols in Cerebrospinal Fluid and the Integrity of Blood-Brain and Blood-Cerebrospinal Fluid Barriers. J. Lipid Res. 2003, 44, 793–799. [Google Scholar] [CrossRef]
- Heverin, M.; Maioli, S.; Pham, T.; Mateos, L.; Camporesi, E.; Ali, Z.; Winblad, B.; Cedazo-Minguez, A.; Björkhem, I. 27-Hydroxycholesterol Mediates Negative Effects of Dietary Cholesterol on Cognition in Mice. Behav. Brain Res. 2015, 278, 356–359. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, C.; An, Y.; Liu, Q.; Rong, H.; Tao, L.; Wang, Y.; Wang, Y.; Xiao, R. Increased Levels of 27-Hydroxycholesterol Induced by Dietary Cholesterol in Brain Contribute to Learning and Memory Impairment in Rats. Mol. Nutr. Food Res. 2018, 62, 1–10. [Google Scholar] [CrossRef]
- Ismail, M.A.M.; Mateos, L.; Maioli, S.; Merino-Serrais, P.; Ali, Z.; Lodeiro, M.; Westman, E.; Leitersdorf, E.; Gulyás, B.; Olof-Wahlund, L.; et al. 27-Hydroxycholesterol Impairs Neuronal Glucose Uptake through an IRAP/GLUT4 System Dysregulation. J. Exp. Med. 2017, 214, 699–717. [Google Scholar] [CrossRef] [PubMed]
- Heverin, M.; Bogdanovic, N.; Lütjohann, D.; Bayer, T.; Pikuleva, I.; Bretillon, L.; Diczfalusy, U.; Winblad, B.; Björkhem, I. Changes in the Levels of Cerebral and Extracerebral Sterols in the Brain of Patients with Alzheimer’s Disease. J. Lipid Res. 2004, 45, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Shafaati, M.; Marutle, A.; Pettersson, H.; Lövgren-Sandblom, A.; Olin, M.; Pikuleva, I.; Winblad, B.; Nordberg, A.; Björkhem, I. Marked Accumulation of 27-Hydroxycholesterol in the Brains of Alzheimer’s Patients with the Swedish APP 670/671 Mutation. J. Lipid Res. 2011, 52, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; An, Y.; Yu, H.; Lu, Y.; Feng, L.; Wang, C.; Xiao, R. Relationship between Oxysterols and Mild Cognitive Impairment in the Elderly: A Case-Control Study. Lipids Health Dis. 2016, 15, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mateos, L.; Ismail, M.A.M.; Gil-Bea, F.J.; Schüle, R.; Schöls, L.; Heverin, M.; Folkesson, R.; Björkhem, I.; Cedazo-Mínguez, A. Side Chain-Oxidized Oxysterols Regulate the Brain Renin-Angiotensin System through a Liver X Receptor-Dependent Mechanism. J. Biol. Chem. 2011, 286, 25574–25585. [Google Scholar] [CrossRef] [PubMed]
- Merino-Serrais, P.; Loera-Valencia, R.; Rodriguez-Rodriguez, P.; Parrado-Fernandez, C.; Ismail, M.A.; Maioli, S.; Matute, E.; Jimenez-Mateos, E.M.; Björkhem, I.; Defelipe, J.; et al. 27-Hydroxycholesterol Induces Aberrant Morphology and Synaptic Dysfunction in Hippocampal Neurons. Cereb. Cortex 2019, 29, 429–446. [Google Scholar] [CrossRef]
- Sandebring-Matton, A.; Goikolea, J.; Björkhem, I.; Paternain, L. Reduction in Circulating 27-Hydroxycholesterol during Multidomaine Lifestyle/Vascular Intervention Is Associated with Improvement in Cognition. Alzheimer Res. Ther. 2021, 13, 56. [Google Scholar] [CrossRef]
- Loera-Valencia, R.; Ismail, M.A.M.; Goikolea, J.; Lodeiro, M.; Mateos, L.; Björkhem, I.; Puerta, E.; Romão, M.A.; Gomes, C.M.; Merino-Serrais, P.; et al. Hypercholesterolemia and 27-Hydroxycholesterol Increase S100A8 and RAGE Expression in the Brain: A Link Between Cholesterol, Alarmins, and Neurodegeneration. Mol. Neurobiol. 2021, 58, 6063–6076. [Google Scholar] [CrossRef]
- Nagata, K.; Seyama, Y.; Shimizu, T. Changes in the Level of 7 Alpha-Hydroxy-3-Oxo-4-Cholestenoic Acid in Cerebrospinal Fluid after Subarachnoid Hemorrhage. Neurol. Med. Chir. 1995, 35, 294–297. [Google Scholar] [CrossRef]
- Saeed, A.A.; Edström, E.; Pikuleva, I.; Eggertsen, G.; Björkhem, I. On the Importance of Albumin Binding for the Flux of 7α-Hydroxy-3-Oxo-4-Cholestenoic Acid in the Brain. J. Lipid Res. 2017, 58, 455–459. [Google Scholar] [CrossRef]
- Sodero, A.O. 24S-Hydroxycholesterol: Cellular Effects and Variations in Brain Diseases. J. Neurochem. 2021, 157, 899–918. [Google Scholar] [CrossRef] [PubMed]
- Relkin, N.; Marmarou, A.; Klinge, P.; Bergsneider, M.; Black, P.M. Diagnosing Idiopathic Normal-Pressure Hydrocephalus. Neurosurgery 2005, 57, S24–S216. [Google Scholar] [CrossRef] [PubMed]
- Hellström, P.; Klinge, P.; Tans, J.; Wikkelsø, C. A New Scale for Assessment of Severity and Outcome in INPH. Acta Neurol. Scand. 2012, 126, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; Staurenghi, E.; Zerbinati, C.; Gargiulo, S.; Iuliano, L.; Giaccone, G.; Fantò, F.; Poli, G.; Leonarduzzi, G.; Gamba, P. Changes in Brain Oxysterols at Different Stages of Alzheimer’s Disease: Their Involvement in Neuroinflammation. Redox Biol. 2016, 10, 24–33. [Google Scholar] [CrossRef]
- Björkhem, I. Crossing the Barrier: Oxysterols as Cholesterol Transporters and Metabolic Modulators in the Brain. J. Intern. Med. 2006, 260, 493–508. [Google Scholar] [CrossRef]
- Lenfeldt, N.; Hauksson, J.; Birgander, R.; Eklund, A.; Malm, J. Improvement after Cerebrospinal Fluid Drainage Is Related to Levels of N-Acetyl-Aspartate in Idiopathic Normal Pressure Hydrocephalus. Neurosurgery 2008, 62, 135–141. [Google Scholar] [CrossRef]
- Eide, P.K.; Hansson, H.A. Astrogliosis and Impaired Aquaporin-4 and Dystrophin Systems in Idiopathic Normal Pressure Hydrocephalus. Neuropathol. Appl. Neurobiol. 2018, 44, 474–490. [Google Scholar] [CrossRef]
- Hasan-Olive, M.M.; Enger, R.; Hansson, H.A.; Nagelhus, E.A.; Eide, P.K. Loss of Perivascular Aquaporin-4 in Idiopathic Normal Pressure Hydrocephalus. Glia 2019, 67, 91–100. [Google Scholar] [CrossRef]
- Trillo-Contreras, J.L.; Ramírez-Lorca, R.; Villadiego, J.; Echevarría, M. Cellular Distribution of Brain Aquaporins and Their Contribution to Cerebrospinal Fluid Homeostasis and Hydrocephalus. Biomolecules 2022, 12, 530. [Google Scholar] [CrossRef]
- Liu, C.; Li, G.; Wang, P.; Wang, Y.; Pan, J. Characterization of Spontaneous Hydrocephalus Development in the Young Atherosclerosis-Prone Mice. Neuroreport 2017, 28, 1108–1114. [Google Scholar] [CrossRef]
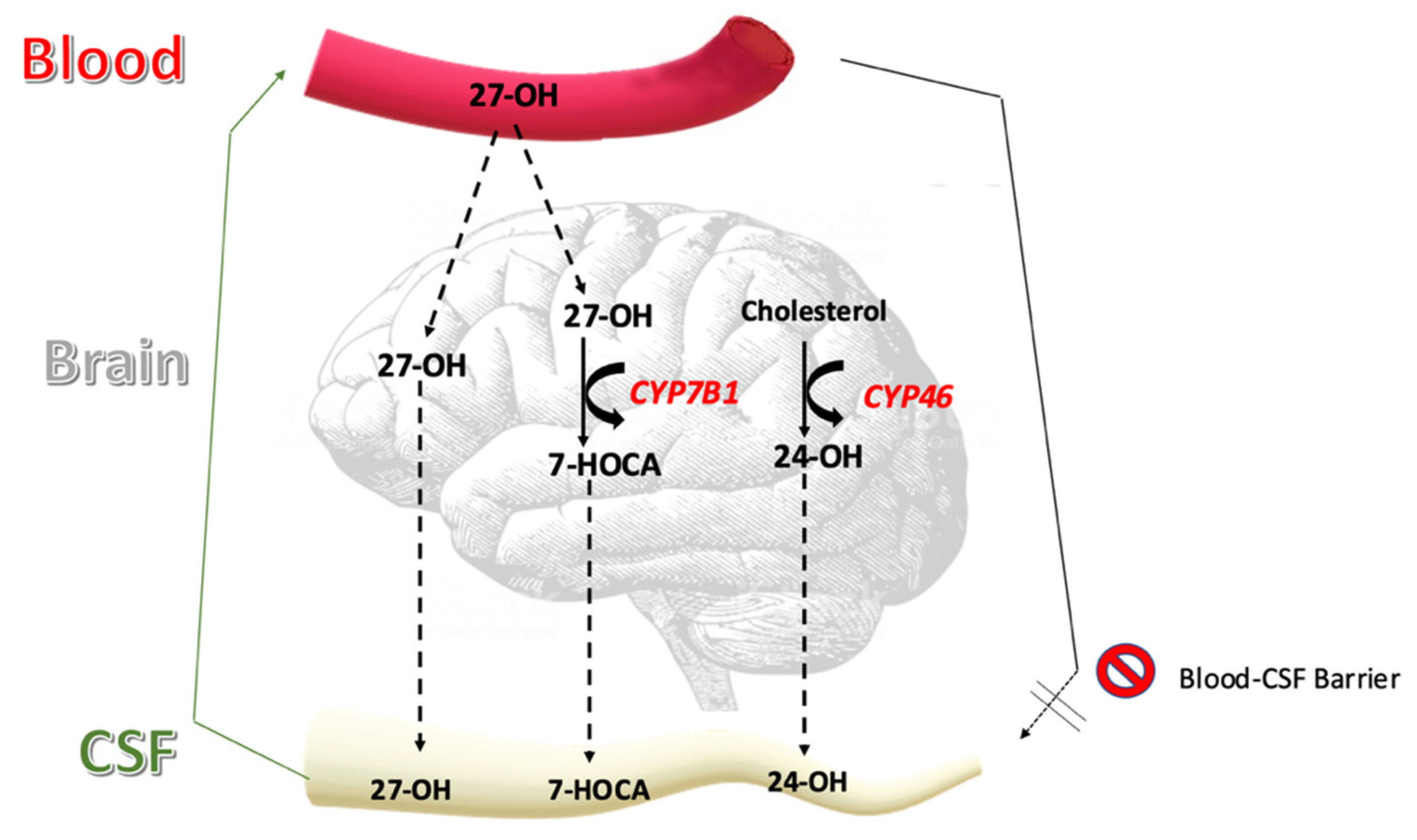
| Oxysterol | Treatment (n = 17) | Control (n = 28) | p-Value |
|---|---|---|---|
| Albumin (mg/L) | 243 (72–529) | 205 (115–483), (5 missing) | 0.547 |
| Cholesterol (µg/mL) | 2.3 (1.3–12), (1 missing) | 3.9 (1.9–7.5) | <0.001 |
| 24-OH (ng/mL) | 1.1 (0.5–4.0) | 1.9 (0.8–5.4) | 0.006 |
| 27-OH (ng/mL) | 0.7 (0.3–1.7) | 1.1 (0.4–2.5) | 0.001 |
| 7HOCA (ng/mL) | 14 (6.8–28) | 17 (10–31), (2 missing) | 0.186 |
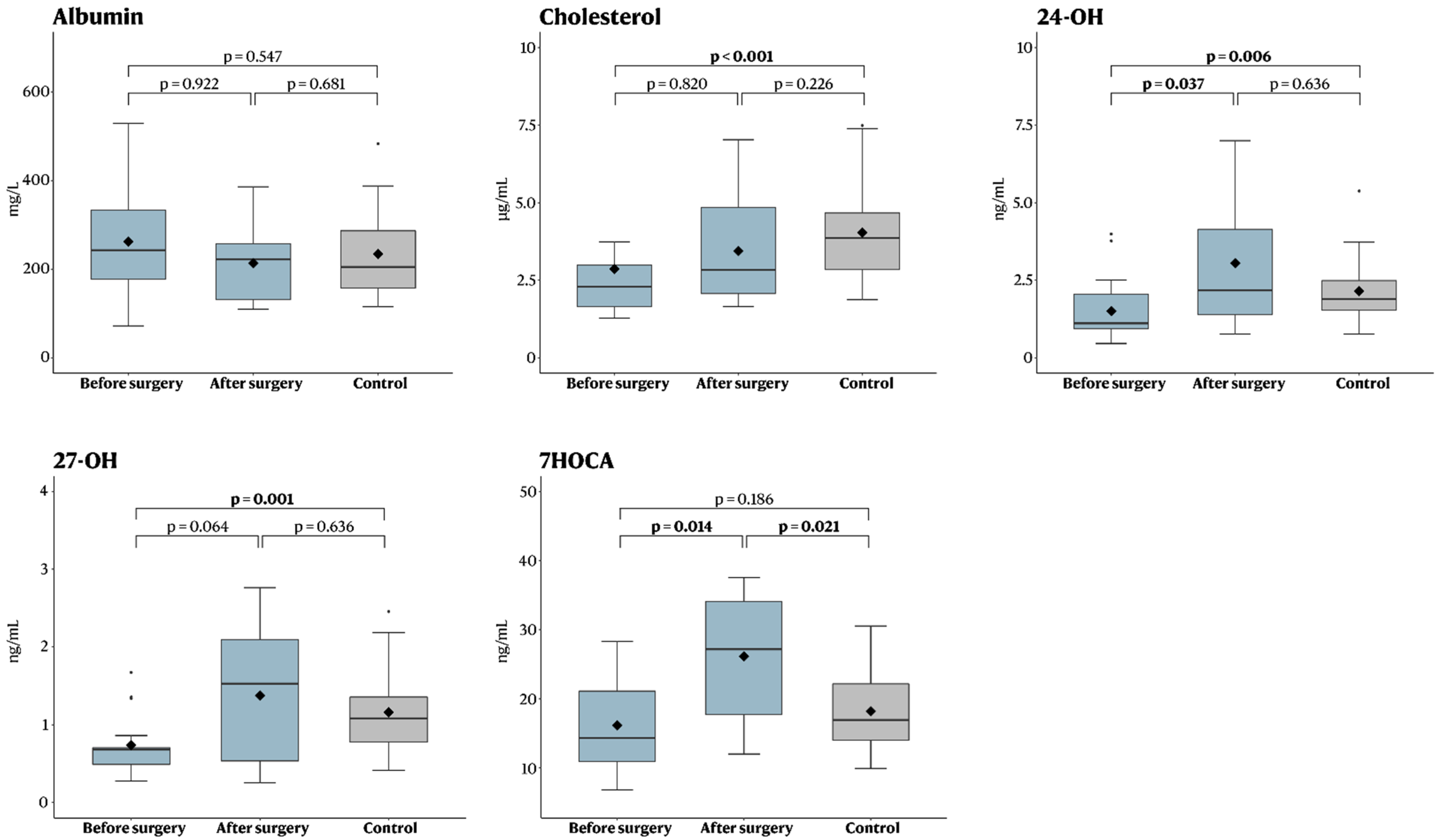
| Oxysterol | Before Surgery (n = 10) | After Surgery (n = 10) | p-Value |
|---|---|---|---|
| Albumin (mg/L) | 215 (72–518) | 222 (110–386) | 0.922 |
| Cholesterol (µg/mL) | 2.5 (1.3–12) (1 missing) | 2.8 (1.7–7.0) (1 missing) | 0.820 |
| 24-OH (ng/mL) | 1.6 (0.6–4.0) | 2.2 (0.8–7.0) | 0.037 |
| 27-OH (ng/mL) | 0.7 (0.4–1.7) | 1.5 (0.2–2.8) | 0.064 |
| 7HOCA (ng/mL) | 17 (6.8–28) | 27 (12–38) | 0.014 |
| Oxysterol | Treatment (n = 17) | Control (n = 28) | p-Value |
|---|---|---|---|
| Albumin (mg/L) | 222 (110–386) (7 missing) | 205 (115–483) (5 missing) | 0.681 |
| Cholesterol (µg/mL) | 2.8 (1.7–7.0) (8 missing) | 3.9 (1.9–7.5) | 0.226 |
| 24-OH (ng/mL) | 2.2 (0.8–7.0) (7 missing) | 1.9 (0.8–5.4) | 0.636 |
| 27-OH (ng/mL) | 1.5 (0.2–2.8) (7 missing) | 1.1 (0.4–2.5) | 0.636 |
| 7HOCA (ng/mL) | 27 (12–38) (7 missing) | 17 (10–31) (2 missing) | 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porru, E.; Edström, E.; Arvidsson, L.; Elmi-Terander, A.; Fletcher-Sandersjöö, A.; Sandblom, A.L.; Hansson, M.; Duell, F.; Björkhem, I. Ventriculoperitoneal Shunt Treatment Increases 7 Alpha Hy-Droxy-3-Oxo-4-Cholestenoic Acid and 24-Hydroxycholesterol Concentrations in Idiopathic Normal Pressure Hydrocephalus. Brain Sci. 2022, 12, 1450. https://doi.org/10.3390/brainsci12111450
Porru E, Edström E, Arvidsson L, Elmi-Terander A, Fletcher-Sandersjöö A, Sandblom AL, Hansson M, Duell F, Björkhem I. Ventriculoperitoneal Shunt Treatment Increases 7 Alpha Hy-Droxy-3-Oxo-4-Cholestenoic Acid and 24-Hydroxycholesterol Concentrations in Idiopathic Normal Pressure Hydrocephalus. Brain Sciences. 2022; 12(11):1450. https://doi.org/10.3390/brainsci12111450
Chicago/Turabian StylePorru, Emanuele, Erik Edström, Lisa Arvidsson, Adrian Elmi-Terander, Alexander Fletcher-Sandersjöö, Anita Lövgren Sandblom, Magnus Hansson, Frida Duell, and Ingemar Björkhem. 2022. "Ventriculoperitoneal Shunt Treatment Increases 7 Alpha Hy-Droxy-3-Oxo-4-Cholestenoic Acid and 24-Hydroxycholesterol Concentrations in Idiopathic Normal Pressure Hydrocephalus" Brain Sciences 12, no. 11: 1450. https://doi.org/10.3390/brainsci12111450
APA StylePorru, E., Edström, E., Arvidsson, L., Elmi-Terander, A., Fletcher-Sandersjöö, A., Sandblom, A. L., Hansson, M., Duell, F., & Björkhem, I. (2022). Ventriculoperitoneal Shunt Treatment Increases 7 Alpha Hy-Droxy-3-Oxo-4-Cholestenoic Acid and 24-Hydroxycholesterol Concentrations in Idiopathic Normal Pressure Hydrocephalus. Brain Sciences, 12(11), 1450. https://doi.org/10.3390/brainsci12111450






